Abstract
Mimicking morphological similarities of the natural extra cellular matrix (ECM), described by ultrafine continuous fibers, high surface to volume ratio, and high porosity is valuable for effective regeneration of injured skin tissue. Electrospun nanofibers, being one of the most favorable and fast developing products of technology today, display a tremendous potential in wound healing and skin tissue engineering. Under the remarkable attention being given to electrospun nanofibrous scaffolds in promoting wound healing and skin regeneration, this review focuses on the potential of the electrospinning technique as a promising tool for constructing polymeric nanofibrous scaffolds with the favorable physicochemical properties needed for skin bioengineering. In addition, current applications of electrospun nanofibrous matrices for skin bioengineering are detailed in this review.
Introduction
The skin is the largest organ of the body in vertebrates, acting mainly as a protective barrier against the environment. Furthermore, it aids in inhibiting body dehydration and creates a physical obstacle, limiting the penetration of potentially injurious agents to internal organs. The dermis is a collection of the predominating ECM, including collagen, elastin, and glycosaminoglycans (GAGs), and frequently, the fewer cellular constituents of fibroblasts. This layer primarily provides the physical strength and elasticity to the skin and supports wide vasculature, nerve bundles, and the lymphatic system (CitationPomahač et al. 1998).
Skin damage is principally caused by acute trauma, burn injuries, chronic wounds, and other dermatological conditions (CitationGroeber et al. 2011). According to WHO, 300 000 deaths annually are attributed to thermal traumas, whereas 6 million patients throughout the world suffer from burns every year. Besides, more than 6 million persons suffer from chronic wounds. More than 3 million patients suffer from chronic skin ulcers in the USA alone (CitationPereira et al. 2013).
The damaged epidermis is normally capable of promoting self-renewal, thanks to the existence of basal-layer epidermal stem cells, a subpopulation of undifferentiated cells with almost limitless proliferative capacity. However, the damaged skin cannot regenerate spontaneously when the damage is severe, such as loss of both epidermis and dermis in full-thickness skin injuries like burn injuries and chronic wounds. Acute large full-thickness skin wounds not only result in an immediate loss of wound coverage, but also initiate physiological instability and cause numerous problems (CitationKun 2009).
At present, split-thickness autologous skin grafting is the clinical “gold standard” in full-thickness wound treatment that includes all of the epidermis but only part of the dermis. These are removed from healthy parts of the body and utilized to treat damaged regions in the same individual. If there is sufficient blood flow in the residual dermis, patients will regenerate an epidermis from the injured sites (CitationBöttcher-Haberzeth et al. 2010). However, this approach is limited in the cure of full-thickness skin wounds, which lack the dermal layer with blood vessels. In addition, if significant amounts of skin are damaged, tissue harvest will be circumscribed by the accessible surface area of unaffected skin and the creation of an additional injury (CitationMetcalfe and Ferguson 2007). Hence, the technique of allogeneic or xenogeneic skin grafts was established. For instance, allografts of cadaver skin are usually applied as a temporary protection for full-thickness burns, but are subject to immune rejection. Xenogeneic skin implanting involves the transfer of tissue among species, and similar to allogeneic transplantation, they have complications of immune rejection and disease transmission (CitationMacNeil 2007, CitationPlacencio et al. 2011).
All of these disadvantages increase the clinical need for the development of engineered tissue substitutes. Tissue-engineered skin substitutes are currently developing as a potential solution to the restrictions of traditional therapies, and represent a reasonable therapeutic choice for the treatment of acute and chronic skin injuries, whereby tissue loss or organ failure is addressed by employing functionally active scaffolds supporting cells like keratinocytes, fibroblasts, adult and embryonic stem cells, growth factors etc., and are grafted in the patient's body where tissue regeneration is needed (CitationBártolo et al. 2011, CitationMansbridge 2009). In this respect, all tissue-engineered skin substitute bioconstructs are required to comply with three major requirements. They must be harmless for the patient, be clinically effective, and be suitable for handling and application.
Recent improvements in methods in tissue engineering and regenerative medicine have sparked interest in the creation of scaffolds with biocompatible and biodegradable polymer nanofibers. The nanofibers represent a potential candidate for scaffold biomaterial, for use in skin bioengineering. The logic for using nanofibers is based on the hypothesis that cells attach and organize appropriately around nanofibers with diameters smaller than the diameter of the cells themselves (CitationLaurencin et al. 1999, CitationPelipenko et al. 2014). More significantly, the non-woven polymeric architecture of nanofibers is morphologically similar to the fibrillar constituents of the native ECM, and mimics the native topography of ECM in the skin. The high surface-to-volume ratio of the nanofibers combined with their microporous organization supports adhesion, proliferation, migration, and differentiation of cells, all of which are extremely preferable properties for tissue engineering applications (CitationMurugan and Ramakrishna 2007).
Naturally, when the skin is injured, the body responds through a complicated dynamic process known as wound healing that results in the restoration of anatomic continuity and function of skin. During the ideal wound healing process, the wound proceeds through four phases including hemostasis, inflammation, proliferation, and finally maturation. These stages may overlap in some
Hemostasis
| (i) | Damaged blood vessels quickly undergo vasoconstriction, stopping blood flow into local tissue | ||||
| (ii) | Platelets interact with the sub-endothelium and fibrillar collagens and also many clotting factors to activate the clotting cascade. | ||||
| (iii) | The resultant fibrin clot formation provides a platform for fibroblast migration and ECM formation. | ||||
Inflammation
| (i) | Vasodilatation occurs due to endothelial products and mast cell-derived factors like histamines. | ||||
| (ii) | Neutrophils and monocytes enter the wounded area to phagocytose cell debris, foreign materials, and bacteria. | ||||
| (iii) | Macrophages produce TGF-α, TGF-β, IGF-1, FGF-2, PDGF and VEGF, which are involved in proliferation, migration, collagen production, and angiogenesis. | ||||
Proliferation
| (i) | With migration of endothelial cells to the site of the wound, angiogenesis happens simultaneously with fibroblast proliferation. Migration of endothelial cells requires collagenases and plasminogen activator to degrade the fibrin clot and part of the ECM. | ||||
| (ii) | Concurrently with angiogenesis, fibroblasts initiate accrual in the wound area and begin to produce collagen, progressively substituting the fibrin matrix. Crosslinking of new collagen matrix occurs. Furthermore, fibroblasts differentiate into myofibroblasts that produce α-smooth muscle actin, which leads to contraction of the wound. | ||||
| (iii) | Macrophages, fibroblasts, and endothelial cells in the wound site form new stroma called ‘granulation tissue’ | ||||
| (iv) | Moreover, keratinocytes begin to migrate from the wound edges and proliferate on the surface of the granulation tissue (re-epithelialization). | ||||
Maturation
In this ultimate phase, the wound has re-epithelialized and the dermis layer has recovered most of its tensile strength, while it is no more as elastic as normal skin tissue, and can be vulnerable. The scar will continue to undergo more remodeling over a time scale of months to years.
The small pore size of nanofiber scaffolds is able to protect the wound from bacterial penetration. Furthermore, nanofiber scaffolds commonly possess high tensile strength, so they can maintain structural integrity and inhibit wound contraction when a surgeon handles and grafts them into the site of the host's lesion (CitationVenugopal et al. 2008).
Due to its operational simplicity, versatility, broad range of materials that can be processed, and the capability to generate nanofibers similar to the collagen structure of the native ECM, there is great attention focused on the application of electrospinning for constructing nanoscale structures for skin tissue regeneration (CitationHeunis and Dicks 2010). The present review focuses on electrospinning as a capable tool in skin bioengineering. Emphasis is being laid on recent advances in the application of electrospun nanofibrous scaffolds fabricated from numerous materials for wound dressing and skin bioengineering.
Potential of nanofibers for wound healing applications
Cells live in native ECM, which is a 3D network organization comprised of multiple fibrils at a nanoscale. The ECM is mainly composed of hierarchically organized collagen, laminin, other fibrils, and proteoglycans in a complex structure in the nanometer size range. Therefore, this hierarchical organization exhibits a defined environment, with nanoscale intermolecular binding interactions that will influence the cellular functions like adhesion, proliferation, migration, differentiation, and cell shape (CitationArnold et al. 2009). Studies have revealed the significance of nanotexture scaffolds for tissue engineering uses (CitationMa et al. 2005). Cells that were cultured on microfibrous scaffolds were flattened, and they were found to spread and disperse if cultured on flat surfaces. Scaffolds with a nanoscale structural design and a higher surface area to adsorb proteins and donate many more binding sites to cell membrane receptors would be more biomimetic, and help better cell–matrix interactions (CitationStevens and George 2005). Hence, the presentation of appropriate topographical cues is a critical feature to consider in designing tissue-engineered scaffolds.
Nanotechnology deals with structures on the nanometer size, that is, in the range from1 μm to 1 nm. Various nanosized structures like nanorods, nanotubes, nanowires, nanocrystals, nanospheres, and nanofibers have gathered a great deal of interest for different high technology applications (CitationNukavarapu et al. 2007, CitationKumbar et al. 2007). Nanofibers are being surveyed for a variety of applications, and study in this field is promptly developing. Nanofibers have demonstrated their potential applications in several areas such as filtration (CitationSambaer et al. 2011), sensors (CitationDing et al. 2009), liquid-crystal display (LCD) (CitationNakagaito et al. 2010), super-efficient and functional catalysts (CitationLaurencin et al. 2008), and biomedical and cosmetic applications (CitationSridhar et al. 2013, CitationNguyen et al. 2013).
In biomedical applications, nanofibers have been applied as carriers for drug delivery (CitationHu et al. 2014), wound dressing materials (CitationAbdelgawad et al. 2014),and 3D scaffolds for engineering numerous tissues.
Nanofibers have been extensively investigated in the area of skin tissue engineering. They have numerous inherent properties, which make them principally attractive for wound healing applications. The remarkable nanotopographic features of the nanofibers show morphological similarities to the native ECM of skin and mimic the structure and the function of ECM, thereby supporting cell adhesion, growth, migration, differentiation, and angiogenesis (CitationKanani and Bahrami 2010). The ECM is the non-cellular section existing within all tissues. It plays a key role during the wound healing process by functioning as a scaffold, physically supporting cells and supplying conditions for adhesion, proliferation, migration, and differentiation of cells (CitationFrantz et al. 2010).
Besides, nanofibers have been revealed that increase the hemostasis of damaged tissues due to presence of small interstices and the high surface area to volume ratio. Further, the large surface area is important for fluid absorption, and also enables the possibility of modifying the surface of the nanofibers with particular chemical functionalities (CitationLannutti et al. 2007).
Nanofibrous scaffolds present high porosity, allowing cell respiration and gas permeation, protecting the wound from fluid and protein loss, and aiding in removal of exudates from the wound site. A nanofibrous mesh having interconnected nanopores inhibits the infiltration of microorganisms from the external environment and unfavorable cell/tissue ingrowth. In addition, nanofibers can improve the aesthetic appearance of the healed skin tissue (CitationAbrigo et al. 2014).
Nanofiber matrices may also be applied as carriers for a variety of bioactive molecules such as antibiotics and antimicrobial and growth factors for promotion of wound healing. Nanofiber matrices encapsulated with bioactive molecules have revealed a great potential for application in skin tissue regeneration (CitationThakur et al. 2008).
Fabrication of nanofibers: Electrospinning technique
Numerous fabrication techniques including drawing, template synthesis, temperature-induced phase separation, molecular self-assembly, and electrospinning have been applied to fabricate suitable polymeric nanofiber scaffolds for tissue engineering applications. Among all the techniques cited above, electrospinning is the most versatile, simple, elegant, reproducible, continuous, and scalable method to produce nanofibers. It is feasible to fabricate fibers with the diameter ranging from a few nanometers to a few micrometers, applying the same experimental set-up (CitationAgarwal et al. 2009). The precise advantage of this method is that it can help create scaffolds with most of the structural features needed for cell growth and subsequent tissue organization. Besides, it offers many benefits over conventional scaffold methodologies. For instance, it can generate ultrafine fibers with spatial orientation and a high surface-to-volume ratio, enable control over fiber diameter, and moreover, provide flexibility for surface alteration. Another benefit of using electrospinning to make ultra fine polymer fibers is that nearly any polymer with a suitably high molecular weight that forms a solution can be electrospun (CitationBendrea et al. 2011, CitationPelipenko et al. 2014). Synthetic and natural polymers, polymer blends, nanoparticles, or drug-impregnated polymers, and also ceramic precursors have been efficaciously electrospun into nanofibers (CitationBose et al. 2012). Furthermore, various nanofiber structures like beaded, ribbon, porous, core-shell, and core sheath fibers have been electrospun (CitationKhayet and Matsuura 2011).
Electrospinning was first presented by Zelency in 1914 (CitationMurugan and Ramakrishna 2007). Subsequently, Formhals greatly aided improvement in the electrospinning technique and achieved numerous patents in the 1930s and 1940s. In those years, it was named electrostatic spinning or electrostatic spray, and was later renamed as electrospinning in the 1990s. It has now attracted progressively increasing global consideration in the academic and industrial communities (CitationZhang et al. 2007). In the area of tissue engineering, electrospun nanofibers have been enthusiastically explored for various uses like skin, tendon, bone, cartilage, blood vessels, and nerves.
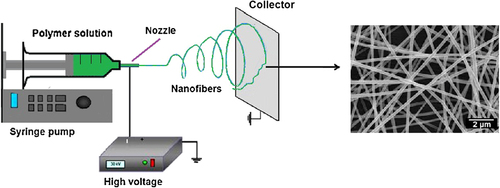
The electrospinning technique applies a high electric field to create ultrafine fibers with diameters ranging from 3 nm to 6 μm and several meters in length. In this method, a high-voltage electric field is set up between the injection needle and the stationary or rotating grounded metallic collector, using a high-power voltage supply and electrodes. The fundamental requirements of an electrospinning apparatus are illustrated in . An increase in the electrical potential initially causes the elongation of the semispherical surface of the polymer solution at the tip of the needle to create a conical shape known as the Taylor cone. An additional increase leads to the electric potential reaching a critical rate, at which it overcomes the surface tension forces to cause the creation of a polymer jet that is ejected from the tip of the Taylor cone. The solvent in the polymer jet vaporizes as it moves to the collector, enhancing the surface charge on the jet. This enhancement in surface charge stimulates instability in the polymer jet when it passes across the electric field. In an attempt to reduce the instability caused by the repulsive electrostatic forces, the jet elongates to undergo a large extent of plastic stretching that subsequently results in a significant reduction in its diameter and produces ultrathin fibers. Finally, the charged jet forms randomly oriented nanofibers that are deposited layer-by-layer on a metallic collector, generating a non-woven nanofibrous scaffold (CitationSharma et al. 2013, CitationBhardwaj and Kundu 2010).
Through the electrospinning process, both external and inherent parameters are recognized to influence the structural morphology of the nanofibers. Particularly, extrinsic parameters including environmental temperature and humidity, and inherent parameters such as applied voltage, distance between tip and collector, molecular weight of the polymer, and conductivity and viscosity/concentration of the polymer solution, need to be improved to create uniform nanofibers (CitationShabafrooz et al. 2014).
Modifications on the conventional electrospinning technique have appeared as novel procedures such as co-axial electrospinning, emulsion (water in oil) electrospinning, and electrospinning using dual spinnerets (co-electrospinning).
Co-axial electrospinning uses a specific needle setup to create core-shell-organized nanofibers. During co-axial electrospinning, a polymer solution in the external parts of the needle will form the shell, and the solution on the internal part of the needle will produce the core, thereby encapsulating the core material in the outer shell. Core polymeric solution will commonly include polymers with bioactive components such as antimicrobials and growth factors (CitationJiang et al. 2014). The shell solution can contain a suitable polymer, which helps to avoid the bioactive components from an undesirable environment and moreover allows sustained release. Core and shell compartments are separated and have different syringe pumps to ensure no miscibility of the solutions before nanofiber creation. When high voltage is utilized, the compound polymeric droplet will undergo the regular electrospinning process and create core–shell nanofibers. In the construction of core–shell nanofibers, the encapsulation of bioactive molecules is influenced by different electrospinning parameters such as viscoelasticity of the polymeric solutions, flow rate, etc. (CitationYarin 2011).
Emulsion electrospinning has been applied to produce core–sheath nanofibers containing bioactive components. Emulsion electrospinning fabricates core–sheath structured nanofibers without the requirement of the particular needle setup. In emulsion electrospinning, an aqueous solution (bioactive components, hydrophilic polymer, or a combination thereof) will be located in an organic polymer solution and emulsified, then electrospun applying the conventional electrospinning setup. Finally, components in the aqueous phase will be encapsulated into an organic polymer with electrospinning and form core–sheath nanofibers (CitationXu et al. 2006, CitationYarin 2011). These nanofibers have moreover been revealed to possess high encapsulation efficiency (CitationLi et al. 2010).
Polymers in electrospun nanofibers
Natural polymers
A broad range of natural and synthetic polymers can be electrospun into nanofibrous scaffold for skin graft and wound dressing. Natural materials that have been applied to produce nanofibrous scaffold skin grafts or wound dressings include collagen, gelatin, chitosan, fibrinogen, silk, hemoglobin, and myoglobin.
Collagen type I, comprised of two α1 chains and one α2 chain, is a good candidate for fabricating nanofibrous scaffolds for skin tissue engineering, since it is the most abundant ECM constituent in human skin tissue. In natural ECM, collagen exists in a 3D network structure comprised of multifibrils in the nanofiber range of 50–500 nm. The fibrillar structure has long been recognized to be vital for cell attachment, proliferation, and differentiation (CitationGaspar et al. 2011).
Studies have revealed the effectiveness of collagen nanofibrous scaffolds in normal human keratinocyte attachment, proliferation, and early-stage wound healing. In identical full-thickness rectangular back injuries using a Sprague-Dawley rat as an animal model, microscopic observation showed that early-stage wound healing in the collagen nanofiber scaffold cluster was quicker than in the control cluster. The wound surface of the control cluster was enclosed with fibrous tissue debris, along with a layer with a dense infiltration of leukocytes and an accumulation of proliferating fibroblasts. In comparison, in the collagen nanofiber scaffolds cluster, there was no surface tissue debris and fibroblast proliferation, indicating the efficiency of collagen nanofibrous scaffolds in improving early-stage wound healing (CitationRho et al. 2006).
The advantage of applying natural polymers such as collagen is that they are biodegradable, have good biocompatibility, have a suitable porous structure with low immunogenicity, are nontoxic, and tend to yield greater cell-binding ability. However, the poor mechanical performance, and weak control of enzymatic degradation of natural electrospun collagen nanofibers limiting the graft instability are the significant disadvantages that hinder their application (CitationGaspar et al. 2011). Hence, to control the degradation, several efforts have been focused on enhancing the mechanical properties of collagen, like using chemical and physical crosslinking methods (CitationTorres-Giner et al. 2008, CitationWang et al. 2013). Other complications in collagen-based nanofibers for wound healing applications, such as the high cost of the purification process, variability in the physicochemical properties, and potential viral contamination still exist (CitationLynn et al. 2004, CitationLee et al. 2001).
Gelatin is a processed form of a natural polymeric protein, collagen, which, as an alternative protein to collagen, is commercially available at a considerably lower cost and retains many advantages of collagen like biological origin, excellent biodegradability, and biocompatibility. Gelatin has been successfully electrospun into nanofibers applying numerous solvents like TFE, HFP, and formate (CitationPowell and Boyce 2008, CitationRujitanaroj et al. 2008). Electrospun gelatin scaffolds have been revealed to be promising as scaffolds for wound healing applications (CitationGu et al. 2009, CitationZhong et al. 2010, CitationRujitanaroj et al. 2008). However, this polymer has the limitation of low mechanical strength, and is efficiently applied only as a merged constituent with other materials to promote or change the biological or mechanical properties. The electrospun gelatin nanofibrous scaffold stabilized by glutaraldehyde vapor crosslinking supports human fibroblast proliferation and is found appropriate for wound dressings (CitationZhang et al. 2006).
Chitin and chitosan have been applied in a wide variety of biomedical applications, for example, as a carrier for drug delivery, healing materials, surgical sutures, and particularly wound dressings (CitationRinaudo 2006). Chitin has structural characteristics resembling GAGs like hyaluronic acid and chondroitin sulfates in the ECM. Furthermore, it has good biodegradability and biocompatibility, in addition to numerous biofunctionalities such as improving hemostatic immunity, and can serve as an antithrombogenic and wound-healing agent (CitationMin et al. 2004b). Chitin and particular derivatives could speed up tensile strength of wounds by prompting the fibroblastic synthesis of collagen in the first days of wound healing (CitationJayakumar et al. 2011). Chitin nanofiber scaffolds have revealed a relatively better attachment and spreading behavior of normal human fibroblasts and keratinocytes. Collagen type I coating improved the effectiveness of the nanofiber scaffolds to support cell attachment and proliferation even more. Chitin nanofiber scaffolds fully degraded within 28 days in rat subcutaneous grafting, devoid of any inflammatory reaction. Such an experiential phenomenon may be a result of the high surface area-to-volume ratio of nanofibers compared to microfibers (CitationNoh et al. 2006).
Chitosan is derivative of deacetylation of chitin, and contains glucosamine units. It has been commonly considered as a favorable natural polymer, and is the most widely used for wound healing next to collagen, because of its many benefits such as biocompatibility, biodegradability, and hemostatic and antibacterial activity, which are principally due to its polycationic nature (CitationChen et al. 2008a, CitationJayakumar et al. 2011, CitationCharernsriwilaiwat et al. 2012). Chitosan can accelerate collagen synthesis and efficiently incorporate into fibroblast growth factor because of its electrostatic role, which can improve the wound healing process (CitationMizuno et al. 2003). It is challenging to fabricate chitosan nanofiber scaffolds because of its weak solubility in broadly used organic solvents and its ionic nature in the dissolved condition. Therefore, chitosan has been physically blended with other natural and synthetic materials like poly(vinyl alcohol) (PVA), gelatin, etc. (Croisier et al. 2011, CitationJayakumar et al. 2011, CitationMak and Leung 2013).
Hyaluronic acid is a component of the native ECM in tissues and has been applied as a biopolymer in wound healing applications, for example, dermal filler scaffolds for skin bioengineering and drug delivery tools (CitationUppal et al. 2011, CitationLai et al. 2014). The biological activities of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid nanofibers have been assessed, and hyaluronic acid molecules noticeably indicated excellent biocompatibility; therefore hyaluronic acid can be valuable in construction of wound-healing nanofibrous scaffolds (CitationCollins and Birkinshaw 2013).
Silk fibroin has attracted much consideration because of its suitable biocompatibility, biodegradability, and the least inflammatory responses (CitationChutipakdeevong et al. 2013). Silk fibroin nanofiber scaffolds with high surface area-to-volume ratio, wide range of pore size distribution, and enhanced mechanical properties are highly desirable as wound dressing materials and for skin tissue-engineered scaffolds. Silk fibroin nanofiber scaffolds were treated with water steam and methanol to provide structural biostability and increase their mechanical properties. It has been demonstrated that silk fibroin scaffolds are beneficial for culturing fibroblasts, normal human keratinocytes, and stem cells, and that they improve the cell adhesion, proliferation, and differentiation (CitationMin et al. 2004a).
Electrospun fibrinogen nanofibers are suitable for fabrication of skin tissue engineering matrices that serve as wound dressings or as hemostatic cover materials (CitationWnek et al. 2003). Fibrinogen is a soluble protein in blood plasma that has been proven to contribute to wound healing as a provisional matrix. In vitro culturing with neonatal rat cardiac fibroblasts demonstrated the electrospun fibrinogen matrices to be extremely bioactive and prompt ready migration of fibroblasts through the matrices, depositing native collagen (CitationMcManus et al. 2007).
Recently, hemoglobin and myoglobin have been electrospun as wound dressing materials aimed to support oxygen delivery to the healing skin. Wound hypoxia is one of the limiting factors in the wound healing process, which hinders the normal skin regeneration. Electrospun nanofibers of globular proteins myoglobin and hemoglobin are able to be saturated with oxygen, and these scaffolds, when located on the wound site, can discharge oxygen and improve wound healing in vivo (CitationBarnes et al. 2007, CitationZhong et al. 2010). Improved oxygen delivery was considered to satisfy the local oxygen requirement because of the enhanced metabolic rate, possibly increasing resistance to infection.
Electrospun nanofibers of natural biomaterials need crosslinking to improve their biostability in aqueous environment. Additionally, crosslinking is able to modify the surface morphology and porosity of the electrospun scaffolds. Thus, the selection of an efficient crosslinker is extremely significant to maintain the structure of nanofibers following crosslinking (CitationZhong et al. 2006). For instance, adding the matrix protein tropoelastin to type I collagen was found to improve the proliferation and migration rates of normal human fibroblasts (CitationRnjak-Kovacina et al. 2012). Crosslinking collagen with chitosan following electrospinning was found to lead to a good potential for migration of cells and re-epithelialization (CitationSarkar et al. 2013). Incorporation of fibroblast growth factor 2 (FGF-2) or vascular endothelial growth factor (VEGF) to heparin-crosslinked collagen scaffolds improved their angiogenic potential (CitationNillesen et al. 2007, CitationBi and Jin 2013).
Synthetic polymers
Various synthetic materials have also been applied to produce nanofibrous scaffolds for wound healing applications due to their good mechanical and biodegradation properties that support new tissue ingrowth. Synthetic materials are inexpensive and represent a more reliable source of raw polymers. Moreover, synthetic materials are soluble in a broader range of solvents, which simplifies their electrospinning process (CitationSundaramurthi et al. 2014). Several synthetic materials such as poly (ε-caprolactone) (PCL), polyurethane (PU), poly (lactide-co-glycolide) (PLGA), and poly (L-lactide) (PLLA) have been electrospun and assessed in skin bioengineering applications.
PU has been widely studied as a material for use in wound dressings due to its unique properties. Electrospun PU nanofibrous scaffolds were found to efficiently supply excellent oxygen permeability, control evaporation water loss, and allow fluid exudation from a wound while inhibiting dehydration of the wound. Additionally, the ultrafine porosity of the PU nanofiber scaffolds prohibits invasion by exogenous microorganisms. These results show that the electrospun PU nanofibrous scaffolds have the potential to be applied as wound dressing materials (CitationKhil et al. 2003).
Because of long-term biostability, PU can just be applied as a temporary dressing in vivo and needs to be removed after completing its functions. Although biodegradable PU has been acquired for use in tissue engineering, its degradation products obtained have been found to be highly toxic, carcinogenic, and mutagenic aromatic diamines (CitationGuelcher 2008). For skin bioengineering, degraded products from the biodegradable polymers need to be nontoxic and should not elicit any significant foreign body reaction, since that would hamper the process of tissue regeneration.
The aliphatic polyesters like PCL, poly(glycolic acid) (PGA), poly(lactic acid) (PLA), and their copolymers are most generally applied for skin bioengineering.
PCL has been extensively utilized as the polymer of choice in skin bioengineering due to its biocompatibility and its desirable mechanical and biodegradable properties (CitationAugustine et al. 2014). PCL is relatively low-cost, generally soluble in the organic solvents, and is a highly elastic polyester that exhibits a lack of toxicity and a slow degradation time (CitationSundaramurthi et al. 2014). PGA has been found to have better than average biocompatibility and reproducible mechanical properties. Nevertheless, it has a very rapid degradation rate and it degrades within 2–4 weeks in vivo because of its hydrophilic nature; therefore, PGA is just applied when fast degradation is needed (CitationBarnes et al. 2007). Due to the presence of the methyl group in PLA structure, it is more hydrophobic than PGA, and is therefore dissolved in organic solvents to achieve electrospinning; it also exhibits a steric hindrance that provides a higher solubility in organic solvents and significantly decelerates hydrolysis, such that it usually degrades within 30–50 weeks (CitationBarnes et al. 2007).
PLGA, which is a copolymer of polyglycolide and polylactide, presents more favorable geometrical, mechanical and biodegradation properties than PGA and PLA alone. Electrospun PLGA nanofibrous scaffolds have been revealed to be the most desired biodegradable polymers for skin bioengineering because they are capable of matching the wound healing rate in injured skin, and support keratinocyte and fibroblast cell growth and ECM creation (CitationBlackwood et al. 2008). In addition to its structural role in increasing natural polymers like collagen, it has been demonstrated that the PLGA knitted matrix displays sufficient internal space for tissue ingrowth (CitationChen et al. 2008b).
Blended polymers
When electrospun nanofibers are produced from single polymers, there is a possibility that the nanofibers have high density, and this is named the fishnet effect. This phenomenon refers to nanofibrous scaffolds with knitted open and diamond forms, leading to fiber density. This is unhelpful since high fiber density in such mats can decrease cellular infiltration. The other disadvantage of synthetic polymers is the lack of cell-recognition signals (CitationJayakumar and Nair 2012). Furthermore, as regenerated natural biopolymers commonly possess poor mechanical properties, combinations of natural and synthetic materials are able to overcome these problems and merge two favorable features, that is, the durability and strength of a synthetic material, and the particular cell affinity of a natural biopolymer (CitationPrabhakaran et al. 2011). Additionally, blended nanofibers of synthetic and natural polymers avoid the fishnet effect and also the necessity for crosslinking. Synthetic polymers may supply the fibrous backbone while the natural biopolymer supports cell attachment and proliferation (CitationGhasemi-Mobarakeh et al. 2008). Blended polymeric nanofibers can be electrospun by applying a mixed solution and/or applying a dual or co-electrospinning method in separate solvent systems (CitationMin et al. 2004c).
A broad variety of reports based on the blending of natural and synthetic polymers for wound healing and skin reconstitution is available in literature. A blended electrospun nanofibrous scaffold of collagen and PCL has been found to have potential for the treatment of skin tissue defects and burn injuries (CitationVenugopal et al. 2006). The blended PCL/collagen nanofiber scaffold was found to support the growth, proliferation and migration of fibroblasts, and the authors suggested that PCL/collagen nanofibrous scaffolds can be suitable scaffold for skin bioengineering. CitationYang et al. (2009) constructed electrospun scaffolds comprising of type I collagen and PLGA, with high porosity. The result showed that the scaffolds with 30% collagen supported good attachment and viability of human dermal fibroblasts. The authors concluded that enhancing the collagen to PLGA ratio in electrospun blended scaffolds improved attachment and proliferation of cells, as well as collagen production. In addition, the high porosity and large pore sizes in the scaffolds provided good opportunity for fibroblasts to migrate into the scaffold, making them suitable candidates for skin bioengineering (CitationYang et al. 2009). In another research study, CitationJin et al. (2011) produced poly(L-lactic acid)-co-poly (ε-caprolactone) (PLLCL), a copolymer of PCL and PLLA nanofibrous scaffolds, with and without collagen, to study the differentiation of bone marrow-derived mesenchymal stem cells (BM-MSCs) into the keratinocytes (CitationJin et al. 2011). Best results were obtained in the case of PLLCL constructs with collagen.
Electrospun nanofibers of PLLCL and gelatin have also been fabricated, and the results have shown that the scaffold may provide a “promising bioartificial extracellular matrix with better biocompatibility and chemical properties for skin regeneration (CitationJeong et al. 2008). Recently, the wound healing capability of blended electrospun gelatin and poly (L-lactic acid)-b-poly (ε-caprolactone) (gelatin/PLLCL) nanofibers was assessed (CitationJin et al. 2014). In vitro evaluation of the study showed that the proliferation of fibroblast cells on gelatin/PLLCL with a weight ratio (w/w) of 60/40 was higher compared to cell proliferation at other weight ratios of gelatin/PLLCL. The healing ability of this scaffold studied in vivo using mouse models revealed that the gelatin/PLLCL greatly facilitates wound closure and regeneration in the first 10 days of grafting, compared to the results in the control group. Furthermore, freshly regenerated epidermis in the nanofibrous scaffold was found to be comparable to the epidermis of normal skin tissue.
Because of the antimicrobial characteristics of chitosan and the cell adhesion property of gelatin, blended nanofibrous scaffolds of chitosan/gelatin have been studied as potential scaffolds for skin tissue engineering applications (CitationDhandayuthapani et al. 2010). Furthermore, nanofibrous scaffolds constructed from chitosan/collagen (CitationChen et al. 2008a), chitosan/PVA (CitationSundaramurthi et al. 2012), chitosan/poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (PHBV) (CitationVeleirinho et al. 2012), and chitosan-grafted PCL/PCL (CitationChen et al. 2011) exhibited good cytocompatibility and antibacterial activity against a broad spectrum of microorganisms, and accelerated the wound healing process.
Polyhydroxybutyrate (PHB) combined with organic-soluble chitosan also revealed beneficial effects on promoting cell attachment and proliferation (CitationMa et al. 2010). Moreover, wound healing and histological tests on PHBV/modified keratin nanofibrous scaffolds showed that the composite mats are capable of facilitating wound recovery. Keratin is a class of fibrous proteins that are found in a wide range of biological tissues, and serve a structural role in skin and its derivatives (CitationYuan et al. 2009).
Recent approaches to overcome weak infiltration of cells have resulted in the development of nanofiber-based layer-by-layer cell assembly (RL 2009). Multilayered, multifunctional skin substitutes were produced by depositing a layer of nanofibrous scaffold and then seeding the nanofibers with cells prior to depositing a new layer of scaffolding. Remarkably, Blackwood et al. (2008) observed that adding keratinocytes to the skin substitute decreased the fibroblast-mediated contraction of the scaffolds (Blackwood et al. 2008). Applying this method, 3D multilayered functional tissue scaffolds consisting of many kinds of cells, different scaffold structures, and a particular microenvironment tailored for cells can be produced. In a recent study by Pan and colleagues in 2014, a bilayer scaffold was constructed using two different biomaterials by underlying the electrospun nanofibrous scaffold (PLLCL/Poloxamer) and casting hydrogel (composed of 10% dextran and 20% gelatin) to simulate the structure and function of natural skin tissue (CitationPan et al. 2014). The upper layer nanofiber gave mechanical support to the scaffold and the lower layer hydrogel provided suitable space to allow cells to proliferate and produce ECM. The results revealed that the bilayer scaffold is biocompatible and could be applied as an acellular scaffold aimed to promote wound healing.
Electrospun nanofibers with bioactive molecules
In addition to the advantages mentioned above, the nanofibers are able to biomimic the structure of ECM, and can be employed for biosimulating the function of ECM. The active components and functional factors can be electrospun into the nanofibers and then successively released from the nanofibers. Electrospun nanofiber scaffolds provide suitable molecular signaling, when merged with and/or immobilized with bioactive components in the 3D structure. Various bioactive molecules such as antibacterial and antifungal agents, silver nanoparticles, analgesics, growth factors, vitamins, as well as herbal extracts, and recently, animal oil extract influencing wound healing have been incorporated into nanofiber scaffolds for controlled release. The polymer–bioactive component interaction and the solubility of the bioactive molecule in the polymer solution will affect distribution of the bioactive molecule in the nanofiber scaffold and therefore will affect the drug release profile. Drug stability in the solution is also a key element to consider, and addition of bioactive molecules to the solution may change the properties of the solution, thereby affecting the electrospinning process (CitationHeunis 2012).
Antibiotics and antimicrobials
The possibility of incorporating antibiotics or antimicrobials into electrospun nanofibers represents a great benefit in the development of systems capable of treating infections in the wound. Several antibiotics and antimicrobials that have been loaded into electrospun nanofibers are listed in .
Table I. Some antimicrobial agents which have been loaded into electrospun nanofibers for wound healing applications.
In one of these investigations, an antibacterial composite scaffold was fabricated by electrospinning a solution consisting of dextran, polyurethane, and ciprofloxacin HCl (CitationUnnithan et al. 2012a). The parameters of interaction between the fibroblasts and the PU/dextran and PU/dextran/drug nanofiber scaffolds, like viability, proliferation, and attachment of cells were studied, and the results showed that the cells interacted well with the nanofibrous scaffold, particularly the drug-containing scaffold. Furthermore, the composite scaffold indicated good bactericidal activity against both Gram-positive and Gram-negative bacteria. The authors conclude that the composite scaffold presented will possibly be an ideal scaffold for wound dressing applications.
Silver (Ag) has been known as an effective antimicrobial agent for centuries, and forms part of different commercial antimicrobial wound dressing materials. Electrospinning of Ag into nanofibers to produce scaffolds with good antimicrobial properties is very well established and keenly investigated by various research groups. In a recent study, Li and coworkers indicated that scaffolds made of electrospun nanofibers of PVA and chitosan oligosaccharides with silver nanoparticles (AgNPs) implanted into Sprague-Dawley rats with full thickness wounds exhibited complete re-epithelialization on day 14 (CitationLi et al. 2013). PVA/chitosan/AgNP nanofibers revealed better wound healing compared to PVA/chitosan/AgNO3 nanofibers, gauze, and commercial wound plast. Thus, the authors suggest that the slower release of Ag ions from AgNPs probably facilitates wound healing through longer duration of protection, compared to AgNO3.
In addition, composite nanofibrous scaffolds with zinc oxide (ZnO) nanoparticles have also been reported to promote the wound healing process. Shalumon and coworkers fabricated sodium alginate/PVA nanofiber scaffolds containing different concentrations of ZnO nanoparticles as an antimicrobial compound (CitationShalumon et al. 2011). Mouse fibroblasts cultured on the scaffolds having 0.5 and 1.0 wt % ZnO nanoparticles revealed good adhesion and migration of the cells. For high concentrations of ZnO nanoparticles (2 and 5 wt %), a reduction in cell spreading was detected as a result of the toxic effect of these particles. The antimicrobial activity of the nanofibers was assessed through diffusion disc tests, applying Staphylococcus aureus and Escherichia coli. These results showed the antibacterial property of the nanofibers, which was bettered by enhancing the concentration of ZnO nanoparticles.
Analgesics and anti-inflammatory drugs
Delivery of analgesics and anti-inflammatory drugs directly into open wounds would have an important impact on pain reduction in wound healing. In a study, paracetamol as a painkilling (analgesic) drug was encapsulated in electrospun acid-labile polymers (CitationQi et al. 2008). These polymers were synthesized by reacting 3, 9-dimethylene-2, 4, 8, 10-tetraoxaspiro [5.5] undecane with 1, 10-decanediol or polyethylene glycol (PEG), which were subsequently copolymerized with D, L-lactide to obtain triblock copolymers. The in vitro release investigation revealed that paracetamol was released from the nanofibers in a biphasic pattern, with an initial burst release, and then a sustained release; the release of paracetamol was enhanced as the pH level reduced.
With the aim of developing a functional wound dressing and accelerating the wound healing process, Bui and colleagues constructed electrospun PCL nanofiber-loaded curcumin (Cur) and PEG (CitationBui et al. 2014). Curcumin (diferuloylmethane), a polyphenol, is an active component in turmeric (Curcuma longa L.), known to exhibit strong anti-inflammatory, antioxidant, antimicrobial (CitationYang et al. 2007), and wound-healing activities (CitationPanchatcharam et al. 2006). Cell viability studies using the mouse myoblast cell line C2C12 demonstrated about 80% viability on the Cur-loaded PCL/PEG nanofiber scaffold. In addition, the inclusion of 0.5 wt% Cur in both the PCL and PCL/PEG blended nanofiber scaffold hindered excessive production of nitric oxide (NO) in RAW264.7 mouse macrophages and displayed good antibacterial activity against Staphylococcus aureus (S. aureus). A preliminary study showed that the treatment using Cur-loaded PCL/PEG nanofiber scaffolds considerably improved the rate of wound closure (99%) on day 10 as compared that employing the PCL nanofiber scaffold (59%). The authors concluded that the PCL nanofibrous scaffold containing Cur and PEG may accelerate wound healing via cell proliferation and anti-inflammatory properties.
Anti-inflammatory drugs including diclofenac sodium and ibuprofen have been incorporated into nanofibers. Diclofenac sodium was released from electrospun poly (ϵ-caprolactone/D, L-lactide) nanofibers with an initial burst, releasing up to 45% during the first 24 h; the release rate after this was negligible (CitationNikkola et al. 2006). These authors concluded that in conditions where instant control of inflammation within a wound is needed, diclofenac- electrospun nanofibers can be valuable. In other experiments, by co-electrospinning ibuprofen into PLGAPEG-g-chitosan nanofibers, enhanced burst release was observed (CitationJiang et al. 2004). More improvements were achieved by covalent binding of ibuprofen to PLGA-PEG-g-chitosan nanofibers. This effort indicated that variations in polymer type and bonding mechanisms to the nanofibers can result in a burst release of drugs.
Growth factors
Some of the most key factors, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), transforming growth factor (TGF-b), VEGF, and FGF-2 were produced at different wound healing stages with certain functionalities (CitationBarrientos et al. 2008). For instance, VEGF is a main mediator for angiogenesis and granulation tissue regeneration in the early stage of wound healing. On the other hand, PDGF is critical for inflammation, granulation, re-epithelialization, and tissue remodeling during the three phases of wound healing (CitationWerner and Grose 2003). Recently, numerous studies focused on investigating and developing nanofibrous scaffolds loaded with growth factors for improved skin regeneration ().
Table II. Growth factor-conjugated electrospun nanofibrous scaffolds applied in skin tissue-engineering applications.
A novel coaxial electrospinning procedure was developed to construct a PLGA nanofibrous scaffold loaded with basic fibroblast growth factor (bFGF). This novel biofunctionalized scaffold was capable of sustained release of growth factors, and moreover, increased adhesion and proliferation of BMSCs (CitationSahoo et al. 2010).
PCL-PEG-PCL amine-terminated block copolymers were electrospun into nanofibers with surface functional amine groups. PEG molecules as crosslinkers were applied to chemically conjugate EGF to the surface of the scaffolds. The influence of these novel EGF nanofibers in wound healing was verified in diabetic mice with dorsal full-thickness wounds (CitationChoi et al. 2008).
Emulsion electrospinning was used to encapsulate bFGF into poly(ethylene oxide-co-lactic acid) (PELA) nanofibers with a novel core–sheath structure to promote skin tissue regeneration. PELA nanofiber scaffolds loaded with bFGF showed meaningfully higher wound recovery rate with complete re-epithelialization and skin appendage regeneration in a diabetic rat model, when compared to control (CitationYang et al. 2011).
To develop a dual growth factor-releasing nanoparticle-in-nanofiber system for skin engineering applications, Xie and colleagues loaded VEGF into an electrospun chitosan and poly (ethylene oxide) (PEO) nanofibrous scaffold to promote angiogenesis in the short term (CitationXie et al. 2013). Moreover, to supply a sustained release of PDGF-BB for enhanced tissue regeneration and remodeling, PDGF-BB-encapsulated poly(lactic-co-glycolic acid) nanoparticles were synthesized and were then embedded inside nanofibers. In vitro studies showed that the nanofibrous composites delivered VEGF rapidly and PDGF-BB in a relayed manner, promoted fibroblast growth, and displayed antimicrobial activities. A preliminary in vivo investigation performed on wound models of normal full-thickness rat skin demonstrated that nanofiber/nanoparticle scaffolds meaningfully enhanced the wound healing process by supporting angiogenesis, promoting re-epithelialization and controlling granulation tissue formation.
Nanofiber scaffolds loaded with multiple wound healing mediators act as favorable medical appliances for skin tissue regeneration. By programmable drug delivery, these medical tools are able to reduce the cytotoxic effects and improve clinical effectiveness. In a study, multiple epidermal induction factors (EIF) such as EGF, retinoic acid (RA), hydrocortisone, and insulin were encapsulated with gelatin and PLLCL solutions, and electrospun by two different methods: blend electrospinning and coaxial electrospinning (CitationJin et al. 2013b). The results did not indicate burst release from EIF-encapsulated core–shell nanofibers; nevertheless, an initial 44.9% burst release from EIF-blended nanofibers was detected. In addition, EIF-encapsulated core–shell nanofibers indicated higher proliferation and differentiation of adipose-derived stem cells (ADSCs) to keratinocytes compared to EIF-blended nanofibers. This study proved that the EIF-encapsulated core–shell nanofibers will possibly act as a favorable bioengineered construct for skin regeneration.
Moreover, Lai and colleagues developed a collagen and hyaluronic acid inter-stacking nanofibrous skin substitute with the controlled release of several growth factors including EGF, VEGF, PDGF and bFGF. The growth factors were either directly loaded in the nanofibers or encapsulated in the gelatin nanoparticles (GNs) by the electrospinning process (CitationLai et al. 2014). The delivery of EGF and bFGF in the initial stage of wound healing is anticipated to increase the speed of re-epithelialization and vascularization, whereas the release of PDGF and VEGF in the late stage of wound healing stimulates maturation of blood vessels. The regenerative effect of the collagen–hyaluronic acid w/4GF scaffold on streptozotocin-induced diabetic rats indicated an enhanced wound closure rate, along with the promotion of collagen deposition and increased maturation of vessels. The authors concluded that the electrospun collagen–hyaluronic acid–GN composite nanofibrous scaffold with a stage-wise release pattern of several angiogenic factors could possibly be an encouraging tissue engineered graft for chronic wound healing in skin regeneration.
Platelet-rich plasma (PRP) was selected as a natural source of various growth factors. Results indicated that PRP accelerates keratinocyte and fibroblast growth in vitro. Bertoncelj and co-workers fabricated hydrophilic nanofibrous scaffold loaded with PRP from chitosan and PEO, employing the electrospinning process, and the effects of these nanofibers on cell survival, proliferation, and mobility were studied (CitationBertoncelj et al. 2014). It was shown that such nanofibrillar support significantly induced cell proliferation, representing the synergistic effect of nanotopography and loaded growth factors. The total results confirmed desirable in vitro properties of constructed nanofibers, showing their high potential as a nanomaterial appropriate for delivery of PRP in skin tissue regeneration.
Natural substances
There is also considerable attention on the production of novel composite nanofibers with improved properties via electrospinning of natural substances, especially plant-derived compounds (CitationVenugopal et al. 2014).
Application of plant extracts for healing of burns and wounds is a usual tradition followed over the centuries. A lot of medicinal plants have a long history of therapeutic properties in wound healing, and electrospinning technology makes it possible to mix the benefits of using these plant extracts in the form of nanofibrous scaffolds to act as scaffolds for skin tissue substitutes. In a study by Jin and co-workers, the potential of the electrospinning technique four different herb extracts including Indigofera aspalathoides, Azadirachta indica, Memecylon edule (ME), and Myristica andamanica, along with a PCL for skin tissue engineering were studied, and the capability of human dermal fibroblasts (HDF) to proliferate on the electrospun nanofibrous scaffolds was also assessed (CitationJin et al. 2013a). Results showed that the proliferation rate of HDF on PCL/ME nanofibers exhibited was the highest amongst all the other electrospun nanofibrous scaffolds. Furthermore, epidermal differentiation of ADSCs on PCL/ME scaffolds was done, and proved the potential of electrospun PCL/ME nanofibers as favorable scaffolds for wound healing applications.
Aloe vera (AV) is one of the oldest medicinal herbs with well-known wound healing properties. To combine the biological activity of aloe vera and the benefits of electrospun mats, Suganya and colleagues recently fabricated electrospun nanofibrous scaffolds of PCL containing 5 and 10 wt % of lyophilized powder of aloe vera (CitationSuganya et al. 2014). The results of biological responses showed that the PCL/AV 10% nanofibrous scaffold favored cell proliferation compared to other control scaffolds, and demonstrated that the nanofibrous scaffold is a potential biomaterial for accelerating wound healing.
Charernsriwilaiwat and colleagues fabricated electrospun chitosan-based nanofibrous scaffolds and incorporated the fruit hull of Garcinia mangostana (GM) extracts into the scaffold (CitationCharernsriwilaiwat et al. 2013). Mangosteen (Garcinia mangostana Linn; GM) is a tropical fruit found in Southeast Asia. People in Southeast Asia have applied the pericarp (peel, rind, hull, or ripe fruit) of GM as a traditional medicine for the cure of abdominal pain, diarrhea, dysentery, infected wound and chronic ulcer. The antioxidative and antibacterial activity, extract release, and stability of the scaffold containing the GM extract were assessed, and the scaffold was found to display antioxidant and antibacterial activity. Also, in vivo wound healing experiments were performed in Wistar rat models. During the wound healing experiments, the scaffold was found to enhance the rate of healing when compared to the control (gauze-covered). These authors suggested that the chitosan-based nanofibrous scaffolds loaded with GM extracts might provide a suitable alternative for accelerating the wound healing process.
Karami and colleagues studied the effects of electrospun PCL/PLA nanofibrous scaffolds containing the herbal drug thymol as wound dressing material (CitationKarami et al. 2013). The results of this study indicated that the nanofibrous scaffolds containing thymol had a higher effectiveness in the wound closure compared to Comfeel®Plus that is available as a commercial wound dressing, and gauze bandages after 14 days post-treatment.
Among natural substances, honey is a very attractive material because of its anti-inflammatory and antimicrobial properties, and contains several beneficial healthful compounds such as minerals, vitamins, antioxidants and polyphenols. Furthermore, honey is imagined to alter the alkaline environment of chronic wounds toward further acidic conditions desirable for wound healing because of its low pH (3.5–4) (CitationGethin et al. 2008). Up to now, intense research has been focused on nanofibers in combination with honey. In one of the studies, Maleki and coworkers fabricated nanofibrous scaffolds from polyvinyl alcohol loaded with honey applying electrospinning (CitationMaleki et al. 2013). Dexamethasone sodium phosphate (as an anti-inflammatory drug) was incorporated into the nanofibers, and its release from nanofibers was investigated. The results revealed burst drug release, which can be extremely effectual in the early stages of wound healing, and honey can act as a natural antibiotic which improves and facilitates wound healing process.
Emu oil has been applied traditionally as a wound healer, and the native Aboriginals and first settlers in Australia rubbed the oil to accelerate wound healing and to relieve pain and disability from various musculoskeletal injuries or disorders (CitationYoganathan et al. 2003). Numerous reported animal studies suggest emu oil curing can promote re-epithelialization in wound areas and possibly will have anti-inflammatory and painkilling properties (CitationYoganathan et al. 2003, CitationWhitehouse et al. 1998). Recently, Unnithan and colleagues successfully fabricated emu oil-blended nanofibrous scaffolds via electrospinning with different composition ratios of PU for tissue skin engineering (CitationUnnithan et al. 2012b). Results showed that the scaffold with 10 wt. % emu oil is the optimum oil content in the electrospun solution to acquire PU/emu oil-blended nanofibers with good morphology. Analysis of the effects of emu oil content in nanofibrous morphology and proliferation properties of 3T3-L1 fibroblasts on blended nanofibers revealed that the composite scaffold was able to create a 3D network of the nanofibers like ECM and support long-term cell growth, as well as provide good antibacterial activity. Therefore, the authors suggest that the scaffold can be successfully applied in various biomedical fields such as wound dressing materials and skin tissue scaffolds ().
Table III. Some naturally derived substances conjugated electrospun nanofibrous scaffolds applied in skin tissue-engineering applications.
Conclusion
Generally, it is not easy to electrospin natural substances into nanofibers unless they are incorporated with synthetic materials, as they lack structural and mechanical stability upon hydration. Thus, for operating the electrospinning, we require one electrospin driving material that will improve spinnability of the natural substance along with good mechanical stability. For skin bioengineering, the high porosity of nanofibers makes more structural space available to accommodate migration and proliferation. Among several manufacturing techniques that are used to fabricate ultrafine fibers, the electrospinning technique is the most cost effective and is the easiest approach to create large volumes of nanofibers. Animal fats such as emu oil also have also been employed to generate novel composite nanofibers through the electrospinning technique.
Authors’ contributions
NZ conceived of the study and participated in its design and coordination. AA participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Department of Medical Biotechnology, Faculty of Advanced Medical Science of Tabriz University, for all support provided.
Declaration of interest
The authors have no declaration of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdelgawad AM, Hudson SM, Rojas OJ. 2014. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr Polym. 100:166–178.
- Abrigo M, McArthur SL, Kingshott P. 2014. Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol Biosci.14: 772–792.
- Agarwal S, Wendorff JH, Greiner A. 2009. Progress in the field of electrospinning for tissue engineering applications. Adv Mater. 21: 3343–3351.
- Arnold M, Schwieder M, Blümmel J, Cavalcanti-Adam EA, López-Garcia M, Kessler H, et al. 2009. Cell interactions with hierarchically structured nano-patterned adhesive surfaces. Soft Matter. 5:72–77.
- Augustine R, Dominic EA, Reju I, Kaimal B, Kalarikkal N, Thomas S. 2014. Electrospun poly ((‐caprolactone)‐based skin substitutes: In vivo evaluation of wound healing and the mechanism of cell proliferation. J Biomed Mater Rest B Appl Biomater. [Epub ahead of print].
- Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. 2007. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 59:1413–1433.
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. 2008. Growth factors and cytokines in wound healing. Wound Repair Regen. 16:585–601.
- Bártolo PJ, Domingos M, Patrício T, Cometa S, Mironov V. 2011. Biofabrication strategies for tissue engineering. Advances on Modeling in Tissue Engineering. Springer.
- Bendrea A-D, Cianga L, Cianga I. 2011. Review paper: progress in the field of conducting polymers for tissue engineering applications. J Biomater Appl. 26:3–84.
- Bertoncelj V, Pelipenko J, Kristl J, Jeras M, Cukjati M, Kocbek P. 2014. Development and bioevaluation of nanofibers with blood-derived growth factors for dermal wound healing. Eur J Pharm Biopharm. 88:64–74.
- Bhardwaj N, Kundu SC. 2010. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 28:325–347.
- Bi H, Jin Y. 2013. Current progress of skin tissue engineering: Seed cells, bioscaffolds, and construction strategies. Burn Trauma. 1:63.
- Blackwood KA, McKean R, Canton I, Freeman CO, Franklin KL, Cole D, et al. 2008. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials. 29:3091–3104.
- Bose S, Roy M, Bandyopadhyay A. 2012. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 30:546–554.
- Böttcher-Haberzeth S, Biedermann T, Reichmann E. 2010. Tissue engineering of skin. Burns. 36:450–460.
- Bui HT, Chung OH, Cruz JD, Park JS. 2014. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol Res. 22: 1288–1296.
- Charernsriwilaiwat N, Opanasopit P, Rojanarata T, Ngawhirunpat T. 2012. Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int J Pharm. 427:379–384.
- Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Sukma M, Opanasopit P. 2013. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int J Pharm. 452:333–343.
- Chen H, Huang J, Yu J, Liu S, Gu P. 2011. Electrospun chitosan-graft-poly (ϵ-caprolactone)/poly (ϵ-caprolactone) cationic nanofibrous mats as potential scaffolds for skin tissue engineering. Int J Biol Macromol. 48:13–19.
- Chen J-P, Chang G-Y, Chen J-K. 2008a. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surfaces A. 313:183–188.
- Chen X, Qi Y-Y, Wang L-L, Yin Z, Yin G-L, Zou X-H, Ouyang H-W. 2008b. Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials. 29:3683–3692.
- Choi JS, Leong KW, Yoo HS. 2008. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials. 29:587–596.
- Chutipakdeevong J, Ruktanonchai UR, Supaphol P. 2013. Process optimization of electrospun silk fibroin fiber mat for accelerated wound healing. J Appl Polym Sci. 130:3634–3644.
- Collins MN, Birkinshaw C. 2013. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr Polym. 92:1262–1279.
- Dai X-Y, Nie W, Wang Y-C, Shen Y, Li Y, Gan S-J. 2012. Electrospun emodin polyvinylpyrrolidone blended nanofibrous membrane: a novel medicated biomaterial for drug delivery and accelerated wound healing. J Mater Sci: Mater Med. 23:2709–2716.
- Dhandayuthapani B, Krishnan UM, Sethuraman S. 2010. Fabrication and characterization of chitosan‐gelatin blend nanofibers for skin tissue engineering. J Biomed Mater Res B: Appl Biomater. 94:264–272.
- Ding B, Wang M, Yu J, Sun G. 2009. Gas sensors based on electrospun nanofibers. Sensors. 9:1609–1624.
- Frantz C, Stewart KM, Weaver VM. 2010. The extracellular matrix at a glance. J Cell Sci. 123:4195–4200.
- Gaspar A, Moldovan L, Constantin D, Stanciuc A, Boeti PS, Efrimescu I. 2011. Collagen–based scaffolds for skin tissue engineering. J Med Life. 4:172.
- Gethin GT, Cowman S, Conroy RM. 2008. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int Wound J. 5: 185–194.
- Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani M-H, Ramakrishna S. 2008. Electrospun poly (ϵ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 29:4532–4539.
- Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K. 2011. Skin tissue engineering—In vivo and in vitro applications. Adv Drug Deliv Rev. 63:352–366.
- Gu S-Y, Wang Z-M, Ren J, Zhang C-Y. 2009. Electrospinning of gelatin and gelatin/poly (l-lactide) blend and its characteristics for wound dressing. Mater Sci Eng C Mater Biol Appl. 29:1822–1828.
- Guelcher SA. 2008. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng Part B Rev. 14:3–17.
- Hadjiargyrou M, Chiu JB. 2008. Enhanced composite electrospun nanofiber scaffolds for use in drug delivery. Expert Opin Drug Deliv. 5:1093–1106
- He CL, Huang ZM, Han XJ, Liu L, Zhang HS, Chen LS. 2006. Coaxial electrospun poly (L‐lactic acid) ultrafine fibers for sustained drug delivery. J Macromol Sci, Part B. 45:515–524.
- Heunis TDJ. 2012. Development of an antimicrobial wound dressing by co-electrospinning bacteriocins of lactic acid bacteria into polymeric nanofibers. Stellenbosch: Stellenbosch University.
- Heunis TDJ, Dicks LMT. 2010. Nanofibers offer alternative ways to the treatment of skin infections. J Biomed Biotechnol. 2010. pii: 510682.
- Heunis TD, Smith C, Dicks LM. 2013. Evaluation of a nisin-eluting nanofiber scaffold to treat Staphylococcus aureus-induced skin infections in mice. Antimicrob Agents Chemother. 57:3928–3935.
- Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. 2014. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 185:12–21.
- Huang ZM, He CL, Yang A, Zhang Y, Han XJ, Yin J, Wu Q. 2006. Encapsulating drugs in biodegradable ultrafine fibers through co‐axial electrospinning. J Biomed Mater Res A. 77:169–179.
- Jannesari M, Varshosaz J, Morshed M, Zamani M. 2011. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int J Nanomedicine. 6:993–1003.
- Jao WC, Yang MC, Lin CH, Hsu CC. 2012. Fabrication and characterization of electrospun silk fibroin/TiO2 nanofibrous mats for wound dressings. Polymers for Advanced Technologies. 23:1066–1076.
- Jayakumar R, Nair S. 2012. Biomedical Applications of Polymeric Nanofibers. Springer.
- Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. 2011. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 29:322–337.
- Jeong SI, Lee A-Y, Lee YM, Shin H. 2008. Electrospun gelatin/poly (L-lactide-co-(-caprolactone) nanofibers for mechanically functional tissue-engineering scaffolds. J Biomater Sci Polym Ed. 19:339–357.
- Jiang H, Fang D, Hsiao B, Chu B, Chen W. 2004. Preparation and characterization of ibuprofen-loaded poly (lactide-co-glycolide)/poly (ethylene glycol)-g-chitosan electrospun membranes. J Biomaterials Sci Polym Ed. 15:279–296.
- Jiang H, Wang L, Zhu K. 2014. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. J Control Release.
- Jin G, Li Y, Prabhakaran MP, Tian W, Ramakrishna S. 2014. In vitro and in vivo evaluation of the wound healing capability of electrospun gelatin/PLLCL nanofibers. J Bioact Compat Pol: Biomed Appl. 0883911514553525.
- Jin G, Prabhakaran MP, Kai D, Annamalai SK, Arunachalam KD, Ramakrishna S. 2013a. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials. 34:724–734.
- Jin G, Prabhakaran MP, Kai D, Ramakrishna S. 2013b. Controlled release of multiple epidermal induction factors through core–shell nanofibers for skin regeneration. Eur J Pharm Biopharm. 85:689–698.
- Jin G, Prabhakaran MP, Ramakrishna S. 2011. Stem cell differentiation to epidermal lineages on electrospun nanofibrous substrates for skin tissue engineering. Acta biomater. 7:3113–3122.
- Kanani AG, Bahrami SH. 2010. Review on electrospun nanofibers scaffold and biomedical applications. Trends Biomater Artif Organs. 24:93–115.
- Kang M, Jung R, Kim H-S, Youk JH, Jin H-J. 2007. Silver nanoparticles incorporated electrospun silk fibers. J Nanosci Nanotechnol. 7:3888–3891.
- Karami Z, Rezaeian I, Zahedi P, Abdollahi M. 2013. Preparation and performance evaluations of electrospun poly ((‐caprolactone), poly (lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J Appl Polym Sci. 129:756–766.
- Katti DS, Robinson KW, Ko FK, Laurencin CT. 2004. Bioresorbable nanofiber‐based systems for wound healing and drug delivery: Optimization of fabrication parameters. J Biomed Mater Res Part B Appl Biomater. 70:286–296.
- Kenawy E-R, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE. 2002. Release of tetracycline hydrochloride from electrospun poly (ethylene-co-vinylacetate), poly (lactic acid), and a blend. J Control Release. 81:57–64.
- Khayet M, Matsuura T. 2011. Membrane distillation: principles and applications. Elsevier.
- Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. 2003. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res Part B Appl Biomater. 67:675–679.
- Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M. 2004. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 98:47–56.
- Kumbar SG, Kofron MD, Nair LS, Laurencin CT. 2007. Cell behavior toward nanostructured surfaces. Biomed Nanostructures. 261–295.
- Kun M. 2009. Biomimetic Nanofiber/Stem Cell Composite for Skin Graft Application. National university of singapore.
- Kwak HW, Kang MJ, Bae JH, Hur SB, Kim I-S, Park YH, Lee KH. 2014. Fabrication of Phaeodactylum tricornutum extract-loaded gelatin nanofibrous mats exhibiting antimicrobial activity. Int J Biol Macromol. 63:198–204.
- Lai H-J, Kuan C-H, Wu H-C, Tsai J-C, Chen T-M, Hsieh D-J, Wang T-W. 2014. Tailor Design Electrospun Composite Nanofibers with Staged Release of Multiple Angiogenic Growth Factors for Chronic Wound Healing. Acta Biomater.
- Lannutti J, Reneker D, Ma T, Tomasko D, Farson D. 2007. Electrospinning for tissue engineering scaffolds. Mater Sci Eng C. 27: 504–509.
- Laurencin CT, Ambrosio AM, Borden MD, Cooper JA Jr. 1999. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1: 19–46.
- Laurencin CT, Kumbar SG, Nukavarapu SP, James R, Hogan MV. 2008. Recent patents on electrospun biomedical nanostructures: an overview. Recent Pat Biomed Eng. 1:68–78.
- Lee CH, Singla A, Lee Y. 2001. Biomedical applications of collagen. Int J pharm. 221:1–22.
- Li C, Fu R, Yu C, Li Z, Guan H, Hu D, et al. 2013. Silver nanoparticle/chitosan oligosaccharide/poly (vinyl alcohol) nanofibers as wound dressings: a preclinical study. Int J Nanomed. 8:4131.
- Li X, Su Y, Liu S, Tan L, Mo X, Ramakrishna S. 2010. Encapsulation of proteins in poly (L-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids Surf B Biointerfaces. 75:418–424.
- Lin J-H, Lu C-T, Hu J-J, Chen Y-S, Huang C-H, Lou C-W. 2012. Property evaluation of Bletilla striata/polyvinyl alcohol nano fibers and composite dressings. J Nano Mat. 2012:5.
- Lynn A, Yannas I, Bonfield W. 2004. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 71:343–354.
- Ma G, Yang D, Wang K, Han J, Ding S, Song G, Nie J. 2010. Organic‐soluble chitosan/polyhydroxybutyrate ultrafine fibers as skin regeneration prepared by electrospinning. J Appl Polym Sci. 118:3619–3624.
- Ma Z, Kotaki M, Inai R, Ramakrishna S. 2005. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue engineering. 11: 101–109.
- MacNeil S. 2007. Progress and opportunities for tissue-engineered skin. Nature. 445:874–880.
- Mak EY-W, Leung WW-F. 2013. Novel Nanofibrous Scaffold to Improve Wound Healing. ASME. 2013 International Mechanical Engineering Congress and Exposition. American Society of Mechanical Engineers.
- Maleki H, Gharehaghaji A, Dijkstra P. 2013. A novel honey‐based nanofibrous scaffold for wound dressing application. J Appl Polym Sci. 127:4086–4092.
- Mansbridge JN. 2009. Tissue-engineered skin substitutes in regenerative medicine. Curr Opin Biotechnol. 20:563–567.
- McManus MC, Boland ED, Simpson DG, Barnes CP, Bowlin GL. 2007. Electrospun fibrinogen: feasibility as a tissue engineering scaffold in a rat cell culture model. J Biomed Mater Res A. 81:299–309.
- Metcalfe AD, Ferguson MW. 2007. Bioengineering skin using mechanisms of regeneration and repair. Biomaterials. 28:5100–5113.
- Min B-M, Lee G, Kim SH, Nam YS, Lee TS, Park WH. 2004a. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 25:1289–1297.
- Min B-M, Lee SW, Lim JN, You Y, Lee TS, Kang PH, Park WH. 2004b. Chitin and chitosan nanofibers: electrospinning of chitin and deacetylation of chitin nanofibers. Polymer. 45:7137–7142.
- Min B-M, You Y, Kim J-M, Lee SJ, Park WH. 2004c. Formation of nanostructured poly (lactic-co-glycolic acid)/chitin matrix and its cellular response to normal human keratinocytes and fibroblasts. Carbohydr Polym. 57:285–292.
- Mizuno K, Yamamura K, Yano K, Osada T, Saeki S, Takimoto N, et al. 2003. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mat Res A. 64:177–181.
- Murugan R, Ramakrishna S. 2007. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Engineering. 13:1845–1866.
- Nakagaito AN, Nogi M, Yano H. 2010. Displays from transparent films of natural nanofibers. MRS Bulletin. 35:214–218.
- Nguyen LT, Chen S, Elumalai NK, Prabhakaran MP, Zong Y, Vijila C, et al. 2013. Biological, chemical, and electronic applications of nanofibers. Macromol Mat Eng. 298:822–867.
- Nguyen TTT, Tae B, Park JS. 2011. Synthesis and characterization of nanofiber webs of chitosan/poly (vinyl alcohol) blends incorporated with silver nanoparticles. J Mater Sci. 46:6528–6537.
- Nikkola L, Seppälä J, Harlin A, Ndreu A, Ashammakhi N. 2006. Electrospun multifunctional diclofenac sodium releasing nanoscaffold. J Nanosci Nanotechnol. 6:3290–3295.
- Nillesen S, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. 2007. Increased angiogenesis and blood vessel maturation in acellular collagen–heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 28:1123–1131.
- Noh HK, Lee SW, Kim J-M, Oh J-E, Kim K-H, Chung C-P, et al. 2006. Electrospinning of chitin nanofibers: degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials. 27:3934–3944.
- Nukavarapu SP, Kumbar SG, Nair LS, Laurencin CT. 2007. Nanostructures for tissue engineering/regenerative medicine. Biomed Nanostruct. 377.
- Pan J-F, Liu N-H, Sun H, Xu F. 2014. Preparation and Characterization of Electrospun PLCL/Poloxamer Nanofibers and Dextran/Gelatin Hydrogels for Skin Tissue Engineering. PloS One. 9:e112885.
- Panchatcharam M, Miriyala S, Gayathri V, Suguna L. 2006. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 290:87–96.
- Pelipenko J, Kocbek P, Kristl J. 2014. Nanofiber diameter as a critical parameter affecting skin cell response. European J Pharma Sci. 66:29–35.
- Pereira RF, Barrias CC, Granja PL, Bartolo PJ. 2013. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine. 8:603–621.
- Placencio K, Jefferson TF, Teran M, Fenn M. 2011. Artificial Skin and Wound. New Mater Technol Healthcare. 201.
- Pomahač B, Svensjö T, Yao F, Brown H, Eriksson E. 1998. Tissue engineering of skin. Crit Rev Oral Biol Med. 9:333–344.
- Powell H, Boyce S. 2008. Fiber density of electrospun gelatin scaffolds regulates morphogenesis of dermal–epidermal skin substitutes. J Biomed Mater Res A. 84:1078–1086.
- Prabhakaran MP, Ghasemi-Mobarakeh L, Ramakrishna S. 2011. Electrospun composite nanofibers for tissue regeneration. J Nanosci Nanotechnol. 11:3039–3057.
- Qi M, Li X, Yang Y, Zhou S. 2008. Electrospun fibers of acid-labile biodegradable polymers containing ortho ester groups for controlled release of paracetamol. Eur J Pharma Biopharm. 70:445–452.
- Rho KS, Jeong L, Lee G, Seo B-M, Park YJ, Hong S-D, et al. 2006. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 27:1452–1461.
- Rieger KA, Schiffman JD. 2014. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly (ethylene oxide) nanofibers. Carbohydr Polym. 113:561–568.
- Rinaudo M. 2006. Chitin and chitosan: properties and applications. Prog Polym Sci. 31:603–632.
- Rnjak-Kovacina J, Wise SG, Li Z, Maitz PK, Young CJ, Wang Y, Weiss AS. 2012. Electrospun synthetic human elastin: collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 8:3714–3722.
- Rujitanaroj P-O, Pimpha N, Supaphol P. 2008. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer. 49:4723–4732.
- Sahoo S, Ang LT, Goh JCH, Toh SL. 2010. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A. 93:1539–1550.
- Said SS, Aloufy AK, El-Halfawy OM, Boraei NA, El-Khordagui LK. 2011. Antimicrobial PLGA ultrafine fibers: Interaction with wound bacteria. Eur J Pharm Biopharm. 79:108–118.
- Sambaer W, Zatloukal M, Kimmer D. 2011. 3D modeling of filtration process via polyurethane nanofiber based nonwoven filters prepared by electrospinning process. Chem Eng Sci. 66:613–623.
- Sarkar SD, Farrugia BL, Dargaville TR, Dhara S. 2013. Chitosan–collagen scaffolds with nano/microfibrous architecture for skin tissue engineering. J Biomed Mater Res A. 101:3482–3492.
- Shabafrooz V, Mozafari M, Vashaee D, Tayebi L. 2014. Electrospun Nanofibers: From Filtration Membranes to Highly Specialized Tissue Engineering Scaffolds. J Nanosci Nanotechnol. 14:522–534.
- Shalumon K, Anulekha K, Nair SV, Nair S, Chennazhi K, Jayakumar R. 2011. Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int J Biol Macromol. 49:247–254.
- Sharma U, Pham Q, Marini J. 2013. Electrospinning process for fiber manufacture. Google Patents.
- Sridhar R, Sundarrajan S, Venugopal JR, Ravichandran R, Ramakrishna S. 2013. Electrospun inorganic and polymer composite nanofibers for biomedical applications. J Biomater Sci Polym Ed. 24:365–385.
- Stevens MM, George JH. 2005. Exploring and engineering the cell surface interface. Science. 310:1135–1138.
- Suganya S, Venugopal J, Mary SA, Ramakrishna S, Lakshmi B, Dev VG. 2014. Aloe vera incorporated biomimetic nanofibrous scaffold: a regenerative approach for skin tissue engineering. Iran Polym J. 23:237–248.
- Sukumar N, Ramachandran T, Lakshmikantha C. 2014. Development and characterization of cactus–dextrin–recombinant human epidermal growth factor based silk scaffold for wound dressing applications. J Ind Text. 43:565–576.
- Sun B, Li S, Zhang H, Li H, Zhao C, Yuan X, Cui Y. 2010. Controlled release of Berberine Chloride by electrospun core/shell PVP/PLCL fibrous membranes. Int J Mater Prod Technol. 37:338–349.
- Sundaramurthi D, Krishnan UM, Sethuraman S. 2014. Electrospun Nanofibers as Scaffolds for Skin Tissue Engineering. Polym Rev. 54:348–376.
- Sundaramurthi D, Vasanthan KS, Kuppan P, Krishnan UM, Sethuraman S. 2012. Electrospun nanostructured chitosan–poly (vinyl alcohol) scaffolds: a biomimetic extracellular matrix as dermal substitute. Biomed Mater. 7:045005.
- Thakur R, Florek C, Kohn J, Michniak B. 2008. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int J Pharm. 364:87–93.
- Torres-Giner S, Gimeno-Alcaniz JV, Ocio MJ, Lagaron JM. 2008. Comparative performance of electrospun collagen nanofibers cross-linked by means of different methods. ACS Appl Mater Inter. 1:218–223.
- Unnithan AR, Barakat NA, Tirupathi Pichiah P, Gnanasekaran G, Nirmala R, Cha Y-S, et al. 2012a. Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym. 90:1786–1793.
- Unnithan AR, Gnanasekaran G, Sathishkumar Y, Lee YS, Kim CS. 2014. Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing. Carbohydr Polym. 102:884–892.
- Unnithan AR, Pichiah P, Gnanasekaran G, Seenivasan K, Barakat NA, Cha Y-S, et al. 2012b. Emu oil-based electrospun nanofibrous scaffolds for wound skin tissue engineering. Colloid Surface A. 415:454–460.
- Uppal R, Ramaswamy GN, Arnold C, Goodband R, Wang Y. 2011. Hyaluronic acid nanofiber wound dressing—production, characterization, and in vivo behavior. J Biomed Mater Res B Appl Biomater. 97:20–29.
- Uttayarat P, Jetawattana S, Suwanmala P, Eamsiri J, Tangthong T, Pongpat S. 2012. Antimicrobial electrospun silk fibroin mats with silver nanoparticles for wound dressing application. Fiber Polym. 13:999–1006.
- Veleirinho B, Coelho DS, Dias PF, Maraschin M, Ribeiro-do-Valle RM, Lopes-da-Silva JA. 2012. Nanofibrous poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/chitosan scaffolds for skin regeneration. Int J Biol Macromol. 51:343–350.
- Venugopal J, Low S, Choon AT, Ramakrishna S. 2008. Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater. 84:34–48.
- Venugopal JR, Sridhar S, Ramakrishna S. 2014. Electrospun plant-derived natural biomaterials for Tissue engineering. Plant Science Today. 1:151–154.
- Venugopal JR, Zhang Y, Ramakrishna S. 2006. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif Organs. 30:440–446.
- Wang H-M, Chou Y-T, Wen Z-H, Wang Z-R, Chen C-H, Ho M-L. 2013. Novel biodegradable porous scaffold applied to skin regeneration. PloS One. 8:e56330.
- Werner S, Grose R. 2003. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 83:835–870.
- Whitehouse M, Turner A, Davis C, Roberts M. 1998. Emu oil (s): A source of non-toxic transdermal anti-inflammatory agents in aboriginal medicine. Inflammopharmacology. 6:1–8.
- Wnek GE, Carr ME, Simpson DG, Bowlin GL. 2003. Electrospinning of nanofiber fibrinogen structures. Nano Lett. 3:213–216.
- Xie Z, Paras CB, Weng H, Punnakitikashem P, Su L-C, Vu K, et al. 2013. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta biomater. 9:9351–9359.
- Xu X, Zhuang X, Chen X, Wang X, Yang L, Jing X. 2006. Preparation of core‐sheath composite nanofibers by emulsion electrospinning. Macromol Rapid Comm. 27:1637–1642.
- Yang K-YL, Lin LC, Tseng T-Y, Wang S-C, Tsai T-H. 2007. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J Chromatogr B. 853:183–189.
- Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, Li X. 2011. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 32:4243–4254.
- Yang Y, Zhu X, Cui W, Li X, Jin Y. 2009. Electrospun composite mats of poly [(D, L‐lactide)‐co‐glycolide] and collagen with high porosity as potential scaffolds for skin tissue engineering. Macromol Mater Eng. 294:611–619.
- Yarin A. 2011. Coaxial electrospinning and emulsion electrospinning of core–shell fibers. Polym Adv Technol. 22:310–317.
- Yoganathan S, Nicolosi R, Wilson T, Handelman G, Scollin P, Tao R, et al. 2003. Antagonism of croton oil inflammation by topical emu oil in CD-1 mice. Lipids. 38:603–607.
- Yuan J, Xing Z-C, Park S-W, Geng J, Kang I-K, Shen J, et al. 2009. Fabrication of PHBV/keratin composite nanofibrous mats for biomedical applications. Macromol Res. 17:850–855.
- Zahedi P, Karami Z, Rezaeian I, Jafari SH, Mahdaviani P, Abdolghaffari AH, Abdollahi M. 2012. Preparation and performance evaluation of tetracycline hydrochloride loaded wound dressing mats based on electrospun nanofibrous poly (lactic acid)/poly (ε‐caprolactone) blends. J Appl Polym Sci. 124:4174–4183.
- Zhang Y, Su B, Venugopal J, Ramakrishna S, Lim C. 2007. Biomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibers. Int J Nanomed. 2:623.
- Zhang Y, Venugopal J, Huang Z-M, Lim C, Ramakrishna S. 2006. Crosslinking of the electrospun gelatin nanofibers. Polymer. 47:2911–2917.
- Zhong S, Teo WE, Zhu X, Beuerman RW, Ramakrishna S, Yung LYL. 2006. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J Biomed Mater Res A. 79:456–463.
- Zhong S, Zhang Y, Lim C. 2010. Tissue scaffolds for skin wound healing and dermal reconstruction. WIREs: Nanomed Nanobiotechnol. 2:510–525.