?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Sphere-capped ferrocene nanospheres with Schiff base spacers have been prepared using a template, and used as carriers to immobilize glucose oxidase (GOx). GOx immobilized on spheres with one C-spacer (APS–Fc) exhibited high binding affinity to the substrate, which was attributed to appropriate position for the GOx conformation. When glucose oxidase was immobilized with spacers of different lengths, it was found that storage stability decreased with increasing the length of the spacer. It has been found that nanospheres, including capped ferrocene, exhibit good performance as the immobilized supporters of GOx. (APS–EtFc–GOx) retain more than 10% of the initial activity after forty-two successive cycles, which is a remarkable result.
Keywords::
Introduction
Immobilization methods have been widely used for biocatalysts (CitationPawar and Yadav 2014) due to their significant advantages such as simplicity of procedure, inexpensive instrumentation, high stability, and automated detection. Methods of immobilization based on nanomaterials have recently attracted considerable attention in the quality control of medicines and food (CitationLiangliang et al. 2015, CitationMani et al. 2014). In particular, due to its usefulness in the diagnostic analysis of diabetes, immobilization of glucose oxidase (GOx) based on nanoparticles has been extensively studied. GOx catalyzes the oxidation of glucose to gluconic acid in the presence of oxygen, and generates hydrogen peroxide. It therefore requires a cofactor, flavin adenine dinucleotide (FAD). In the catalysis reaction, FAD functions as the electron acceptor and is then reduced to FADH2.
Recently, Colak et al. used electrochemical methods for the immobilization of GOx on composite film; Arslan and Beskan have reported excellent results as electron transfer mediators of potassium ferricyanide for determination of glucose. Generally, mediators are used for determination of glucose (CitationColak et al. 2012, CitationArslan and Beskan 2014, CitationStege et al. 2010). Due to their value in the electron transfer reaction between GOx and electrodes, several redox-active polymers have been prepared (CitationJiang et al. 2008, CitationDursun et al. 2012). Ferrocene and its derivatives are excellent electron transfer mediators (CitationArmada et al. 2004), due to their high chemical stability.
However, soluble mediators such as ferrocene can easily diffuse away from the study media into the bulk solution (CitationLiang et al. 2011). A method has been proposed to solve this problem and retain the covalently attached mediator on the polymers (CitationHodak et al. 1997, CitationDursun et al. 2012).
In 1997, Hodak et al. used cationic poly(allylamine) modified by ferrocene to facilitate electron transfer. The attachment of ferrocene as a mediator to poly(allylamine hydrochloride) has been reported by Jian-Ding CitationQui et al. (2011). Cong Liang and coworkers (CitationLiang et al. 2012) successfully modified Fe3O4 nanoparticles with ferrocene for a magneto-controlled glucose biosensing system.
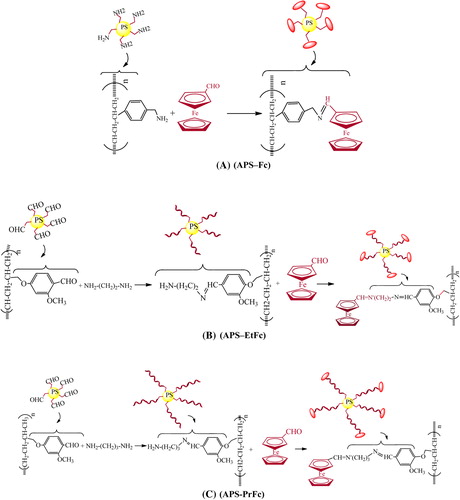
In the present work, we report a simple route for the synthesis of nanospheres with ferrocene-tagged polystyrene–ANH2/(4-Formyl-3-methoxyphenoxy methyl)polystyrene for immobilization of GOx. We propose that the tagged mediator () might be important for the performance of storage stability and reusability of the immobilized enzyme.
Materials and methods
Materials and physical measurements
All chemicals were reagent grade (Sigma-Aldrich Company). Glucose oxidase (β-D-glucose: oxygen-l-oxidoreductase, EC 1.1.3.4) from Aspergillus niger was purchased from Sigma Chemical Company (SIGMA, 49180). Its molecular weight and pI were 160 000 Da and 4.2, respectively. 4-aminoantipyrene (4-AAP), phenol, (aminomethyl)polystyrene [(APS);100–200 mesh, extent of labeling: 1.0 mmol/g N loading, 1% cross-linked (Aldrich)], (4-Formyl-3-methoxyphenoxymethyl)polystyrene [(FMPS); 1 g, 100–200 mesh, 1.0–1.5 mmol/g –CHO loading, 1% cross-linked with divinylbenzene], ferrocenecarboxaldehyde, ethylenediamine, and 1,3-diaminopropane were purchased from Sigma (St. Louis, MO). Elemental analyses were carried out with a LECO, CHNS-932 instrument. IR spectra were recorded on a Mattson-5000 FT-IR instrument with KBr pellets. TGA of the polymeric Schiff bases and their complexes were recorded by a Setaram simultaneous model thermal analyzer under a nitrogen atmosphere between 25 and 900°C at a heating rate of 10°C min− 1. Scanning electron microscopy of the Au-Pd-coated compounds was done using a JEOL JEM 100 CX II scanning electron microscope (JEOL, Peabody, MA) equipped with a Link analytical system. The electron energy used was 20 keV. The gel permeation chromatography (GPC) measurements were recorded using Waters 1500 Series GPC.
Synthesis of polymer-attached ferrocene nanospheres with one C-spacer (APS–Fc)
Nanospheres containing ferrocene were prepared by reacting (aminomethyl) polystyrene (APS) (1 g, 100–200 mesh, 0.5–1.0 mmol/g –NH2 loaded, 1% cross-linked (Aldrich)) in hot DMF (15 mL) with ferrocenecarboxaldehyde (1.0 mmol) in DMF (5 mL), and were stirred for 2 h under a reflux condenser at 50°C (). After the mixture cooled to room temperature, polymer–ferrocene complexes were poured into acetone and washed by adding more acetone. The resulting solid was filtered and dried in a oven.
Synthesis of polymer-attached ferrocene nanospheres with two C-spacers (APS–EtFc)
Nanospheres containing ferrocene were prepared by reacting of 4-Formyl-3-methoxyphenoxymethyl)polystyrene (FMPS) (1 g, 100–200 mesh, 0.5–1.0 mmol/g –NH2-loaded, 1% cross-linked (Aldrich)) in hot DMF (15 mL) with ethylenediamine (1.0 mmol) in DMF (5 mL). Ethylenediamine was slowly added dropwise on FMPS (in hot DMF, 15 mL) while stirring, through a period of 30 min. This mixture was stirred under a reflux condenser ca. 2 h, at 50°C. ().Then, ferrocenecarboxaldehyde solution was added to this reaction mixture while stirring, through a period of 1 h. The reaction mixture was boiled and stirred under a reflux condenser ca. 2 h, at 50°C. After the mixture cooled to room temperature, polymer–ferrocene complexes were poured into acetone and washed by adding more acetone. The resulting solid was filtered and dried in a oven.
Synthesis of polymer-attached ferrocene nanospheres with three C-spacers (APS–PrFc)
Nanospheres containing ferrocene were prepared by reacting 4-Formyl-3-methoxyphenoxymethyl)polystyrene (FMPS) (1 g, 100–200 mesh, 0.5–1.0 mmol/g –NH2 loaded, 1% cross-linked (Aldrich)) in hot DMF (15 mL) with ethylenediamine (1.0 mmol) in DMF (5 mL). 1,3-diaminopropane was slowly added dropwise on FMPS (in hot DMF, 15 mL) while stirring, through a period of 30 min. This mixture was stirred under a reflux condenser ca. 2 h, at 50°C. (). Then, ferrocenecarboxaldehyde solutions were added to this reaction mixture while stirring, through a period of 1 h. The reaction mixture was boiled and stirred under a reflux condenser ca. 2 h, at 50°C. After the mixture cooled to room temperature, polymer–ferrocene complexes were poured into the acetone and washed by adding more acetone. The resulting solid was filtered and dried in a oven.
Immobilization of GOx on (APS–Fc), (APS–EtFc), and (APS–PrFc)
The nanospheres encapsulating ferrocene (0.0125 g) were placed in a 10 mL DMF:water solution (9:1) of 0.010 gL− 1 of GOx at 30°C in a shaking water bath for 2 h. The immobilized polymer was separated and the free enzyme was removed by washing with phosphate buffer (pH 7.0, 20 mL). The immobilized enzyme was used fresh and then stored at 4°C. The saturation ratio was determined as 97.13%, from the absorbance value at 504 nm.
Assay for measurement of enzyme activity
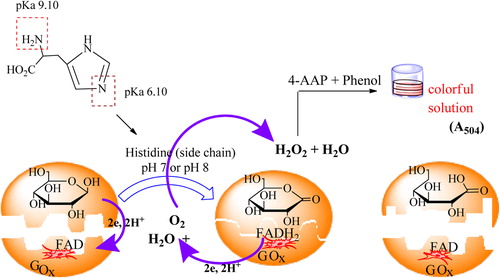
A colorimetric method based on Trinder's reaction was used to determine glucose concentration. Glucose is enzymatically oxidized to gluconic acid and hydrogen peroxide in the presence of GOx. The hydrogen peroxide reacts with 4-aminoantipyrene (4-AAP) and phenol to form a pink-colored quinoneimine dye, which has an absorption maximum at 504 nm (A504). The following reaction was started by adding 16 mg of glucose, after pre-incubating at 30°C for 15 min. This mixture was removed after incubating the reaction mixture at 30°C for 75 mins under continuous stirring. Then, it was transferred to a quartz cuvette for measurement.
The following recipe was used for the assay of free enzyme/immobilized enzyme: 4 mL of the buffer studied (pH 3.0–8.0) + 10 mg of 4-aminoantipyrene + 20 mg of phenol + 0.5 mg of horse radish peroxidase (HRP) + 0.010 g/L, 6 mL of free GOx/immobilized glucose oxidase in the buffer studied + 16 mg of glucose.
Effect of pH and temperature on activity of free and immobilized GOx
The optimum pH for immobilized GOx was determined by measuring the activity of immobilized enzyme in buffers of different pH values ranging from 3.0 to 10.0 (Sari et al. 2012) at room temperature, in the presence of 20 mM of glucose. In case of temperature studies, immobilized enzymes were incubated in the reaction mixtures at different temperatures ranging from 25°C to 90°C. The activities of free and immobilized enzymes were plotted against their respective temperature values.
Effect of substrate
To determine the extent to which immobilization affects the enzyme activity, Km and Vmax were determined at an optimum pH of 7 (at t = 60°C) and at a pH of 8 (at t = 40°C). Immobilized enzyme was incubated with different substrate concentrations (0.5–35 mM) in the buffer studied (pH 7and pH 8), and assayed for enzyme activity at 60°C/40°C, the recommended temperature values for enzyme assays.
Storage stability and reusability of immobilized enzyme
Storage stability experiments were carried out to determine the stability of immobilized enzyme after storage in dry conditions at + 4°C during a period of 16 months. The enzyme activity was measured every month. To evaluate the reusability, the GOx-immobilized polymer supports were also washed with buffer solution after each run, and re-introduced into a fresh solution. Reaction cycles under the conditions (pH = 8.0 or pH = 7, at room temperature) described above were performed. The enzyme activity was measured every 5 mins.
Results and discussion
Analytical data, heterogenic index, and some of the physical properties of all polymers studied have been given in , along with some of the physical properties of all modified polymers. The weight and average molecular weight (Mw) were suggested from elemental analyses. Molecular weight and molecular weight distribution (Mw/Mn) were determined by GPC. According to GPC, modified polymers have a very narrow molecular weight distribution (PDI: 1.09, 1.15, 1.30 and 1.08, for APS–Fc, APS–EtFc and APS–PrFc respectively).
Table I. Elemental analysis, thermal data, and some of the physical properties.
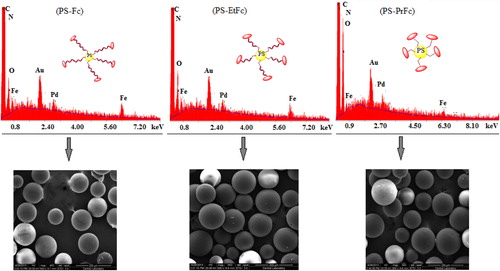
IR spectra and SEM-EDX of (APS–Fc), (APS–EtFc), and (APS–PrFc)
The characteristic peaks of IR spectra of the APS polymer and all modified polymers are given in . Three overtone peaks are seen at 1943, 1873, and 1800 cm− 1 for all polymers with nanospheres. In the spectra of polymers with nanosphere ((APS–Fc), (APS–EtFc) and (APS–PrFc)), bands appearing at 3423–3448, 3005–3043, 2900–2930, 523–76, 682–762 and 523–541 cm− 1 regions are characteristic of υ(OH) (–OH in crystal H2O), υ(CH) aromatic, υ(CH) aliphatic, υ(CH) buckling out of plane, and υ(CH) buckling, respectively. Imine bands are observed in the range of 1630–1634 cm− 1. This situation was evaluated as being due to binding of aldehyde to polymer. Also, a medium intensity band appeared at 1404–1402 cm− 1 and 510–508 cm− 1, belonging to υring-Fc. This confirms the binding of ferrocenecarboxyaldehyde with the polymer.
Table II. Kinetic parameters (Vmax/Km; mMmin− 1/mM) for free GOx and immobilized GOx.
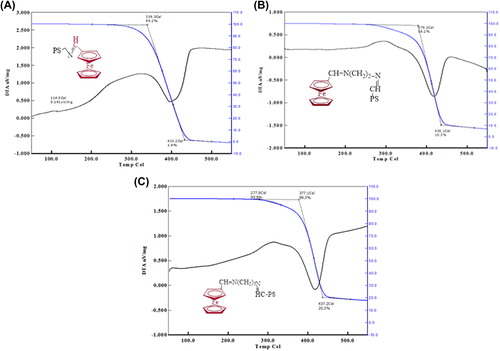
The TGA and DTA thermograms for poly(styrene)-attached ferrocene were recorded in a N2 atmosphere, and the percent weight loss and the corresponding temperature range are given in . The TGA thermograms of all polymers studied exhibit a one-step weight in the range of 339–279°C. The values of Ti and Tf are 339 and 433°C, respectively for (APS–Fc). Initial (Ti) and final (Tf) decomposition temperature of (APS–EtFc) are higher than those of others. According to this result (APS–EtFc) is more thermally stable (CitationEşsiz and Sari 2014). The DTA thermograms of the polymers studied have exothermic peaks ().
The SEM image indicates that the polymers studied have nanospherical structures. presents the elemental compositions of the (APS–Fc), (APS–EtFc), and (APS-PrFc) synthesized, as obtained from analysis using energy-dispersive X-ray sectroscopy (EDX). EDX is not the technique of choice for analyzing polymers, because the intensities of the carbon atom are often erratic in modified polymers. However, an EDX spectrum gives an excellent elemental analysis for all elements above beryllium in the periodic table, in a modified polymer. Moreover, the amount of oxygen in (APS–Fc) is more than that in (APS–EtFc) and (APS–PrFc). This excellent result may be due to difference of the arm length in polymers.Thus, the combined information from SEM and EDX indicate the genuinely modified polymer formation with the ferrocenecarboxyaldehyde group ().
Immobilization studies for biocatalysis
There are active centers in GOx which have FAD. FAD is a prosthetic group in enzyme structure, and is reduced to FADH2 by taking an electron. While this reduction occurs, glucose is oxidized to gluconic acid and then to gluconolactone. In this study, the maximum activity was obtained at pH 5.0 for the free enzyme, as in previous studies.
Influence of pH and temperature on the enzyme activity
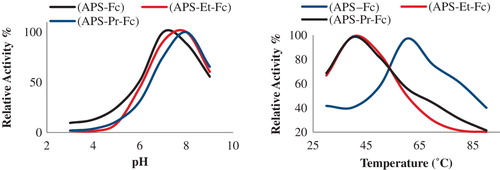
The pH is an important parameter capable of altering enzyme activity in aqueous solutions. While (APS–Fc–GOx) has an optimum pH of 7, (APPS–PrFc–GOx) and (APS–EtFc–GOx) have an optimum pH of 8. This can be attributed to the immobilization of the enzyme, which is likely to result in conformational changes in the enzyme, resulting in a variation of optimum pH. As presented in , the relative activity increased from pH 5.0 to 7.0/8.0 to reach the maximum, and then decreased at pH levels over 8.0. That is, enzyme activity for the materials studied will decrease in a strong acidic or alkaline medium.
It is known that the active site of the enzyme contains three amino acid side chains that are intimately involved in catalysis: His516, with pKa = 6.9, and Glu412, with pKa = 3.4, which are hydrogen-bonded to His559, with pKa > 8. In this study, the optimum pH level being 7 and 8 for GOx immobilized on (APS–Fc), (APS–EtFc), and APS–PrFc) may be due to the support of His516 and His559 residues of the enzyme in catalysis (CitationSarı et al. 2012).
We know that histidine has two groups including the –N atom, and their pKa values are 9.18 and 6.10, respectively, for the amino (–NH2) and imidazole group (). The reason for the optimum pH of 7 and 8 for the support studied may be attributed to the effect of hydrogen ions in the amino and imidazole groups.
The effect of temperature on the activity of immobilized GOx is shown in . The optimum temperature values for GOx immobilized on (APS–Fc), (APS–EtFc), and (APS–PrFc) have been shown to be 40°C, 40°C, and 60°C at optimum pH, respectively. Starting at room temperature, the relative activity increases gradually with temperature, reaching a maximum value at the optimum temperature, after which it decreases. This result shows that the entrapment of GOx into (APS–Fc), (APS–EtFc), and (APS–PrFc) spheres stabilizes the enzyme. The decrease of current-response at temperatures above the optimum temperature can be attributed to the loss of enzymatic activity caused by the denaturation of GOx (Qiu et al. 2011).
Kinetic parameters for free GOx and immobilized GOx
The kinetic parameters were studied for free GOx and immobilized GOx at all optimum pH (pH 6.0 and pH 7.0) and temperature (40°C and 80°C) values. The effect of the substrate concentration on the reaction rate was studied using varying initial concentrations (0.5–50 mM) of the β-D-glucose substrate. As presented in and , the activities of the free and immobilized enzymes with various substrate concentrations were plotted as Lineweaver-Burk graphs to calculate Vmax and Km values (CitationDeLousie and Miller 2005). The Km/Vmax values were calculated from Lineweaver-Burk plots for GOx immobilized in the (APS–Fc), (APS–EtFc) and (APS–PrFc), as 0.274/2.47, 76.92/4.61, and 71.43/3.75°CmM/Mmin− 1, respectively (pH = 7.0, t = 60°C, pH = 8.0, t = 40). The Km value is known as the enzyme's binding affinity. We know that a low Km value indicates a large binding affinity. Even though the optimum conditions are similar, the Km value of (APS–Fc) is lower than those for (APS–EtFc) and (APS–PrFc). As seen in , the Km value of (APS–EtFc) indicates that the enzyme does not bind as efficiently with the substrate. However, the Vmax value of (APS–EtFc) is higher than that of the others. In other words, the catalytic rate of (APS–EtFc) is greater.
Storage stability and reusabilty
Dry forms of the immobilized GOx were stored at 4°C for 12 months without a signicant loss of activity, whereas the corresponding free GOx lost more than 60% of its initial activity. Furthermore, immobilized GOx with a shorter arm (APS–Fc) shows more stable activity than that with a longer spacer (). As can be seen from , after 9 months, GOx immobilized on the (APS–Fc), (APS–EtFc), and (APS–PrFc) retained 77.88%, 69.19% and 57.66% of its original activity, respectively. High activity was found for a short-arm polymer. This result suggests that when there is a short-arm polymer support, the polymer imparts a higher structural stability upon the immobilized enzyme.
The reusability was tested because of its importance in industrial applications. The immobilized (APS–Fc), (APS–EtFc), and (APS–PrFc) were used repeatedly, 12 times an hour, due to the short incubation time. The reusability of the immobilized GOx showed a significant decrease in activity after two runs. After ten runs, the relative activity did not show a decrease in any of the GOx polymers studied. After the twenty-fifth use of the (APS–Fc), (APS–EtFc), and (APS–PrFc) complexes that were studied, the immobilized enzyme was found to have retained nearly 17.61%, 24.12%, and 21.13% of the original activity at the determinated optimum conditions, respectively. The reusability number for (APS–EtFc) was determined as 42 (10.0% of its original activity).
Conclusions
In this study, newly synthesized ferrocene tagged by polymeric Schiff bases were used as support material for the determination of glucose. The enzyme was immobilized by means of the adsorption method into the ferrocene tagged by the polymeric Schiff bases. According to the Km values of the nanospheres studied, efficient binding of the enzyme with the substrate is indicated in (APS–Fc). Furthermore, (APS–Fc) nanospheres have a higher structural stability for 10 months. Interestingly, the reusability number of (APS-EtFc) has been shown to be greater.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding
This research was supported by the Gazi University Research Fund (Project number: 05/2011-59).
References
- Armada MPG, Losada J, Cuadrado I, Alonso B, González B, Casado CM, Zhang J. 2004. Preparation of biosensors based in a siloxane homopolymer with interacting ferrocenes for the amperometric detection of peroxides. Sens Actuator B-Chem. 101:143–149.
- Arslan F, Beskan U. 2014. An amperometric biosensor for glucose detection from glucose oxidase immobilized in polyaniline-polyvinylsulfonate-potassium ferricyanide film. Artif Cell Nanomed Biotechnol. 42:284–288.
- Colak O, Yasar A, Cete S, Arslan F. 2012. Glucose biosensor based on the immobilization of glucose oxidase on electrochemically synthesized polypyrrole-poly(vinyl sulphonate) composite film by cross-linking with glutaraldehyde. Artif Cells Blood Substit Immobil Biotechnol. 40:354–361.
- DeLouise LA, Miller BL. 2005. Enzyme ımmobilization in porous silicon: quantitative analysis of the kinetic parameters for glutathione-S-transferases. Anal Chem. 77:1950–1956.
- Dursun F, Ozoner SK, Demirci A, Gorur M, Yilmaz F, Erhan E. 2012. Vinylferrocene copolymers based biosensors for phenol derivatives. J. Chem Techno Biotechno. 87:95–104.
- Eşsiz S, Sarı B. 2014. Temperature-sensitive composite films: synthesis and characterization of poly(vinyl acetate)/polystyrene/polypyrrole ternary composites. Polymer Adv Tech 33:21446.
- Hodak J, Etchenique R, Calvo EJ, Singhal K, Bartlett PN. 1997. Layer-by-layer self-assembly of glucose oxidase with a poly(allylamine)ferrocene redox mediator. Langmuir. 13:2708–2716.
- Jiang L, Liu H, Liu, J, Yang Q, Cai X. 2008. A sensitive biosensor based on Os-complex mediator and glucose oxidase for low concentration glucose determination. J Electroanal Chem. 619–620:11–16.
- Liang C, Jing L, Shi X, Zhang Y, Xian Y. 2012. Magnetically controlled bioelectrocatalytic system based on ferrocene-tagged magnetic nanoparticles by thiol-ene reaction. Electrochim Acta. 69:167–173.
- Liang RP, Fan LX, Huang DM, Qiu JD. 2011. A label-free amperometric immunosensor based on redox active ferrocene-branched chitosan/multiwalled carbon nanotubes conductive composite and gold nanoparticles. Electroanal. 23:719–727.
- Liangliang L, Jingang Y, Xiaoqing C. 2015. Enchanced stability and reusability of alcohol dehydrogenase covalently ımmobilized on magnetic graphene oxide nanocomposites. J Nanosci Nanotechnol. 15:1213–1220.
- Mani V, Devasenathipathy R, Chena SM, Huang ST, Vasanthac VS. 2014. Immobilization of glucose oxidase on graphene and cobalt phthalocyanine composite and its application for the determination of glucose. Enzyme Microb Technol. 66:60–66.
- Pawar SV, Yadav GD. 2014. Enantioselective enzymatic hydrolysis of rac-mandelonitrile to Rmandelamide by nitrile hydratase immobilized on poly(vinylalcohol)/chitosan− glutaraldehyde support. Ind Eng Chem Res. 53:7986–7991.
- Sarı N, Antepli E, Nartop D, Kurnaz Yetim N. 2012. Polystyrene attached Pt(IV)-azomethine, synthesis and immobilization of glucose oxidase enzyme. Int J Mol Sci. 13:11870–11880.
- Stege PW, Messina GA, Bianchi G, Olsina A, Raba J. 2010. Determination of β-glucosidase activity in soils with a bioanalytical sensor modified with multiwalled carbon nanotubes. Anal Bioanal Chem. 397:1347–1353.
- Qui JD, Huang H, Liang RP. 2011. Biocompatible and label-free amperometric immunosensor for hepatitis B surface antigen using a sensing film composed of poly(allylamine)-branched ferrocene and gold nanoparticles. Microchim Acta. 174:97–105.