Abstract
Quantum dots (QDs), as a new class of fluorescent tags, have been widely used for biomedical applications. Despite their various advantages, QDs do not efficiently enter cells on their own, and aggregation often occurs following internalization. In the present study, we have designed three QD–cell-penetrating peptide (CPP) complexes to increase the uptake of QD into cells. The results demonstrated that R9 and R5W3R4 form relatively stable noncovalent complexes with QDs, considerably increasing the rate and efficiency of QD uptake by A549 cells. These data suggest that cationic CPPs could efficiently transfer QDs into cells in a non-toxic manner.
Introduction
Quantum dots (QDs), which are semiconductor nanocrystals, have been widely used as a new class of fluorescent tags for cellular labeling and tissue imaging (CitationWilliam et al. 2006). In contrast with conventional fluorescent dyes (for example, calcein) and fluorescent proteins (for example, green fluorescent protein), QDs have advantages including high quantum yield, narrow emission spectra, broad size-dependent photoluminescence, and excellent resistance to photo and chemical degradation (CitationDerfus et al. 2004, CitationGoldman et al. 2002, CitationResch-Genger et al. 2008). QDs are typically between 2 and 10 nm in diameter and consist of elements from groups II and VI (CdTe, CdSe), groups IV and VI (PbS), or groups III and V (GaAs) of the periodic table (CitationErogbogbo et al. 2011). These nanomaterials have been used for many biomedical applications including optical imaging, sentinel lymph node mapping, studying live cells, and multiplex imaging (CitationErogbogbo et al. 2011, CitationMichalet et al. 2005, CitationJamieson et al. 2007, CitationLarson et al. 2003). However, QDs do not efficiently enter cells on their own, and aggregation often occurs following internalization (CitationLiu et al. 2011). Improving the efficiency of cellular uptake and solubility of QDs could increase their utility in biological applications (CitationLiu et al. 2010). Recently, to overcome these problems, QDs have been surface-modified by covalent or noncovalent linkage with cell-penetrating peptides (CPPs) (CitationLiu et al. 2011). In one study, it was found that SR9 increased the cellular uptake of QDs by noncovalent binding between QDs and SR9 (CitationXu et al. 2010). In another study, it was demonstrated that histidine- and arginine-rich CPPs (HR9) stably and noncovalently combined with QDs, are able to efficiently enter cells in an extremely short period (CitationLiu et al. 2011).
CPPs are short cationic sequences that possess the ability to penetrate the cell membrane, and are thus used to transport biologically active proteins, nucleic acids, nanoparticles, and drugs across plasma membranes (CitationFonseca et al. 2009, CitationZorko and Langel 2005). Nona-arginine (R9), TAT, and penetratin are examples of CPPs (CitationLiu et al. 2011). These peptides are heterogeneous in size (10–27 amino acids in length) and sequence (CitationLindgren and Langel 2011). They have the ability to interact with cellular membranes and to adopt a significant secondary structure to bind to membranes, and are capable of internalizing hydrophilic cargoes (CitationTemsamani and Vidal 2004). CPPs generally have a high content of positively charged residues, such as arginine and lysine. These positive charges interact with negative charges of the cell membrane and drastically influence cellular uptake and membrane interactions (CitationRydberg et al. 2012). CPPs and their cargoes usually retain their biological activities after entry into cells. The advantages of these short peptides as carriers include rapid transduction rate, ease of use, and high efficiency of transduction (CitationTünnemann et al. 2008).
In this work, we studied the ability of three synthesized CPPs, including R9, R5W3R4, and [RW]4 for the delivery of QDs into cells following a noncovalent association. Furthermore, the toxicity of QD alone and of the QD–CPP complexes were evaluated.
Materials and methods
Fluorenylmethyloxycarbonyl (Fmoc)-Rink amide resin and amino acid derivatives were purchased from AAPPTec (Louisville, USA). Coupling agents (TBTU, DIPEA), scavengers (ethanedithiol, phenol, TIPS) and cleavage reagents (piperidine, TFA) were purchased from Sigma (St. Louis, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), RPMI 1640, fetal bovine serum (FBS), trypsin-EDTA, and penicillin/streptomycin were purchased from Invitrogen (Carlsbad, USA). Tellurium (Te) powder, sodium borohydride, and cadmium chloride (CdCl2), were obtained from Sigma (St. Louis, USA).
Peptide synthesis
All peptides were synthesized manually by the solid-phase peptide synthesis method on a Fmoc-Rink amide AM resin by Fmoc strategy in a fritted glass vessel. The resin was swelled in anhydrous DMF for about 1 h under dry nitrogen. Fmoc deprotection of resin was carried out using piperidine in DMF (20% v/v, 2 mL, 30 min). Fmoc-Arg (Pbf)-OH (0.14 mmol) was coupled to the Rink amide resin in the presence of TBTU (0.12 mmol) and DIPEA (50 μL) in DMF (2 mL) by mixing for 2 h. After the coupling was completed, the reaction solution was filtered off and the resin was washed with DMF (4 × 2 mL) and DCM (4 × 2 mL), followed by Fmoc deprotection using piperidine in DMF. The resin was washed with DMF and DCM. The ninhydrin test was used to monitor Fmoc deprotection and coupling of amino acids. After the coupling of all amino acids, the resin was washed with DMF, DCM, and ethanol respectively (each 2 × 2 mL). The resin was dried under vacuum for 24 h. Fresh cleavage cocktail, reagent B, TFA/TIPS/phenol/water (88/2/5/5 v/v/v/v, 3 mL), was added to the resin for side-chain deprotection and the final cleavage of the synthesized peptide from the solid support. The mixture was shaken at room temperature for 2 h. The resin was collected by filtration and washed with another 2 mL of fresh cleavage cocktail. Combined filtrates were evaporated to reduce the volume under dry nitrogen. The crude peptide was precipitated by adding 100 mL of diethyl ether and centrifuged at 4000 rpm for 5 min, followed by decantation to obtain the solid precipitates. The peptide obtained was further washed with ether (2 × 50 mL) 2 times, and then lyophilized.
Quantum dot synthesis
NaHTe was prepared as described earlier, with minor modification. Briefly, a mixture of 64 mg of tellurium (Te) powder and 56.70 mg of sodium borohydride (molar ratio of Te/NaBH4 was 1/3) was added into a 50 mL three-necked flask. Then, 2 mL of ultrapure deoxygenized water was added. The mixture was stirred for 2 h until the black Te powder disappeared.
The synthesis of thioglycolic acid (TGA)-capped colloidal CdTe QDs was carried out using CdCl2 and NaHTe as precursors, followed by previously reported methods, with some modifications. Briefly, 150.98 mg (0.75 mmol) of CdCl2.H2O and 134 mL of TGA were dissolved in 150 mL of ultrapure deoxygenized water, and the pH of the mixture was adjusted to 11.5 using 1 M NaOH. The mixture was then transferred into a three-necked flask and deaerated by argon stream for 30 min. Under continuous stirring, 5 mL of freshly prepared 0.05 M NaHTe was injected into the flask. The initial molar ratio of Cd2+/Te2-/TGA was therefore about 1/0.5/2.4. The resulting colloidal CdTe was refluxed under argon atmosphere at 100°C for different times to obtain CdTe nanocrystals of different sizes. The mixture under reflux was sampled at different times (1, 2, 3, 4, 6, and 15 h) for spectroscopic characterization. The resulting products were precipitated by acetone, and superfluous TGA and Cd2+ that did not participate in the reaction were removed with centrifugation at 5000 rpm for 5 min. Then, the precipitate was redispersed in water, and reprecipitated by acetone more than two times. Finally, the resultant precipitate was redispersed in water and stored for further use.
Noncovalent interaction of QD and CPP
Noncovalently associated QD–CPP complexes were prepared by gently mixing each peptide with QDs at room temperature for 2 h.
Cell culture
The A549 lung carcinoma cell line was obtained from Pasteur Institute (Iran). The cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin, and grown at 37°C in a 5% CO2 humidified atmosphere.
Cell proliferation assay
A549 cells were seeded into 96-well plates at a density of 1.5 × 104 cells/well and pre-incubated for 24 h at 37°C in a humidified atmosphere of 5% CO2 in air. The next day, different concentrations of QD or QD–CPP conjugates were added to the cells in the culture medium. Cells were incubated at 37°C for 24 h. The medium was removed and the wells were washed with PBS. The MTT assay was performed by adding 20 μl of MTT for 4 h. The formazan crystals were dissolved in DMSO and the absorbance of individual wells was noted at 570 nm.
Study of cellular uptake of QD–CPP complexes
The cellular uptake of the QD–CPP complexes was examined in the A549 cell line. A549 cells were seeded with RPMI 1640 medium on coverslips in 6-well plates and allowed to adhere overnight. Then, the medium was removed and washed with PBS. The cells were treated with 100 and 250 nM of QD and QD–CPP conjugates (at a ratio of 1/40) for 1 h at 37°C. After incubation, the media were removed and washed with PBS three times. The cells were fixed in formaldehyde solution for 10–15 min at room temperature and then washed with PBS. Coverslips were placed on a microscope slide (Olympus IX81 fluorescence microscope, Olympus Optical Co., Ltd., Tokyo, Japan).
Results and discussion
Characterization of QDs and QD–CPP complexes
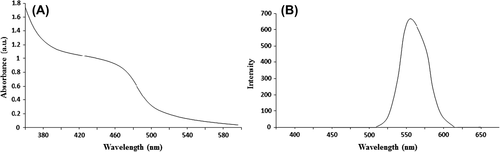
Fmoc solid-phase peptide synthesis was used for synthesizing the linear peptides R5W3R4, R9, and [RW]4 with hydrophobic (tryptophan, W) and charged (arginine, R) residues. Completion of each coupling step was controlled with a ninhydrin test. displays the UV absorption spectra of CdTe QDs, indicating a distinguished excitation absorption peak at 460 nm, and then fluorescence spectroscopy captured after 2 h of reflux time, indicating the emission peak at 560 nm (). Zeta potential was employed for characterization of each of the QD–CPP nanoconjugates. The data showed that CdTe have a zeta potential of –23 mV alone. However, the surface property was significantly changed after peptide coupling, reflected by the changing of zeta potential that is shown in .
Cytotoxicity study
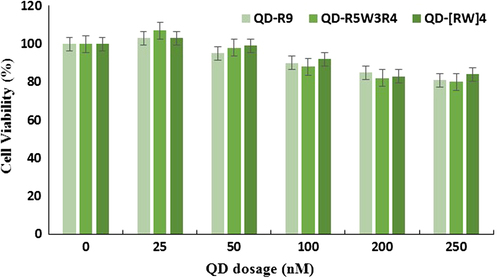
To test the effect of QD–CPP complexes on cell viability, cells were treated for 24 h with different amounts of conjugates, and cell viability was determined using the MTT assay. The data showed that none of the QD–CPP complexes at concentrations ranging from 25 to 250 nM with R9, R5W3R4, or [RW]4 at a ratio of 1/40 caused any significant toxicity on cell viability ().
Uptake study
It has been shown that covalent linkage with cationic CPP increases the efficiency of cellular entry of QDs. However, there have been few reports of improved cellular uptake of QDs following noncovalent coupling with CPPs. In this study, we demonstrated that R9 and R5W3R4 form a relatively stable noncovalent complex with QDs that considerably increases the rate and efficiency of QD uptake by A549 cells.
To determine the ability of CPPs to facilitate QD delivery, A549 cells were treated with 150 and 250 nM of QDs alone or 150 and 250 nM of QDs premixed with the three CPPs. After incubation for 1 h, QD-treated cells were observed under a fluorescent microscope. The images obtained display enhanced uptake of the QD–CPP complexes compared to that of QD alone (). A few cells emitted green fluorescence when they were treated with QD only, indicating a limited cellular uptake. The image demonstrates that among the three peptides, R9 has the best effect on enhancing QD delivery into cells. R9 has a net positive charge and could interact with negatively charged QD, causing a stable interaction with R9. Furthermore, R9 is known as an efficient vector that is capable of delivering a lot of cargo into cells. The improvement of internalization by the basic CPP is thought to reflect an electrostatic interaction between the cationic R9 and the negatively charged polar heads of the phospholipids of the cellular membrane (CitationSnyder and Dowdy 2004). In one study, it was shown that the uptake of QD–R9 is highly energy-dependent, and this supports the idea that endocytosis is the major uptake process. Their data displayed that internalization involves lipid raft and depends on micropinocytosis (CitationXu et al. 2010). For investigating the effect of tryptophan presence and its position within poly-arginine on the uptake efficiency of QDs, two peptides, including R5W3R4 and [RW]4 were designed. The data revealed that R5W3R4 has an effect similar to that of R9 on the uptake of QDs, and significantly increases delivery of QD into the cell. Although [RW]4 is also effective in the improvement of QD delivery to the cell, its effects are less than those of R9 and R5W3R4. [RW]4 probably could not carry out a stable interaction with QDs and enhance its uptake into cells. QDs were observed mainly in the perinuclear area; their entry into the nucleus limited within the first hour of treatment.
Conclusion
In conclusion, our findings are consistent with those of other studies suggesting that cationic CPPs containing arginine and tryptophan in sequence efficiently improve intracellular delivery of QDs. Most of the transferred QDs were observed mainly in the perinuclear area. It is possible that with increasing the time that cells were treated with complexes, the complexes entered the nucleus. Overall, CPPs are excellent carriers for the delivery of hydrophilic QDs.
Acknowledgments
The authors acknowledge the financial support by the “Drug Applied Research Center” and “Research Center for Pharmaceutical Nanotechnology” of Tabriz University of Medical Sciences. This study is part of an MSc thesis submitted by SM. Farkhani in the Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Derfus AM, Chan WCW, Bhatia SN. 2004. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 4:11–18.
- Erogbogbo F, Tien C-A, Chang C-W, Yong K-T, Law W-C, Ding H, et al. 2011. Bioconjugation of luminescent silicon quantum dots for selective uptake by cancer cells. Bioconjug Chem. 22:1081–1088.
- Fonseca SB, Pereira MP, Kelley SO. 2009. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv Drug Deliv Rev. 61:953–964.
- Goldman ER, Balighian ED, Mattoussi H, Kuno MK, Mauro JM, Tran PT, Anderson GP. 2002. Avidin: a natural bridge for quantum dot-antibody conjugates. J Am Chem Soc. 124:6378–6382.
- Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. 2007. Biological applications of quantum dots. Biomaterials. 28:4717–4732.
- Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, Webb WW. 2003. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 300:1434–1436.
- Lindgren M, Langel Ü. 2011. Classes and prediction of cell-penetrating peptides. Cell-Penetrating Peptides: Springer; p. 3–19.
- Liu BR, Huang Y-w, Winiarz JG, Chiang H-J, Lee H-J. 2011. Intracellular delivery of quantum dots mediated by a histidine-and arginine-rich HR9 cell-penetrating peptide through the direct membrane translocation mechanism. Biomaterials. 32:3520–3537.
- Liu BR, Li J-F, Lu S-W, Lee H-J, Huang Y-W, Shannon KB, et al. 2010. Cellular internalization of quantum dots noncovalently conjugated with arginine-rich cell-penetrating peptides. J Nanosci Nanotechnol. 10:6534–6543.
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. 2005. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 307:538–544.
- Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. 2008. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 5:763–775.
- Rydberg HA, Matson M, Åmand HL, Esbjörner EK, Nordén B. 2012. Effects of tryptophan content and backbone spacing on the uptake efficiency of cell-penetrating peptides. Biochemistry. 51: 5531–5539.
- Snyder EL, Dowdy SF. 2004. Cell penetrating peptides in drug delivery. Pharm Res. 21:389–393.
- Temsamani J, Vidal P. 2004. The use of cell-penetrating peptides for drug delivery. Drug Discov Today. 9:1012–1019.
- Tünnemann G, Ter‐Avetisyan G, Martin RM, Stöckl M, Herrmann A, Cardoso MC. 2008. Live‐cell analysis of cell penetration ability and toxicity of oligo‐arginines. J Pept Sci. 14:469–476.
- William WY, Chang E, Drezek R, Colvin VL. 2006. Water-soluble quantum dots for biomedical applications. Biochem Biophys Res Commun. 348:781–786.
- Xu Y, Liu BR, Lee H-J, Shannon KB, Winiarz JG, Wang T-C, et al. 2010. Nona-arginine facilitates delivery of quantum dots into cells via multiple pathways. J BioMed Res Int. 2010.
- Zorko M, Langel Ü. 2005. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 57:529–545.

![Figure 2. Zeta potential of QD (red) and (A) QD–R9 (B) QD–R5W3R4 and (C) QD–[RW]4. Each of the QD–CPP conjugates is shown in blue.](/cms/asset/be69aeb6-41bf-4e56-8f8e-ae1efe9d7276/ianb_a_1031906_f0002_oc.jpg)
![Figure 4. Images of cellular uptake of QD–CPP complexes. Cells were treated with 100 and 250 nM of QDs, or QD–R9, QD–R5W3R4 and QD–[RW]4 mixture (1/40).](/cms/asset/366adee7-d489-4a76-8f8c-2f2ee9949036/ianb_a_1031906_f0004_oc.jpg)