Abstract
Electrospinning is a well-established technique that uses a high electric field to fabricate ultrafine fibrous scaffolds from both natural and synthetic polymers to mimic the cellular microenvironment. Collagen is one of the most preferred biopolymers, due to its widespread occurrence in nature and its biocompatibility. Electrospinning of collagen alone has been reported, with fluoroalcohols such as hexafluoroisopropanol (HFIP) and trifluoroethanol (TFE), but the resultant collagen lost its characteristic ultrastructural integrity of D-periodicity 67 nm banding, confirmed by transmission electron microscopy (TEM), and the fluoroalcohols used were toxic to the environment. In this study, we describe the use of glacial acetic acid and DMSO to dissolve collagen and generate electrospun nanofibers of collagen type 1, which is non-toxic and economical. TEM analysis revealed the characteristic feature of native collagen triple helical repeats, showing 67 nm D-periodicity banding pattern and confirming that the ultrastructural integrity of the collagen was maintained. Analysis by scanning electron microscopy (SEM) showed fiber diameters in the range of 200–1100 nm. Biocompatibility of the three-dimensional (3D) scaffolds was established by MTT assays using rat skeletal myoblasts (L6 cell line) and confocal microscopic analysis of immunofluorescent-stained sections of collagen scaffolds for muscle-specific markers such as desmin and actin. Primary neonatal rat ventricular cardiomyocytes (NRVCM) seeded onto the collagen scaffolds were able to maintain their contractile function for a period of 17 days and also expressed higher levels of desmin when compared with 2D cultures. We report for the first time that collagen type 1 can be electrospun without blending with copolymers using the novel benign solvent combination, and the method can be potentially explored for applications in tissue engineering.
Introduction
Tissue engineering is an interdisciplinary approach combining the fields of materials engineering and life sciences to develop laboratory-grown substitutes, which can support, maintain, or improve the function of defective or injured body parts (CitationLanger and Vacanti 1993). There is an ever increasing clinical need for biocompatible materials that simulate the extracellular matrix (ECM), and a major focus of the last decades has been on the use of natural and synthetic polymeric scaffolds in regenerative medicine (CitationQi-Zhi et al. 2008). Collagen is a component of the ECM of numerous tissues and is the predominant structural protein found in animals. Numerous types of collagens are known to exist and have been characterized, of which type 1 collagen is the principal choice for the production of collagen-based biomaterials in tissue engineering applications. The unit of collagen type 1 is a triple helix consisting of three polypeptide chains, two identical α1 chains, and one distinct α2 chain, held in place by hydrogen and disulfide bonds (CitationShoulders and Raines 2009). Aggregates of triple helices together form collagen fibrils, and their molecular arrangement of helical repeats results in a characteristic 67 nm banding pattern or D-periodicity banding, which is evident under transmission electron microscopy (TEM) (CitationVanderby and Provenzano 2003).
Electrospinning is a facile technique that is used to generate nanofibers by using an electric field between the polymer solution and grounded collector (CitationReneker and Chun 1996). Electrospun collagen has been produced using fluoroalcohols such as 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) (CitationLi et al. 2005) and 2,2,2-trifluoroethanol (TFE) (CitationBurck et al. 2013). Fluoroalcohols are the preferred solvents as they are proposed to play an important role in solubilizing collagen to electrospin (CitationMatthews et al. 2003). However, fluoroalcohols are corrosive and also expensive, and therefore there is a need for alternative solvent systems that are economical and non-toxic to the environment (CitationDror et al. 2008). Zeugolis et al. have also confirmed that collagen electrospun nanofibers produced using fluoroalcohols lose their native typical triple helical ultrastructure of 67 nm banding, as observed by TEM. Moreover, co-spinning of collagen with synthetic polymers using fluoroalcohols does not prevent its denaturation to gelatin either (CitationZeugolis et al. 2008).
Previous studies have not shown electrospinning of type I collagen alone with aqueous solvents. Yogeshwar et al. and Chen et al. studied electrospinning of type I collagen in combination with carrier polymers such as polycaprolactone (PCL) (CitationYogeshwar et al. 2012) and polyethylene oxide (PEO) (CitationChen et al. 2011), to enhance the spinnability. Dong et al. have reported that collagen can be electrospun in a benign solvent combination of water/alcohol/salt (CitationDong et al. 2009). Buttafoco and colleagues have shown that fibers are generated from 1–2% collagen in weak acidic solutions only with the addition of copolymers of high molecular weight, which increase the viscosity of the solution and lead to formation of stable jets (CitationButtafoco et al. 2006). We report, for the first time, electrospinning of type I collagen alone, without a carrier polymer, using a benign binary solvent of glacial acetic acid and DMSO. Fibers of collagen type 1 were generated in the nanometer range and maintained their native integrity of triple helical repeats after electrospinning, as the TEM examination of the 3D scaffolds revealed in the 67 nm D-periodicity banding pattern. The developed 3D collagen nanofibrous scaffolds were characterized with a suitable modulus for contracting cardiomyocytes. The collagen scaffold showed biocompatibility and favored the adhesion and maintenance of primary neonatal cardiomyocytes, with the expression of the contractile protein desmin for long term survival of more than two weeks in static culture.
Methodology
Materials
Collagen type I of fish origin, commercially known as Helisorb. The solvents glacial acetic acid and dimethylsulfoxide (DMSO) were of analytical grade and obtained from SRL, Mumbai, and Merck, USA.
Electrospinning of collagen
A custom-built electrospinning setup consisting of a stabilized high voltage power supply, Sri Ramachandra University, Chennai; a programmable peristaltic pump, Ravels Tech, Chennai; and silicon tubing with a 26-G needle and a grounded metal plate as a stationary collector were used to generate nanofibers. Collagen type 1 was dissolved in glacial acetic acid/DMSO at a ratio of 93/7 to prepare a 10% weight/volume solution by overnight agitation. Nanofibers were generated at a constant voltage of 17 KV and the collagen solution was supplied to the 26G needle at a flow rate of 0.60 ml/h. The working distance between the needle and the collector was 23 cm. Fibers were collected for defined durations and the matrices were crosslinked with 1-ethyl-3(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC, SRL Mumbai) to stabilize the collagen fibers (CitationMichael et al. 2010).
Electron microscopy
Characterization of the collagen nanofibers was carried out by scanning electron microscopy (SEM) (S-3400N). The crosslinked scaffolds were carefully cut into 1 cm square mats and the samples were coated with gold using a sputter coater. Multiple digital images of three different scaffolds were taken and the average fiber diameter was determined by taking the average of 30 measurements chosen at random across each image set. Digital images were analyzed using ImageJ software (version 1.49f). For TEM, the scaffolds were fixed with 3% glutaraldehyde using standard protocols, rinsed in cacodylate buffer, and post-fixed in 1% osmium tetroxide for 1 h and embedded with epoxy resin. Ultrathin sections were examined with a Philips Tecnai T-12 instrument.
Porosity measurement
The porosity of the collagen nanofiber mats was analyzed by examining the SEM images using ImageJ software, as described previously (CitationGhasemi-Mobarakeh et al. 2007). The three upper, middle, and lower layers of nanofibrous membranes were obtained by adjusting the thresholds of the SEM image. The original 256 gray scale image was first converted to a binary image to obtain the total number of pixels (N) in the binary image. The number of white pixels (n) in each layer of binary images is also noted to calculate the porosity percentage of each layer, using the formula P = (1 – n/N) × 100. The average pore size of each layer (P1, P2, and P3) gives the total porosity of the membrane. Four SEM images of magnifications of 2000 and 5000 were analyzed for P1, P2, and P3, and the overall percentage of porosity was estimated by calculating the mean and standard deviation.
Tensile property
Crosslinked collagen scaffolds that had been collected for 8 hours were cut into strips with dimensions of 3 cm length and 1.5 cm width, and were roughly 0.3 mm in thickness. Tensile strength of the electrospun collagen matrices was measured using a universal tensile tester, INSTRON 3365, with a 10N load cell, and was performed at a speed of 1 mm/min (CitationZorlutuna et al. 2009). A total of 4 samples were used for the analysis to calculate the mean and standard deviation.
Cell culture
Collagen nanofibrous scaffolds were cut into 1 cm squares, washed with sterile phosphate-buffered saline (PBS), followed by sterilization with serial dilutions of ethanol, and stored in PBS supplemented with antibiotics, at 4°C. Rat skeletal myoblasts (L6 cell line) were cultured in DMEM high glucose medium supplemented with 10% fetal bovine serum and antibiotics. Myoblasts were seeded onto scaffolds in non-adherent culture dishes at a seeding density of 2.5 × 104 cells/scaffold and incubated at 37° C, in an atmosphere containing 5% carbon dioxide. The culture medium was refreshed every 2 days. The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide, a tetrazole) test assesses the cytotoxicity by measuring the cell viability and proliferation (CitationMi et al. 2000). The scaffolds were tested by MTT assay at different time points of 2, 4, and 6 days, and the color development measured at 595 nm using a spectrophotometer. The mean and standard deviation for 3 scaffolds per time point were calculated to determine cell viability and proliferation on the scaffolds.
Primary cardiomyocyte culture
The guidelines were followed as stipulated by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), and the study was officially permitted by the Institutional Animal Ethics Committee of Sri Ramachandra University (Study no: IAEC/XXII/SRU/174/2011). Culture of primary cardiomyocytes from 2–3-day-old Sprague Dawley rats was performed, as described earlier (CitationSrinivasan et al. 2012). Briefly, the hearts of a total of 10 neonatal rats were chopped and subjected to trypsin digestion, and the isolated cardiomyocytes were pooled together prior to seeding. The scaffolds were seeded with primary ventricular cardiomyocytes from neonatal rats (NRVCM) in nonadherent culture dishes, at 5 × 105 cells/scaffold, and incubated at 37° C with 5% carbon dioxide; also seeded were 1 × 105 cells/cover glass coated with 2.5% gelatin solution for 2D culture. The culture medium (DMEM/F12 in the ratio of 4/1) was changed at intervals of 2 days. Microscopic examinations of 10 fields in three different scaffolds were done, to count the average number of beats per 2 minutes. The scaffolds were examined at the 3, 5, 7, 9, 11, 14, and 17-day time-points. The images were captured with a Nikon TE2000 Eclipse inverted microscope fitted with a CCD camera. The rate of capture was 30 frames per second, for conversion into a video format with Image Pro Software.
Immunocytochemistry and confocal microscopy
The cell-seeded scaffolds were cryopreserved at defined time points. Immunostaining of the cryosections was carried out as described previously (CitationSrinivasan et al. 2012). Briefly, thin sections were fixed in 3.7% formaldehyde, and permeabilized with Triton X-100, after the addition of the primary antibodies—muscle-specific actin and desmin (both from BioGenex, USA). The sections were incubated, washed, and stained with goat anti-mouse FITC (Merck), counterstained with propidium iodide to emphasize the nuclei, and analyzed on a Zeiss LSM 510 laser scanning confocal microscope.
Reverse transcription polymerase chain reaction (RT-PCR)
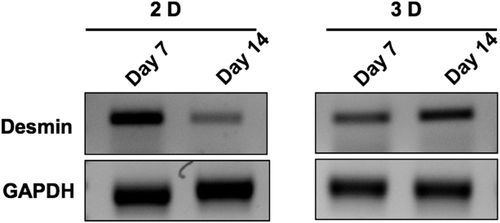
RNA Plus (MP Biomedicals) was used to isolate the total RNA, performed according to the instructions, and the Verso cDNA synthesis kit (Abgene) was used for cDNA synthesis. PCR steps included denaturation, annealing and extension at 94˚C for 30 seconds, 60˚C/63˚C for 30 seconds, and 72˚C for 30 seconds, for 25/29 cycles, of GAPDH/desmin respectively. Primers with product size of 556bp for GAPDH 5’-ACC ACA GTC CAT GCC ATC-3’ (forward) and 5’-TCC ACC ACC CTG TTG CTG-3’ (reverse) and of 224bp for desmin 5’-CAA CCT TCC GAT CCA GAC CT-3’ (forward) and 5’-GAG TGG AAA AGG CTG GCT TC-3’ (reverse) were used for PCR analysis.
Statistical analysis
All numerical data have been expressed as mean ± standard deviation (SD).
Results
Characterization of collagen fibrous matrix
Analysis of the SEM image revealed the deposition of fibers in a random manner () onto the stationary collector, with the average fiber diameters ranging from 200 nm to 1100 nm (). The collagen fibrous matrices also exhibited a porosity percentage of 44.3 ± 1.79, when measured as described by Ghasemi-Mobarakeh et al. (). Recent studies have also shown that fluoroalcohols commonly used to electrospin collagen type 1 lead to a denaturation of the collagen, where the ultrastructural integrity of the triple helix repeats in the form of the 67 nm D-periodicity banding pattern characteristic to native collagen is no longer present in TEM (CitationBurck et al. 2013). We have successfully electrospun collagen type I nanofibrous scaffolds using the binary benign solvent, and they were stabilized successfully using EDC to support contracting cardiomyocytes for long term survival in static culture, without the influence of external electrical stimuli. The matrices, stored in phosphate-buffered saline (PBS) for 2 months, were examined by TEM. Ultrathin sections of scaffolds revealed the presence of the 67 nm D-periodicity banding pattern, a characteristic feature of native collagen. (). The TEM images prove that the native properties of collagen were maintained after electrospinning in glacial acetic acid and DMSO, and this is the highlight of the study. The mechanical properties of the scaffolds were optimal to withstand the forces of the contracting cardiomyocytes. We measured the Young's modulus, elongation at break, and tensile strength of the electrospun scaffolds using a uniaxial tensile testing apparatus (). The obtained modulus of 3.45 ± 1.66 kPa, a tensile strength of 1.57 ± 0.41 kPa, and an elongation of 95.2 ± 28.38%, were found approximately suitable for the human myocardium.
Figure 1. A represents the electrospun collagen nanofibrous sheet stored in PBS. B represents the SEM image of electrospun collagen type I. The matrix illustrates the porous and fibrous nature of the scaffold. Scale bar = 20 μm.
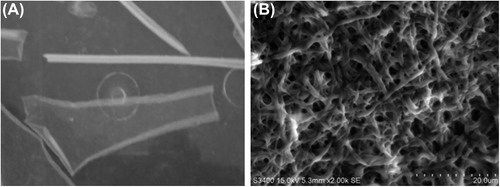
Figure 2. Graphic representation of the distribution of fiber diameters measured from SEM images of four independent scaffolds (n = 4) using ImageJ software. The fiber diameter ranges from 200 nm– 1100 nm.
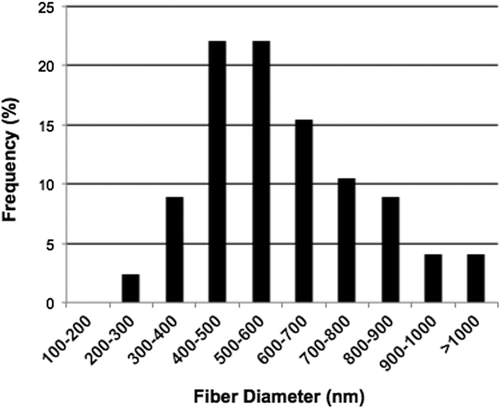
Figure 3. Transmission electron micrographs of electrospun collagen nanofibrous scaffolds exhibit the triple helical repeats of 67 nm D-periodicity banding pattern typical of native collagen (inserted scale bar 250 nm and 50 nm).
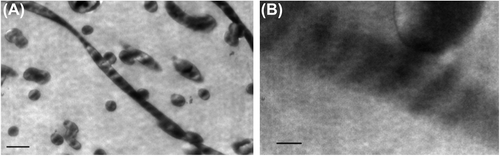
Table I. Represents the porosity measurements of SEM images of four different scaffolds (n = 4) at two different magnifications using ImageJ software. Porosity percentages of three thresholds that help in distinguishing top, middle and lower layers of the nanofiber mats were measured as P1, P2, and P3. Total porosity percentage was the average of the three, and the overall percentage of porosity was estimated by calculating the mean and standard deviation.
Table II. Mechanical properties of the collagen nanofiber scaffold. The tabular column represents the mean and standard deviation of tensile strength, stress, and Young's modulus of 4 different samples of collagen nanofibrous membrane.
Biocompatibility studies with cell lines and primary cardiomyocytes cultures
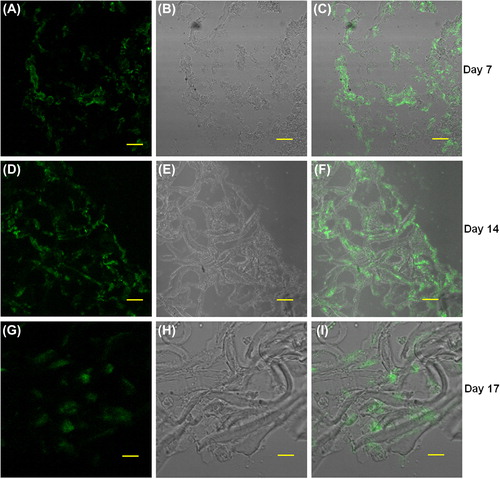
We investigated the biocompatibility of the electrospun scaffolds using cell lines by analyzing the proliferation of L6 rat skeletal myoblasts at various time points, using the MTT assay. We found that the OD values after two days in culture on the nanofiber scaffolds were 1.59 ± 0.17, and increased at later time points to 2.41 ± 0.04 on day 4 and 2.53 ± 0.25 on day 6, respectively (). Scaffolds seeded with myoblasts for six days were cryosectioned and examined for muscle-specific proteins using immunofluorescence staining. Confocal immunofluorescence analysis revealed a prominent expression of desmin and muscle-specific actin throughout the matrices (), indicating the suitability of the scaffold for muscle cells. We also characterized the scaffolds for NRVCM and seeded them onto 3D collagen scaffolds, to study them for applications in cardiac tissue engineering. Two days post seeding, the NRVCMs adhered well onto flat cultureware and 3D collagen scaffolds and organized themselves into groups of contracting cells that began to beat synchronously by day 3 (see Supplementary Videos 1 and 2, to be found online at http://informahealthcare.com/doi/abs/10.3109/21691401.2015.1029629). On observing the contracting cells under the microscope, we noticed that the number of beats per 2 minutes was initially lower for 3D scaffolds, with 120 ± 3.9 and 92 ± 16 beats for day 3 and day 7, when compared to 2D cultures that showed 200 ± 8.3 and 250 ± 12.9 respectively (). The initial difference in beating frequency could be due to cell acclimatization, to adhere, communicate, and organize on the 3D scaffolds. 2D cultures continued to beat well on day 9, with 250 ± 7 beats, but the beating frequency was drastically reduced in the 2D cultures after ten days. The contractility of cardiomyocytes on days 11 and 14 showed 170 ± 1.4 and 174.2 ± 2 beats, and no beating was observed on day 17. In contrast, cardiomyocytes on 3D collagen scaffolds showed contractility with 300 ± 1.6, 310 ± 5.7, 297.1 ± 9.7, and 301 ± 3.6 beats on days 9, 11, 14, and 17 respectively (). Confocal immunofluorescence analysis of cardiac marker troponin T revealed a strong expression on 3D scaffolds at different time points post seeding (). The expression of desmin using RT-PCR at all the time points was also studied. Briefly, 100 ng of cDNA of each sample was amplified using primers for desmin and GAPDH, as described. We observed that there was a reduction in expression levels of desmin in cardiomyocytes cultured on 2D surfaces on day 14 post seeding when compared with those of day 7 (), which is in accordance with the beating data. The cardiomyocytes that were grown on 3D collagen scaffolds had sustained expression of desmin on days 7 and 14, when compared with the 2D surfaces, which also corroborates our beating data. Our work indicates that electrospinning collagen using glacial acetic acid and DMSO can generate favorable 3D nanofibrous scaffolds for both cell line and primary cultures, and hence is suitable for applications in tissue engineering.
Figure 4. Biocompatibility of the collagen scaffold evaluated by MTT assay using L6 rat skeletal myoblasts, cell growth tested at 3 different time points (2, 4 and, 6 days in culture). Myoblasts adhered and proliferated well on collagen nanofiber scaffolds.
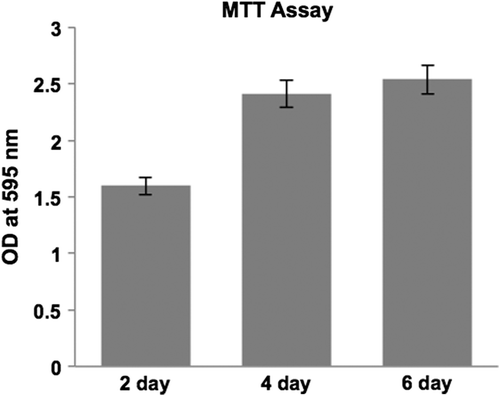
Figure 5. Rat skeletal myoblasts (cell line) were seeded on the collagen nanofibrous scaffold and cultured for 6 days, cryosectioned, and stained. Collagen matrices showed prominent cell adherence and proliferation throughout when examined by immunofluorescence staining using confocal microscopy. A. anti- muscle-specific actin FITC, B. Nuclear staining with propidium iodide, and C. Merged image, D. anti-desmin FITC, E. nuclear staining with propidium iodide, and F. merged image. Scale bar = 50 μm.
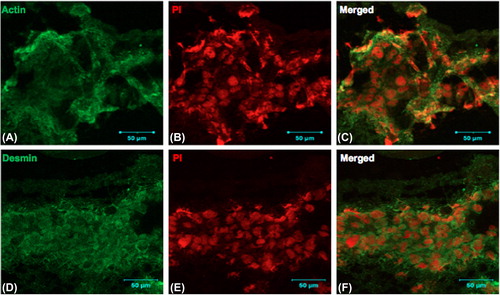
Figure 6. Graphic representation of beating (contracting) of cardiomyocytes on 2D tissue cultureware versus 3D collagen scaffolds, at defined time points.
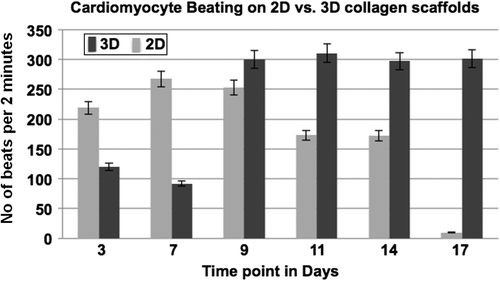
Figure 7. Cryosections of collagen nanofiber scaffolds seeded with (primary cells); neonatal rat cardiomyocytes were stained with the cardiac-specific marker troponin T and examined by immunofluorescence confocal microscopy; A, D, G: anti-troponin-FITC, B, E, H: phase contrast, and C, F, I: merged images. Images A to C (7-day time point), D to F (14-day time point), and G to I (17-day time point) respectively. Scale bar = 50 μm.
Discussion
Acetic acid is widely used for the extraction of collagens from natural sources and is a solvent that does not denature the favorable properties of collagen. DMSO is known as a cosolvent to increase the conductivity and viscosity (CitationLuciana et al. 2010, CitationChoktaweesap et al. 2007) of electrospinning solutions. Electrospinning of collagen type 1 to produce nanofibers has been commonly achieved with fluoroalcohols, which are expensive, toxic to the environment, and also denature the native properties of collagen. Weak acetic acid and PBS ethanol-based solvents have also not maintained the 67 nm D-banding property of collagen after electrospinning (CitationBurck et al. 2013). We provide conclusive evidence to show that it is possible to successfully electrospin collagen type 1 alone, without the need for carrier polymers, using acetic acid and DMSO as solvents. Our study is also the first to show that the benign binary solvent maintains its molecular structure and favorable properties, which are essential for use as a biomaterial in the field of tissue engineering.
The biocompatibility study showed that the myoblasts proliferated well on the collagen scaffolds. The cytoskeleton of muscle cells includes proteins whose primary function is to connect, anchor, and coordinate structural components such as the myofibrils, mitochondria, nuclei, and organelles. It is composed of three integral components—intermediate filaments (IFs), microfilaments, and microtubules. (CitationStromer 1998). Desmin is the major IF protein found in skeletal and heart muscle that has a vital role in maintaining the structural integrity of myocytes and contributes to force transmission. Actin forms the microfilaments and is also an integral part of the cytoskeleton and contractile apparatus (CitationGloria et al. 2009). Engler and coworkers have shown that the elasticity and rigidity of scaffolds directly affects the maintenance and differentiation of cells (CitationEngler et al. 2006). Neonatal ventricular cardiomyocytes cultured on substrates of ∼10 kPa developed aligned sarcomeres and produced maximal force, whereas those on stiffer scaffolds had more stress fibers and unaligned sarcomeres (CitationJeffrey et al. 2008). The mechanical property analysis data proved that the tensile strength appears relatively nearer to native cardiac tissue measures between 3–15 kPa (CitationVenugopal et al. 2012); Engler et al. have also shown contracting cardiomyocytes on hydrogels with matrix stiffness of 1 kPa. Scaffolds developed with low modulus can also be potentially used in soft-tissue engineering (CitationKim et al. 2012). The collagen scaffolds developed with these features favor cardiomyocytes to maintain their contractility for 3 weeks, which was not so in the case of the 2D culture. This static 3D cardiac model can also be further improved by incorporating electrical stimulation strategies for prolonged studies in drug discovery. The results of our study show that collagen type 1 can be electrospun using a simple benign solvent system. The collagen nanofiber maintains its native integrity and is a promising candidate for providing the support and microenvironment required for cardiac tissue-engineered constructs in vitro.
Conclusion
Thus, the electrospinning of collagen with a benign solvent combination can be utilized to reduce the cost of producing electrospun collagen scaffolds that preserve the native integrity and can be potentially applied tissue engineering and regenerative medicine. The developed collagen scaffold was also characterized to support contracting cardiomyocytes in vitro, which can be further developed as in vitro 3D models for applications in cardiac tissue engineering. These collagen scaffolds developed, along with stem cell technology, are considered to be a potential source in the fields of tissue engineering and regenerative medicine.
Supplementary material available online
Supplementary Videos 1 and 2 Video microscopy of neonatal cardiomyocytes seeded on 2D and 3D cultures.
1. Movie clips of beating cardiomyocytes on 2D (culture flask) culture at different time points. Magnification (20X).
2. Beating cardiomyocytes on 3D collagen nanofibrous scaffold on the 17th day time point, under magnification (20X). Cardiomyocytes were beating continuously well on all time points in the collagen scaffolds.
ianb_a_1029629_sm5603.zip
Download Zip (11.5 MB)Acknowledgement
We are grateful to the Department of Biotechnology, Government of India, for funding this work. Grant no: DO No. BT/PR11228/BRB/10 677/2008. We thank Dr. Suresh R, Department of Periodontology, Sri Ramachandra University, for his kind gift of collagen. We are also grateful to the faculty and technical experts at the Biomedical Testing Wing at Sri Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, for their help in tensile testing and confocal microscopy.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Burck J, Heissler S, Geckle U, Ardakani MF, Schneider R, Ulrich AS, Kazanci M. 2013. Resemblance of electrospun collagen nanofibers to their native structure. Langmuir. 29:1562–72.
- Buttafoco L, Kolkman NG Engbers-Buijtenhuijs P, Poot A, Dijkstra A, Vermes PJ, Feijen I. 2006. Electrospinning of collagen and elastin for tissue engineering applications. J Biomater. 27:724–734.
- Chen L, Zhu C, Fan D, Liu B, Ma X, Duan Z, Zhou Y. 2011. A human-like collagen/chitosan electrospun nanofibrous scaffold from aqueous solution: electrospun mechanism and biocompatibility. J Biomed Mater Res A. 99:395–409.
- Choktaweesap N, Kunawan A, Duangdao A, Chidchanok M, Pitt S. 2007. Electrospun gelatin fibers: effect of solvent system on morphology and fiber diameters. Polym J. 39:622–631.
- Dong B, Arnoult O, Smith ME, Wnek G. E. 2009. Electrospinning of collagen nanofiber scaffolds from benign solvents. Macromol Rapid Commun. 30:539–542.
- Dror Y, Ziv T, Makarov V, Admon A, Zussman E. 2008. Nanofibers made of globular proteins. Biomacromolecules. 9:2749–2754.
- Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell. 126:677–689.
- Ghasemi-Mobarakeh L, Semnani D, Morshed M. 2007. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J Appl Polym Sci. 106:2536–2542.
- Gloria MC, Syerra NH, Carol CG. 2009. A Myopathy-linked Desmin Mutation Perturbs Striated Muscle Actin Filament Architecture. Mol Biol Cell. 20:834–845.
- Jeffrey GJ, Andrew D, McCulloch AD, Jeffrey HO. 2008. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 95:3479–3487.
- Kim HN, Kang DH, KiM MS, Jiao A, Kim DH, Suh KY. 2012. Patterning methods for polymers in cell and tissue engineering. Ann Biomed Eng. 40:1339–1355.
- Langer R, Vacanti JP. 1993. Tissue engineering. Science. 260:920–6.
- Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. 2005. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 26:5999–6008.
- Luciana M, Jianjun M, Jonathan SD, Robert JL. 2010. Electrospinning from room temperature ionic liquids for biopolymer fiber formation. Green Chem. 12:1883–1892.
- Matthews JA, Boland ED, Wnek GE, Simpson DG, Bowlin GL. 2003. Electrospinning of collagen type II: a feasibility study. J Bioact Comp Polym. 18:125–134.
- Mi FL, Sung H, Shyu S. 2000. Synthesis and characterization of a novel chitosan-based network prepared using naturally occurring crosslinker. J Polym Sci Part A: Polym Chem. 38:2804–2814.
- Michael JM, Scott AS, David GS, Beat HW, Gary LB. 2010. A three-layered electrospun matrix to mimic native arterial architecture using polycaprolactone, elastin, and collagen: a preliminary study. Acta Biomaterialia. 6:2422–2433.
- Qi-Zhi C, Sian EH, Nadire NA, Alexander RL, Aldo RB. 2008. Biomaterials in cardiac tissue engineering: ten years of research survey. Mat Sci Eng R. 59:1–37.
- Reneker DH, Chun I. 1996. Nanometre diameter fibers of polymer, produced by electrospinning. Nanotechnology. 7:216.
- Shoulders MD, Raines RT. 2009. Collagen structure and stability. Annu Rev Biochem.78:929–958.
- Srinivasan A, Punnoose AM, Nagarajan N, Kuruvilla S, Sehgal PK, Balakrishnan K. 2012. Collagen 3D fleece as a scaffold for cardiac tissue engineering. Indian J Thorac Cardiovasc Surg. 28:1–5.
- Stromer MH. 1998. The cytoskeleton in skeletal, cardiac and smooth muscle cells. Histol Histopathol. 13:283–291.
- Vanderby R, Provenzano PP. 2003. Collagen in connective tissue: from tendon to bone. J Biomech. 36:1523–1527.
- Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. 2012. Biomaterial strategies for alleviation of myocardial infarction. J R Soc Interface. 9:1–19.
- Yogeshwar Chakrapani V, Gnanamani A, Giridev VR, Madhusoothanan M, Sekaran G. 2012. “Electrospinning of Type I collagen and PCL nanofibers using acetic acid.”J Appl Polym Sci. 125:3221–3227.
- Zeugolis DI, Khew ST, Yew ESY, Ekaputra A, Tong KYW, Yung LYL, et al. 2008. Electro-spinning of pure collagen nano/fibers-Just an expensive way to make gelatin? Biomaterial. 29:2293–2305.
- Zorlutuna P, Elsheikh A, Hasirci V. 2009. Nanopatterning of collagen scaffolds improve the mechanical properties of tissue engineered vascular grafts. Biomacromolecules. 10:814–821.