 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: Candida albicans is a common symbiotic fungus in the oral cavity, which can easily adhere to the surface of implanted materials. Highlighted by a broad antibacterial spectrum and potent antibacterial effects, nanosilver-based inorganic antibacterial agents (NSBIAA) are currently being hotly discussed with regard to their influences on biofilm formation of Candida albicans. Purpose: This paper aims to explore the influence of NSBIAA on biofilm formation of Candida albicans. Method: The XTT reduction method and the method of crystal violet determination were applied in measuring the influence of NSBIAA on biofilm formation of Candida albicans. In addition, biofilm morphology was determined by crystal violet staining. Result: It was observed that with the application of liquid antibacterial agent, at a concentration of 0.62 mg/ml, the biofilm activity of Candida albicans reduced (96.1 ± 3.0) %, along with a reduction in the biomass (95.4 ± 2.7) %, and biofilm formation was not observed under an inverted microscope. Conclusion: NSBIAA are able to inhibit biofilm formation.
Introduction
The micro-ecological environment in the healthy oral cavity is simultaneously influenced by various factors, such as biochemistry and electrochemistry (CitationJiang and Wang 2010). With proper temperature, humidity and nutrients, microbes in the oral cavity grow and reproduce at a rapid speed. Located in different positions of the cavity, microbes coexist, compete, and rival each other to create a microbalance with the host. Such a balance is affected by a variety of factors, including the application of antibacterial materials that change the type, quantity, and host position of microbes, to cause different diseases in the cavity (CitationZhang and Liu 2013). With the rapid growth in microbiology and molecular biology, the correlation between the occurrence of diseases in the oral cavity and the application of antibacterial materials is receiving great attention and has become a hot research topic.
To date, silver antibacterial material is widely accepted because of its great heat endurance and remarkable effects in killing different bacteria. Wang Fang et al. introduced the different types of silver antibacterial materials and their antibacterial mechanisms, especially their prospective uses and applications, which has led to progress in research on building materials, medical treatment, textiles, and home appliances, with the ability to direct future research studies on silver antibacterial materials (CitationWang and Li 2014). Nanosilver-based inorganic antibacterial agents (NSBIAA) have been widely applied in the field of medical biochemistry in recent years. Studies by Chai Zhiguo et al. showed that NSBIAA are characterized by a wide antibacterial spectrum, with lasting effect, good heat resistance, and biological safety, when compared with traditional organic antibacterial agents (CitationChai et al. 2014). Besides, Jia Chunli proposed to solidify PMMA by increasing NSBIAA's room temperature, which resulted in a significant antibacterial effect, with the antibacterial rate increasing with the addition of antibacterial agents. Once the antibacterial agent occupied 2.0%, the clinical antibacterial demand was satisfied, and the agent did not impact the mechanical performance of the room-temperature-solidified PMMA material (CitationJia et al. 2010).
This study carried out research on Candida albicans, a common microbe in denture plaque biofilms, to observe the influence of NSBIAA on the formation and development of Candida albicans biofilms. In this research, several different methods were adopted, such as the 2,3,bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) method of biofilm activity detection, the crystal violet determination method, crystal violet staining, confocal laser scanning microscopy (CLSM), and the method of labeling with a sudden-light probe.
Materials and method
Experimental materials
Type strains of Candida albicans ATCC 90038 were provided by the caries laboratory from the stomatological research institute in our city.
Major instruments and agents
FUMAT T200-5, a nanosilver-based inorganic antibacterial agent (Shanghai Weilai New Material Co., Ltd., mean particle diameter < 0.8 μm and layered zirconium phosphate carrier with 2.0% silver); QYC-200 temperature air table (Shanghai Fuma Laboratory Instrument Co., Ltd.); HH.B11-426 electroheating cultivator with constant temperature (Tianjin Zhonghuan Lab Furnace Co., Ltd.); NEST 96-well microplate; Eppendorf micropipette; OLYMPUS IX-71 inverted microscope; DHG-9421 electrothermal dry box with constant temperature (Shanghai Jing Hong Laboratory Instrument Co., Ltd.); Gibco® RPMI1640 culture media; culture media of yeast peptone dextrose (YPD) with 1% yeast extract, 2% peptone extract, and 2% dextrose; XTT and Vitamin K3 (menadione, Sangon Bio-tech (Shanghai) Co., Ltd.); gram staining kit (Solarbio Life Science Co., Ltd.); UCICOTMWFZUV-2100 ultraviolet–visible spectrophotometer; and Thermo Multiskan MK3 enzyme-labeled meter.
Experimental method
Bacterial liquid preparation
Frozen Candida albicans strains were thawed and inoculated into YPD culture media at 37°C for 48 h. Selected monoxenic strains were inoculated into 5 ml of YPD culture media and kept in a shaking bath at 37°Cwith 100 rpm/min for 12 h. The thallus obtained by centrifugation at 5000g × 3 min was re-suspended in RPMI1640 culture media after two washes with PBS.
The preparation of liquid antibacterial agent for application
The NSBIAA was kept in a high-temperature oven at 180°C for 2 h, for disinfection. Taking RPMI1640 as the solvent, 20 mg/ml fluid was obtained and stored at 4°C for later use. For the experiment, the liquid antibacterial agent for application, whose concentration gradient was varied from 10 mg/ml to 19.5 μg/ml, was double-diluted by PRMI1640. Two reference groups were designed in this research, namely the negative control group without bacterial liquid, and the positive control group of RPMI1640 without antibacterial agent and with bacterial liquid. As for the experimental group, it was equipped with bacterial liquid only.
In vitro culture of Candida albicans biofilms
The ratio of the volume of the bacterial liquid mentioned above to that of RPMI1640 was calculated, once the ultraviolet–visible spectrophotometer detected 1 × 106 CFU/ml bacterial liquid, based on which, bacterial liquid was added to different application liquids of the antibacterial agent. While the bacterial liquid was added to the positive control group according to the ratio, the negative control group was maintained as it was. Then, using a micropipette, 200 μl of the application liquid antibacterial agent in the experimental group, 200 μl of bacterial liquid in the positive control group, and 200 μl of the application liquid antibacterial agent in the negative control group were withdrawn. The liquids were transferred to a 96-well micro-plate with 3 rows, which was then kept in an incubator at 37°C for 24 h. The culture medium was removed after the 24-hour culture of biofilms that were then washed with 200 μl of PBS 3 times, and dried naturally. Then, 100 μl of crystal violet staining solution was used for 5 min for staining, and then discarded. This was followed by 3 washes with 200 μl of PBS to remove remaining crystal violet. Re-dried naturally, the biofilms were observed under the inverted microscope.
Measurement of biofilm activity
In the XTT reduction method designed to measure biofilm activity, XTT was dissolved using preheated 0.5 g/L Ringer’s solution at 60°C, filtered and sterilized using filters with 0.22 μm aperture, and separately stored in a refrigerator at − 80°C until further use. Before the measurement, 1 mmol/l menadione, with acetone as solvent, was added to make 1 μmol/l concentration. After the steps mentioned above were completed, the culture medium was discarded once the 24-hour Candida albicans biofilm culture was completed. After the biofilms were washed with 200 μl of PBS 3 times, 100 μl of PBS was added to each well. Then 50 μl of a mixture of XTT and menadione was withdrawn with a micropipette and placed in each well for culture, at 37°C for 2 h. Finally, the biofilm absorbance (A) was measured in the 490 nm wavelength of the enzyme-labeled meter.
Determination of the biomass of biofilm
The biomass of the Candida albicans biofilm was determined using the crystal violet method. After the above steps, the biofilms were washed with 200 μl of PBS with the removal of the mixture of XTT and menadione; when the biofilms were completely dried naturally, 100 μl of crystal violet solution was added in each well to stain for 5 min. Then, the solution was removed and the biofilms were washed with 200 μl of PBS 3 times; each well was filled with 100 μl of 95% ethanol that was constantly blown by micropipette to completely dissolve the crystal violet. Finally, ethanol with crystal violet was transferred into a new 96-well microplate and absorbance (A) was measured in the 560 nm wavelength of the enzyme-labeled meter.
The above experiment was repeated 3 times on a super-clean operating surface.
Statistical analysis
Firstly, we assumed that the values for A in the experimental group, positive control group, and negative control group were T0, T1, and T2 respectively, and P was the rate of decrease in activity and biomass of Candida albicans biofilms under the effect of the NSBIAA. Based on Formula (1) below, the rate of reduction in Candida albicans biofilm activity and biomass under the application of NSBIAA was calculated. SPSS 13.0 was used for the statistical analysis of the rate of reduction. The absorbance (A), in comparison with the values obtained for antibacterial agents of different concentrations, was analyzed by one-way analysis of variance (one-way ANOVA), while paired-comparisons adopted the least significant difference (LSD). The significant level of ɑ equaled 0.05, and therefore if P < 0.05, the difference was considered statistically significant.
Results
The influence of the application of liquid antibacterial agents at different concentrations on Candida albicans biofilm activity.
The effect of inhibition shown by the antibacterial agent on Candida albicans biofilm activity was related to concentration, demonstrating a reduction of activity with an increase in concentration. However, when the concentration reached the levels of 0.312 mg/ml, 1.230 mg/ml and 5.000 mg/ml, the rate of reduction of biofilm activity was not stable, and showed ups and downs; while a concentration of 0.625 mg/ml effected the maximum rate of reduction, at 96.1%. With statistical analysis, shows that the effects seen at concentrations of 78.1 μg/ml, 39.0 μg/ml and 19.5 μg/ml differed from those seen in other groups.
Table I. The influence of application of liquid antibacterial agents of different concentrations on Candida albicans biofilm activity.
The influence of application of liquid antibacterial agent of different concentrations on the biomass of the Candida albicans biofilm
Impacted by antibacterial agents, the biomass of the Candida albicans biofilm reduced in all groups. To be more specific, the increase in concentration of the antibacterial agent resulted in the decrease in biofilm biomass. The influence of antibacterial agent on biomass was most remarkable at concentrations of 0.019 mg/ml and 0.039 mg/ml; but the rate of reduction did not show any difference with concentrations of 5.000 mg/ml, 2.500 mg/ml, and 250 mg/ml. shows that the results in groups with concentrations of 39.0 μg/ml and 19.5 μg/ml differed from those of other groups, with statistical significance.
Table II. The influence of application of liquid antibacterial agents of different concentrations on the biomass of the Candida albicans biofilm.
The influence of application of liquid antibacterial agents of different concentrations on the morphology of the Candida albicans biofilm, observed under the inverted microscope
Due to the effect of the application of liquid antibacterial agents at different concentrations, the morphology of the biofilm in the positive control group was observed to be a dense mesh system, whose major structure consisted of the mycelial phase. Numerous blastospores were located in the system, and gaps could be noticed among the thalli. In the experimental group, the dense mesh structure was destroyed by the antibacterial agent at a concentration of 19.5 μg/ml, leading to the appearance of pseudohyphae and the sparse distribution of blastospores. In the groups treated with antibacterial agent at concentrations of 39 μg/ml and 0.31 mg/ml, the mesh of pseudohyphae and blastospores was completely destroyed by antibacterial agents as the concentration was increased. It can be observed from that the morphology of the Candida albicans biofilm disappeared totally in the group treated with the agent at a concentration of 0.62 mg/ml.
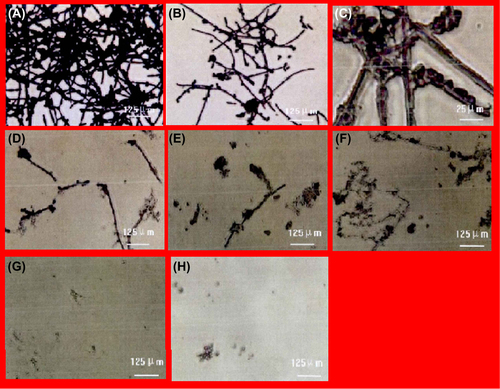
Discussion
For a long time, silver has been applied in speeding up wound recovery, and in treating infection, purifying water, and preserving drinks, and silverware keeps bacteria away from food. It has been reported that silver ions with positive charge can attract negatively charged bacterial membranes to restrain bacterial movement. In such a way, the microenvironment for bacterial survival becomes disordered, which causes cell membrane perforation and cytoplasm overflow, resulting in death of the bacteria when they come into contact with silver (CitationMa et al. 2010). In addition, silver ions seep into cells to attract enzymes and DNA to kill bacteria by affecting bacterial proliferation and metabolism. Based on nanomaterials, the NSBIAA is a novel antibacterial agent that is made by the chemical combination of inorganic ion exchanger and silver ion (CitationChen et al. 2009). The particle diameter of the NSBIAA is in the nanoscale range, which enhances the contact surface with microbes and is useful for the NSBIAA to seep into the thalli (CitationMorones et al. 2005). Moreover, the NSBIAA possesses excellent bio-safety. In detail, after testing cellular toxicity in vitro, the MTT colorimetric method proves that it is safe to apply NSBIAA at a concentration below 25 g/L (CitationZhang et al. 2005). Recently, scholars have attempted to improve mold resistance of the resin base plate with an antibacterial masterbatch formed by putting NSBIAA into polymethyl methacrylate (PMMA). By in vitro cultivation of Candida albicans biofilms on an antibacterial resin test block, observing the structure of biofilms with a microscope, and detecting quantity of microbial adhesion by the plating method, Sun Yan found that the adhesion of Candida albicans on the surface of antibacterial resin was significantly decreased within 72 h, and lowered gradually with the extension of time, compared with ordinary resin (CitationSun 2007). Li Gang, et al. observed the adhesion of bacteria on the surface of resin by building an in vitro dental plaque model on the antibacterial test block, and discovered that the control group, the 5% antibacterial agent group and 10% antibacterial agent group had coccus and bacillus adhesion on the 8th and 16th days (CitationLi et al. 2007). Although the experimental results were different, it should be noticed that the NSBIAA in the resin base plate was substantially reduced on the surface of contact with the outside atmosphere, compared with its powder form, and it cannot move in a fluid medium like antibacterial nanoparticles, which appreciably impacted the antiseptic effect of the NSBIAA by electrostatic adsorption. However, the influence of NSBIAA powder on Candida albicans biofilms has been seldom reported.
As for quantitative research of biofilm, biofilm activity and biomass need to be determined. Biofilm activity was measured by the XTT reduction method, in this study (CitationWang et al. 2010). XTT, a yellow water-soluble substance, degrades into brown formazan under the effect of mitochondrial dehydrogenase. Color changes in the above process reflect the amount of surviving cells, and the cell growth of Candida albicans biofilm can be determined by spectrophotometry and reflected as optical density values (CitationRamage et al. 2001). This study found that concentration is related to the rate of reduction of biofilm activity in different antibacterial agent groups, suggesting the decrease of live cells inside the biofilm and the reduction of Candida albicans growth activity, due to the impacts of NSBIAA. It can be gathered from that biofilm activity reduced (96 ± 3.0) % when the concentration increased to 0.62 mg/ml, indicating the death of biofilms.
The crystal violet method of determination was adopted to determine biofilm biomass. Crystal violet, a basic dye, can combine with negatively charged molecules on the cell surface and with polysaccharides in the extracellular matrix, to stain live or dead bacterium and ground substances (CitationPeeters et al. 2008). shows the correlation between concentration and the decrease of biofilm biomass with the application of liquid antibacterial agent at different concentrations, which indicates that biomass reduction was affected by the NSBIAA. Besides, biofilms were basically removed, with a reduction rate of 91.1 ± 5.6%, with antibacterial agents at a concentration of 0.15 mg/ml. Compared to biofilm activity, biomass showed more significant reductions, which is likely to have been caused by the sensitive reaction of Candida albicans to XTT–menadione (CitationYang et al. 2012).
Morphological observation of biofilms found that NSBIAA at a concentration of 39 μg/ml led to pseudohyphae and blastospores being swaddled by some transparent material. Once concentration reached 0.31 mg/ml, the pseudohyphae and blastospores were smashed or destroyed, and left without complete structures, showing that silver ions had killed Candida albicans by contacting and dissolving through the electrostatic adsorption and sterilization effects. This is in accordance with reports of studies conducted abroad. Jose et al. (Jose et al. 2005) cultured bacteria, such as Bacillus coli and Pseudomonas aeruginosa, in LB culture medium containing nanosilver for 30 min, and found that nanosilver separated from the materials to cling to bacterial cell membrane and seep into cells so as to damage the bacterial membrane. Biofilm morphology was invisible with NSBIAA at a concentration of 0.62 mg/ml; and the XTT reduction method proved the death of biofilms in 0.62 mg/ml NSBIAA. The results of biofilm morphology and activity commonly witnessed the failed formation of Candida albicans biofilms in 0.62 mg/ml NSBIAA, and proved the high sensitivity of the XTT reduction method in measuring biofilm activity (CitationZhang and Wu 2011).
Conclusion
The decreased activity of Candida albicans biofilms, and changes in the biomass of biofilms and their morphology indicate that NSBIAA can inhibit the in vitro formation of Candida albicans biofilms, which provides a scientific foundation for improving mold resistance of the resin base plate with NSBIAA. However, NSBIAA in the resin base plate cannot give play to good antiseptic effect by electrostatic adsorption like antibacterial agent, if it is in a powder state. At this point, the diffusion ability of water molecules and precipitation ability of silver ions in materials will affect antibacterial efficiency of base resin remarkably (CitationWady et al. 2012). Therefore, the specific antibacterial mechanism of NSBIAA in the resin base plate is obviously different compared with the antibacterial mechanism of its powder form, and stable and moderate precipitation of silver ions in the resin base plate will enhance antibacterial function. Methods to improve the molecular structure of base resin and realize the combination of NSBIAA and base resin need further exploration, to advance mold resistance of the resin base plate.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chai ZG, Zhang N, Xiao YH. 2014. Research progress on nano-silver base inorganic antibacterial agents used in denture resin base. J Pract Stomatol. 30:853–856.
- Chen NL, Feng HX, Wang Y, Zhang JQ. 2009. Advances of nano-inorganic antibacterial agents loaded silver. Appl Chem Ind. 38: 717–720.
- Jia CL, Wang XR, Zhang CT, Sun SQ, Yang Y. 2010. Evaluation on in vitro antibacterial effect of room curing polymethylmethacrylate material adding nano-silver base inorganic antibacterial agents. J Jilin Univ (Medicine Edition). 38:899–903.
- Jiang XF, Wang HL. 2010. Research progress of normal reference values of human body. Inner Mongolia Medical Journal. 42:26–28.
- Li Y, Chen ZQ, Wu XH. 2007. The effect of adding silver-bearing inorganic antibacterial agent in resins on common bacteria adhesion in oral cavity. West China J Stomatol. 25:280–284.
- Ma WS, Cui Y, Zhao YY, Zheng WF, Zhang W, Jinag XY. 2010. Progress of antibacterial mechanisms study on nanoparticles. Acta Biophysica Sinica. 26:638–648.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ. 2005. The bacterial effect of silver nanoparticles. Nanotechnology. 16:2346–2353.
- Peeters E, Nelis HJ, Conye T. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods. 72:157–165.
- Ramage G, Walle KV, Wickes BL, López-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicro Agents Chemother. 45:2475–2479.
- Sun Y. 2007. Research on related performance effects of adding nano-silver base inorganic antibacterial agent on denture base resins and baked porcelain with glaze. The Fourth Military Medical University. Xi'an.
- Wady AF, Machado AL, Zucolotto V, Zamperini CA, Berni E, Vergani CE. 2012. Evaluation of Candida alhicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J Appl Microbiol. 112:1163–1172.
- Wang DM, Jin Y, Dong XQ, Liu LY. 2010. Analysis on the growth kinetics and observation on the morphology of Candida albicans biofilm. Acta Academiae Medicine CPAF. 9:698–700 + 676.
- Wang F, Li J. 2014. Application research progress of the silver-typed antibacterial materials. Sci Technol Vis. 20:6–7 + 74.
- Yang J, Zhang TT, Zhu JX. 2012. In vitro formation of biofilm by candida albicans and its relationship with genotype. Int J Lab Med. 33:1539–1541.
- Zhang FQ, She WC, Fu YF. 2005. Comparison of the cytotoxicity in vitro among six types of nano-silver base inorganic antibacterial agents. Chin J Stomatol. 40:504–507.
- Zhang YJ, Liu KL. 2013. Antibacterial properties of oral nano-silver inorganic antibacterial materials. Chinese J Tissue Engg Res. 17:544–551.
- Zhang YP, Wu FM. 2011. Comparison of the effects of five denture cleansers on cleaning of Candida albicans biofilms. J Oral Sci Res. 27:368–371.
