Abstract
Background: The role of dobutamine in the relief of pulmonary edema during septic shock-induced acute respiratory distress syndrome (ARDS) remains undetermined, due to a lack of controllable and quantitative clinical studies. Our objective was to assess the potential effects of dobutamine on extravascular lung water index (ELWI) in septic shock-induced ARDS, reflecting its importance in pulmonary edema. At the same time, ventilator function and perfusion parameters were evaluated. Methods: We designed a prospective, non-randomized, non-blinded, controlled study to compare the differences in PiCCO parameters after 6 h of constant dobutamine infusion (15 μg/kg/min), in the baseline parameters in 26 septic shock-related ARDS patients with cardiac index ≥ 2.5I/min/m2 and hyperlactatemia. These patients (12 survivors/14 non-survivors) were monitored using the PiCCO catheter system within 48 h of onset of septic shock. The dynamic changes in ELWI, which is typically used for quantifying the extent of pulmonary edema, were evaluated, and the corresponding ventilator function and tissue perfusion parameters were also measured. Results: Decreasing ELWI (p = 0.0376) was accompanied by significantly decreased SVRI (p < 0.0001). Despite a significant increase in cardiac output (p < 0.0001), no differences were found in ITBI or GEDI. Moreover, the required dose of norepinephrine was decreased (p = 0.0389), and urine output was increased (p = 0.0358), accompanied by stabilized lactacidemia and MAP. Additionally, airway pressure was moderately improved. Conclusion: During the early stage of septic shock-induced ARDS, dobutamine treatment demonstrated a beneficial effect by relieving pulmonary edema in patients, without a negative elevation in preload or hemodynamics, which might account for the improvements in ventilator function and tissue hypoperfusion.
| Abbreviations | ||
| ACCP | = | American College of Chest Physicians |
| SCCM | = | Society of Critical Care Medicine |
| APACHE | = | Acute Physiology and Chronic Health Evaluation |
| SOFA | = | Sequential Organ Failure Assessment scores |
| SAPS | = | Simplified Acute Physiology Score |
| CHF | = | chronic heart failure |
| MAP | = | mean arterial pressure |
| CO | = | cardiac output |
| ITBI | = | intrathoracic blood volume index |
| GEDI | = | global end-diastolic volume index |
| ELWI | = | extravascular lung water index |
| PVPI | = | pulmonary vascular permeability index |
| HR | = | heart rate |
| SV | = | stroke volume |
| SVRI | = | systemic vascular resistance index |
| dPmx | = | index of left ventricular contractility |
| Crs | = | respiratory-system compliance |
Introduction
Acute respiratory distress syndrome (ARDS), which is characterized by noncardiogenic pulmonary edema due to the accumulation of extravascular lung water (EVLW), remains the leading complication for morbidity and mortality in patients with severe sepsis and septic shock. Thus, reducing EVLW or balancing intravascular volume expansion against the negative effects of causing pulmonary edema might be critical for patients with ARDS and septic shock (CitationMitchell et al. 1992).
Patients with severe ARDS exhibit greater pulmonary edema (CitationChew et al. 2012). Indeed, both persistent hypoperfusion and pulmonary edema are strongly correlated with outcomes in septic shock-induced ARDS. Dobutamine is the first- choice inotrope in patients with low cardiac output or ongoing signs of persistent tissue hypoperfusion after initial adequate fluid resuscitation (CitationDellinger et al. 2013), owing to its predominantly vasodilatory properties. Previous experiments (CitationMinnear et al. 1993, CitationGoodman et al. 1984, CitationMason et al. 1982, CitationMcAuley et al. 2004, CitationBerthiaume et al. 1987, Citation1988, CitationSaumon et al. 1987), including recent data from an ex vivo human lung experiment (CitationSakuma et al. 1994), have demonstrated that the β2 receptor is crucial for recovery from pulmonary edema, based on a mechanism linked to water transport across alveolar type II cells in septic shock (CitationMutlu et al. 2004, CitationPerkins et al. 2007). It has also been shown that patients who cleared EVLW early experienced more rapid resolution of lung injury, shorter duration of mechanical ventilation (CitationWyncoll and Evans 1999), and improved survival. Thus, it is critical and essential to focus on resolution of EVLW to control the formation of pulmonary edema and to avoid the aggravation of hypoperfusion during the early stage of ARDS in septic shock. Although dobutamine has been shown to be beneficial for persistent hypoperfusion, it remains unclear whether dobutamine might relieve pulmonary edema during sepsis-induced ARDS (CitationLi et al. 2013).
Due to a lack of controlled and dynamic monitoring, very few studies or direct evidence have assessed the development of pulmonary edema in patients with ARDS and septic shock. Recently, more studies have demonstrated that ELWI, a more sensitive and specific criterion for quantifying pulmonary edema, could be monitored with PiCCO devices (CitationMichard et al. 2004, CitationGalstian et al. 2011, CitationMartin et al. 2005). In most cases, increased ELWI, as a hallmark of early ARDS, has been shown to be the best pulmonary-specific index of disease severity and outcome prediction (CitationSakka 2013, CitationAman et al. 2012). Indeed, patients with septic shock are usually equipped with a PiCCO catheter device, allowing for the easy access of dynamic ELWI changes (CitationNeumann 1999). Using PiCCO parameters, we demonstrated here that dobutamine has a specific potential contribution to hemodynamically stable septic shock patients who have ongoing signs of tissue hypoperfusion and are at risk for pulmonary edema.
Materials and methods
This study was conducted in the medical and surgical adult intensive care units (ICUs) of 4 tertiary care hospitals in China and was approved by the Regional Ethical Review Board and registered with a database (ChiCTR-ONC-13003320, UTN: U1111-1145-9384). Informed consent was obtained from the next of kin of each patient.
Study subjects
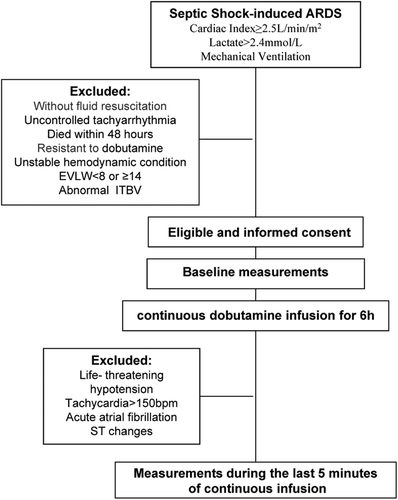
Adult ICU patients were recruited into this prospective, non-randomized, open interventional study. The diagnosis of septic shock was defined according to the criteria of the ACCP/SCCM Consensus Conference (CitationBone et al. 1992). All of the patients were monitored using a transpulmonary thermodilution device (PiCCO, Pulsion Medical System, Munich, Germany). To be eligible, patients were required to have developed septic shock and secondary ARDS onset within the preceding 48 h. ARDS was defined according to the criteria recommended by the Berlin definition (2012). Mechanical ventilation was performed according to the protocol described in the ARDSNet study (CitationNo Authors Listed 2000). Sedation was provided with propofol, and analgesia with remifentanil.
All patients were required to have both a right femoral artery catheter and a right central venous catheter in place. The correct placement of the catheter for insertion was further confirmed by chest radiography. Dobutamine was administered to increase CO because of the persistence of signs of hypoperfusion (oliguria, mottled skin, central venous oxygen saturation [SvO2] < 70% despite a hemoglobin > 8 g/dl). Achieving adequate intravascular volume, adequate mean arterial pressure (MAP > 65 mmHg), and ELW1 ≥ 8 were required (CitationSaumon et al. 1987, CitationGalstian et al. 2011). In addition, patients meeting the criteria for normal ITBV and significantly elevated SVR (SVR ≥ 2000) could be admitted within the precedent of dobutamine infusion.
We excluded patients according to the following criteria: pregnancy; age less than 18 years; absence of fluid resuscitation; unstable hemodynamic condition (change in vasoactive drug dosage or fluid administration within 1 h preceding the protocol); acute coronary syndrome within the last 72 h, and uncontrolled tachyarrhythmia (heart rate > 140 beats/min). In addition, patients who died within 48 h after the implementation of the PiCCO system, had hemodialysis, or were resistant to dobutamine were also excluded.
Study protocol
The baseline clinical characteristics and laboratory values of subjects were obtained upon admission (). Thermodilution parameters and pulse contour parameters were obtained with the PiCCO monitor, based on triplicate central venous injections of 20 ml of iced (4°C) 0.9% saline solution, and were recorded as the average of the three measurements. The corresponding ventilator function and perfusion parameters were observed. Sedation drugs were kept constant during the 6-h period preceding the measurements. The patients were kept in the horizontal position.
Table I. Clinical features of the subjects with ARDS associated with septic shock.
PiCCO measurement was performed before admission, to capture a high ELWI, conforming to the criteria for pulmonary edema. After baseline measurements, an infusion of dobutamine was initiated at 5 μg/kg/min and was increased by 5 μg/kg/min at every 20-min interval, reaching a level up to 15 μg/kg/min, and continuous infusion with 15 μg/kg/min was administered for 6 h. During infusion, additional noradrenaline support (beyond baseline needs) to maintain a MAP of 65 mmHg was mandated. No new vasopressors or inotropes were administered after starting the protocol. During the study period, all participants had the same environmental control system and followed the same control protocol. As a safeguard, in case of any transient adverse effects such as life-threatening hypotension, tachycardia > 150 bpm, acute atrial fibrillation, or ST changes on the cardiac monitor, the study was stopped and treatment was begun immediately. A diagram of the study protocol is shown in . After completing the study, dobutamine was tapered over 20 min and was then discontinued. All subjects were continuously monitored with continuous electrocardiographic monitoring and pulse oximetry, according to the standard protocols of the ICU. The patients’ subsequent intensive care and hospital course were directed by normal clinical practice.
Data analysis
Date at baseline (before dobutamine but after fluid resuscitation) and at the end of the dobutamine infusion for all of the subjects were pooled as the mean ± SD and were analyzed. Fisher's exact test was used for proportional analysis, and the independent-samples t-tests with Levene's correction for unequal group variances were used for measurements (). Statistical analyses were performed with SAS/STAT software, version 9.4. All of the significance tests used were two-tailed, and statistical significance was defined as p < 0.05.
Table II. Measurements of PiCCO, ventilator function, and perfusion parameters.
Results
Subject characteristics
From July 2013 to June 2014, 26 patients (15 male, 11 female) met the criteria for septic shock-induced ARDS and were eligible to be enrolled in the study. The median time between diagnosis of septic shock-induced ARDS and enrollment was 8.2 (4–15) h. Seventy-five percent of the patients were admitted to the ICU from the emergency unit. The overall ICU mortality was 42% (11/26). Death was caused by multiple organ dysfunction syndrome and/or irreversible septic shock. Baseline characteristics and laboratory values of the subjects are summarized in . Treatment was generally well tolerated in all of the patients, and no clinically significant adverse effects occurred during the course of the constant dobutamine administration (except that, in 6 patients, there was a trend toward higher heart rates during the initial period of dobutamine administration, but the rates then rapidly returned to normal levels).
Hemodynamic and thermodilution parameter variables
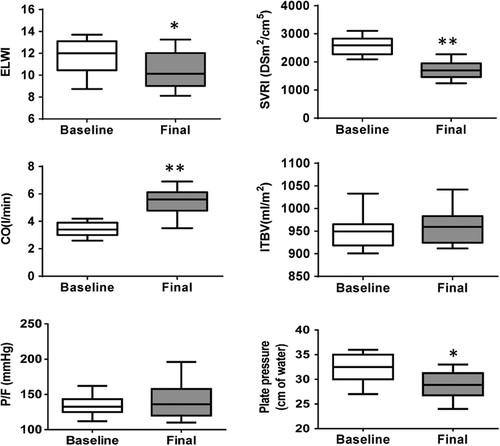
Hemodynamic and thermodilution parameters measured with the PiCCO system are listed in and . ELWI decreased at the final assessment (after 6 h of constant dobutamine administration), compared with baseline (11.2 ± 2.6 vs 9.1 ± 1.8, respectively, p = 0.0376). This decrease was accompanied by a significant decrease in SVRI (2685 ± 416 vs 1532 ± 309, respectively, p < 0.0001), suggesting beneficial effects in the relief of pulmonary edema and afterload during septic shock-induced ARDS. Moreover, cardiac output was significantly increased (3.2 ± 0.6 vs 5.8 ± 0.6, respectively, p < 0.0001), which was paralleled by a statistically significant increase in oxygen delivery (512 ± 215 vs 676 ± 220, respectively, p = 0.0090). However, the intrathoracic blood volume index (ITBI) and global end-diastolic volume index (GEDI) remained unchanged (p = 0.1485, p = 0.2656, respectively), instead of showing the expected elevation that, in theory, would be caused by an intravascular volume increase. There was no difference in pulmonary blood volume (PBV, calculated as the difference between ITBV and GEDV, data not shown). Despite an initial increase in heart rate in some patients, heart rates returned to baseline and stabilized shortly thereafter (p = 0.2011). Additionally, no differences were observed in MAP. Indeed, MAP decreased in only 2 cases, but it rapidly increased and stabilized quickly after administering additional norepinephrine, accompanied by decreased doses of norepinephrine (0.26 ± 0.12 vs 0.20 ± 0.08, respectively, p = 0.0389).
Figure 2. Classification of pulmonary edema as measured using the PiCCO system. The results of categorization by the PiCCO system of pulmonary edema patients in whom a precise diagnosis of pulmonary edema could not be made.
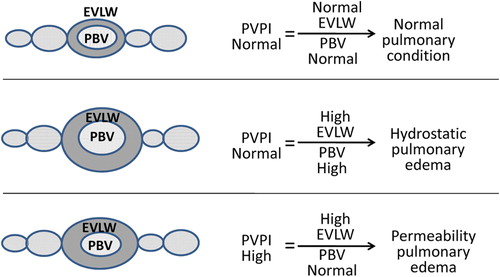
Figure 3. Changes in ELWI, SVRI, Co, ITBI, PaO2/FiO2, and Pplat in patients. Data in the final 6 h corresponding to baseline values. P values were calculated by comparing the differences between two groups (Baseline- Final). The symbols * and ** represent significant differences (P < 0.05, P < 0.001). Data are expressed as medians, interquartile ranges, and 5th/95th percentiles.*.
Ventilator function and perfusion parameter variables
The ventilator function and tissue perfusion parameters were measured, and they are listed in and . Although there were no differences in mixed venous oxygen saturation, arterial lactate, or oxygen consumption, both urine output and oxygen delivery were increased (p = 0.0358, p = 0.0090, respectively). Accordingly, there was a decrease in the dose of norepinephrine required (p = 0.0389), indicating an improvement in tissue perfusion.
Ventilator function variables were also assessed, and they are described in and . Indeed, the PaO2/FiO2 ratio, mixed venous oxygen saturation (SvO2), respiratory-system compliance (Crs), and minute volume (MV) remained unchanged, and there was a moderate reduction in airway pressure indicators (positive end-expiratory pressure [PEEP], peak inspiratory pressure [PIP], and plateau pressure [Pplat]), indicating an improvement in ventilator function (p = 0.0337, p = 0.0227, and p = 0.0047, respectively) (, ).
Discussion
Consistent with previous animal studies (CitationWu et al. 2009), our data provided the evidence that dobutamine treatment diminishes ELWI. The differences in ELWI values in our study were small, however, with an early decrease associated with more rapid improvements in ARDS, reduced durations of ventilation, and improved survival (CitationWare and Matthay 2001). Therefore, the augmentation of resolved ELWI with dobutamine administration during the early stage of ARDS associated with septic shock seems promising and could be important for clinical strategies to prevent pulmonary edema. This finding was in contrast to some previous reports (CitationPittet et al. 1994) that found elevated alveolar fluid clearance (AFC) in an animal experiment on sepsis onset and no improvement in peripheral perfusion following general concentrations of dobutamine intervention (5 μg/kg/min) (CitationHernandez et al. 2013). The reasons for these differences could be as follows:
In this clinical trial, patients with ARDS caused by severe septic shock had more severe alveolar epithelial cell damage than that observed in animal experiments, because the self-cleaning protection of the alveolar epithelium, which increases during light infection, could result in temporarily elevated AFC.
Patients received a bolus of dobutamine, followed by continuous infusion at a constant rate (15 μg/kg/min), in contrast to the low concentrations used in animal experiments.
Early resolution of edema is critical for recovery from ARDS due to the development of pulmonary edema, which can impair gas exchange, causing refractory hypoxemia, and an early diagnosis of the pathological accumulation of EVLW ()during resuscitation could allow for earlier intervention times and considerable changes in the therapeutic plan (CitationPino-Sanchez et al. 2009). In this study, we chose 8 ml/kg (CitationMichard et al. 2012) as a cut-off for normal EVLW, not the higher threshold (EVLW > 14 ml/kg) (CitationEisenberg et al. 1987)used in previous studies. This lower threshold (EVLW > 8 ml/kg) represented the relatively early stage of permeability pulmonary edema in ARDS patients, and it could be utilized to monitor early dobutamine intervention, whereas the higher threshold might represent the aggravate period and a missed opportunity for interventions (CitationLeTourneau et al. 2012). In addition, only in this situation, without the interference of diuretics and nitroglycerin preparation, the effect of dobutamine could be objectively evaluated, theoretically.
At study entry, we noted significantly increased SVRI, which might be connected to the pathophysiology of persistent shock status and the use of norepinephrine during septic shock. Increasing SVRI—a negative effect caused by norepinephrine—can aggravate hypoxia, leading to reduced alveolar fluid clearance, even if CO and MAP are normal after initial adequate fluid resuscitation. Because the signs of persistent tissue hypoxia/hypoperfusion (hyperlactacidemia) were present at enrollment, the high SVRI must be integrated into treatment considerations. We also demonstrated that there was a trend toward higher sensitivity to dobutamine, as shown by the significant decrease in SVRI, indicating that parallel mechanisms acted through dobutamine as a pulmonary vasodilator to reduce pulmonary pressures, thereby reducing one of the driving forces of edema formation (CitationGarcia-Delgado et al. 2001). Additionally, as a pulmonary vasodilator, dobutamine inhibited hypoxic pulmonary vasoconstriction, thereby increasing the shunt and overcoming the beneficial effects on oxygenation caused by the reduction in alveolar edema. Although dobutamine infusion increased CO because of its predominantly inotropic properties, which could theoretically increase the preload and pulmonary perfusion volume and result in elevated ITBV or GEDV, there were no actual changes in ITBV or GEDV, making large changes in pulmonary hemodynamics less likely and avoiding the risk of hydrostatic pulmonary edema.
In this study, we found that pulmonary blood volume (PBV), a value calculated by the difference in value between ITBV and GEDV, was also unaffected by the increase in CO caused by dobutamine infusion. Chew et al. (CitationChew et al. 2012)showed aggravated permeability of pulmonary edema due to a higher pulmonary vascular permeability index (PVPI), which is the ratio of high EVLW to normal PVB. In this study, although CO increased, PVPI decreased according to decreased EVLW and stable PVB, resulting in the resolution of permeability pulmonary edema. Based on further analysis of dobutamine as a pulmonary vasodilator, if it influenced pulmonary perfusion, it would have increased, rather than decreased ELWI. One possibility to tie these observations together is the role of water transportation, instead of vasodilation, in parallel with dobutamine infusion to relieve the abnormal accumulation of fluid in the extravascular compartments of the lung, in accordance with a component of the molecular mechanisms that potentially link to active water transport across the epithelial barrier and that might be responsible for decreasing ELWI after dobutamine treatment (CitationWu et al. 2009).
We also examined hemodynamic and hypoperfusion parameters in these patients. Interestingly, MAP was not decreased and it remained more stable during treatment, as evidenced by the reduced dose of norepinephrine and stabilization of lactacidemia, suggesting that there were no negative or aggravated signs in hypotension or hemodynamic parameters (CitationSingh and Pai 2014). Despite the lack of differences in SvO2, arterial lactate, and oxygen delivery, the improvement in urine output and in the doses of norepinephrine indicate beneficial effects on tissue hypoperfusion. In addition, both the stable ITBV and the decrease in norepinephrine demonstrated improved fluid responsiveness (CitationDonati et al. 2008), although no obvious improvement in PaO2/FiO2, Crs, or SvO2 were noted, which could have been due to three reasons: the infusion time was short, the sample size was small, and the recovery from ventilator function and tissue hypoperfusion might have been a late response related to the critical pathophysiology of septic shock. In addition, this finding indicates that monitoring of the risk of pulmonary edema via bedside ELWI measurements is more sensitive than PaO2/FiO2. One possible explanation is that the delay in oxygenation response observed reflected the time necessary for regeneration and repair of the damaged alveolar–capillary barrier.
Furthermore, some corresponding improvements in ventilator function (PEEP, PIP, and Pplat) were also noted, consistent with the possibility that the presence of cardiovascular response and ELWI reserve were critical for recovery from hypoxia/hypoperfusion and lung dysfunction in septic shock, thus providing clinicians with additional information on ventilator function and with the hypotension parameters that are concerned with promising outcomes in parallel with dobutamine administration.
Therefore, it seems that the reversion of pulmonary edema, cardiovascular response, ventilator function, and tissue hypoperfusion might potentially be accelerated by more rapid decreased in ELWI and SVRI, resulting in promising outcomes parallel to dobutamine administration in ARDS associated with septic shock. The challenges with dobutamine by PiCCO monitoring include understanding the multitude of variables that are measured and integrating the clinically relevant and adequately validated variables with the appropriate therapeutic interventions. These results are important for at least three reasons. First, they support the clinical observation with quantitative measurements that intravenous dobutamine can potentially reduce ELWI with normal PBV, thereby reducing the formation of edema to prevent ARDS development. This finding confirmed the potential effects suggested by previous studies (CitationPittet et al. 1994) that showed that the stimulation of fluid clearance from the distal airspaces of the lung was mediated by beta-adrenergic active water transport across the epithelial barrier. Second, this study might also have demonstrated that there is no clear proof that reduced MAP is accompanied by stable hemodynamics, despite a significant decrease in SVRI. Third, despite a lack of evidence for increases in PaO2/FiO2 and Crs, the data in this study suggested beneficial effects on ventilator function and tissue perfusion, suggesting promising effects of dobutamine administration. Based on these results, our observations provided important integrated insights regarding the cardiovascular and volume mechanisms underlying the effects of dobutamine infusion on pulmonary edema in early ARDS onset, associated with septic shock.
Our study showed a correlation between the statistical reductions in the amount of ELWI and dobutamine treatment. This study showed improved tissue perfusion, without the negative effects of increased hydrostatic pulmonary edema and unstable hemodynamics, suggesting both prognostic and therapeutic utility in the early clinical stage of ARDS associated with septic shock. We only sought to evaluate the incremental value of adding constant infusion of dobutamine to preliminary treatment, which would be a logical next step in future research. Further studies are needed to confirm these findings in an appropriately powered, randomized, and controlled trial.
Limitations
There were several significant limitations to this study. First, the very small number of patients in this study represented its main limitation and the primary source of possible error. Second, we could not ensure that the same volume status was present in the entire patient population that was enrolled because volume status could have been affected by time-dependent compensatory fluid shifts. These factors, and unaccounted compensatory fluid changes in perfusion, might have influenced the results. Finally, because the guidelines did not specify the dose for dobutamine, a relatively large dose (15 μg/kg/min) was adopted in this study. Thus, we were unable to select the optimal dose due to a lack of comparisons between different concentrations.
Author contributions
M.Z. contributed to the study concept, research design, and data analysis and wrote the manuscript; J.D., M.D., W.W., C.G., Y.W., R.T., F.X., and Z.R., participated in performing the study and the acquisition of data; G.S. contributed to the research design.
Trial registration: ChiCTR-ONC-13003320, UTN: U1111-1145-9384
Funding statement
This research article and the work were supported by the National Natural Science Foundation of China (No. 81201488 to M.Z., No. 81370170 to G.S.). The funders played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aman J, Groeneveld AB, van Nieuw Amerongen GP. 2012. Predictors of pulmonary edema formation during fluid loading in the critically ill with presumed hypovolemia. Crit Care Med. 40:793–799. doi: 10.1097/CCM.0b013e318236f2df. [Published online first: Epub date].
- Berthiaume Y, Broaddus VC, Gropper MA, Tanita T, Matthay MA. 1988. Alveolar liquid and protein clearance from normal dog lungs. J Appl Physiol (1985). 65:585–593.
- Berthiaume Y, Staub NC, Matthay MA. 1987. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest. 79:335–343. doi: 10.1172/JCI112817. [Published online first: Epub date].
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 2009. 136(5 Suppl):e28.
- Chew MS, Ihrman L, During J, Bergenzaun L, Ersson A, Undén J, et al. 2012. Extravascular lung water index improves the diagnostic accuracy of lung injury in patients with shock. Crit Care. 16:R1. doi: 10.1186/cc10599. [Published online first: Epub date].
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock 2012. Intensive Care Med. 39:165–228. doi: 10.1007/s00134-012-2769-8. [Published online first: Epub date].
- Donati A, Nardella R, Gabbanelli V, Scarcella M, Romanelli M, Romagnoli L, et al. 2008. The ability of PiCCO versus LiDCO variables to detect changes in cardiac index: a prospective clinical study. Minerva Anestesiologica. 74:367–374.
- Eisenberg PR, Hansbrough JR, Anderson D, Schuster DP. 1987. A prospective study of lung water measurements during patient management in an intensive care unit. Am Rev Respir Dis. 136:662–668. doi: 10.1164/ajrccm/136.3.662. [Published online first: Epub date].
- Galstian GM, Bychinin MV, Gorodetskii VM, Aleksanian MZh. 2011. Assessment of cardiac output and intrathoracic blood volume by means of transpulmonary thermodilution and ultrasound dilution: similarities and differences. Anesteziol Reanimatol. 48–53.
- Garcia-Delgado M, Colmenero-Ruiz M, Fernandez-Sacristan MA, Rus-Mansilla C, Fernández-Mondéjar E. 2001. Effect of a catecholamine-induced increase in cardiac output on extravascular lung water. Critical care medicine. 29:931–935.
- Goodman BE, Brown SE, Crandall ED. 1984. Regulation of transport across pulmonary alveolar epithelial cell monolayers. J Appl Physiol Respir Environ Exerc Physiol. 57:703–710.
- Hernandez G, Bruhn A, Luengo C, Regueira T, Kattan E, Fuentealba A, et al. 2013. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med. 39:1435–1443. doi: 10.1007/s00134–013-2982-0. [Published online first: Epub date].
- LeTourneau JL, Pinney J, Phillips CR. 2012. Extravascular lung water predicts progression to acute lung injury in patients with increased risk. Crit Care Med. 40:847–854. doi: 10.1097/CCM.0b013e318236f60e. [Published online first: Epub date].
- Li T, Zhang Z, Wu W, Liao D, Chen Y, Li S, et al. 2013. Resuscitation with polymerized human placenta hemoglobin attenuated hemorrhagic shock-induced lung injury. Artif Cells Nanomed Biotechnol. 41:27–31. doi: 10.3109/10731199.2012.696061. [Published online first: Epub date].
- Martin GS, Eaton S, Mealer M, Moss M. 2005. Extravascular lung water in patients with severe sepsis: a prospective cohort study. Critical Care. 9:R74–82. doi: 10.1186/cc3025. [Published online first: Epub date].
- Mason RJ, Williams MC, Widdicombe JH, Sanders MJ, Misfeldt DS, Berry LC Jr. 1982. Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc Natl Acad Sci U S A. 79: 6033–6037.
- McAuley DF, Frank JA, Fang X, Matthay MA. 2004. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 32:1470–1476.
- Michard F, Fernandez-Mondejar E, Kirov MY, Malbrain M, Tagami T. 2012. A new and simple definition for acute lung injury. Crit Care Med. 40:1004–1006. doi: 10.1097/CCM.0b013e31823b97fd. [Published online first: Epub date].
- Michard F, Zarka V, Alaya S. 2004. Better characterization of acute lung injury/ARDS using lung water. Chest. 125:1166. author reply 1167.
- Minnear FL, DeMichele MA, Leonhardt S, Andersen TT, Teitler M. 1993. Isoproterenol antagonizes endothelial permeability induced by thrombin and thrombin receptor peptide. J Appl Physiol (1985). 75:1171–1179.
- Mitchell JP, Schuller D, Calandrino FS, Schuster DP. 1992. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis. 145:990–998. doi: 10.1164/ajrccm/145.5.990. [Published online first: Epub date].
- Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, et al. 2004. Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling. Circ Res. 94:1091–1100. doi: 10.1161/01.RES.0000125623.56442.20. [Published online first: Epub Date].
- Neumann P. 1999. Extravascular lung water and intrathoracic blood volume: double versus single indicator dilution technique. Intensive Care Med. 25:216–219.
- No Authors Listed. 2000. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. the acute respiratory distress syndrome network. N Engl J Med. 342:1301–1308. doi: 10.1056/NEJM200005043421801. [Published online first: Epub Date].
- Perkins GD, Nathani N, McAuley DF, Gao F, Thickett DR. 2007. In vitro and in vivo effects of salbutamol on neutrophil function in acute lung injury. Thorax. 62:36–42. doi: 10.1136/thx.2006.059410. [Published online first: Epub Date].
- Pino-Sanchez F, Lara-Rosales R, Guerrero-Lopez F, Chamorro-Marín V, Navarrete-Navarro P, Carazo-de la Fuente E, Fernández-Mondéjar E. 2009. Influence of extravascular lung water determination in fluid and vasoactive therapy. J Trauma. 67:1220–1224. doi: 10.1097/TA.0b013e3181a5f1f1. [Published online first: Epub Date].
- Pittet JF, Wiener-Kronish JP, McElroy MC, Folkesson HG, Matthay MA. 1994. Stimulation of lung epithelial liquid clearance by endogenous release of catecholamines in septic shock in anesthetized rats. J Clin Invest. 94:663–671. doi: 10.1172/JCI117383. [Published online first: Epub date].
- Sakka SG. 2013. Extravascular lung water in ARDS patients. Minerva Anestesiol. 79:274–284.
- Sakuma T, Okaniwa G, Nakada T, Nishimura T, Fujimura S, Matthay MA. 1994. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med. 150:305–310. doi: 10.1164/ajrccm.150.2.8049807. [published online first: Epub date].
- Saumon G, Basset G, Bouchonnet F, Crone C. 1987. cAMP and beta-adrenergic stimulation of rat alveolar epithelium. Effects on fluid absorption and paracellular permeability. Pflugers Arch. 410: 464–470.
- Singh G, Pai RS. 2014. In vitro and in vivo performance of supersaturable self-nanoemulsifying system of trans-resveratrol. Artif Cells Nanomed Biotechnol. 21:1–7. doi: 10.3109/21691401.2014.966192. [Published online first: Epub date].
- Ware LB, Matthay MA. 2001. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 163: 1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [Published online first: Epub date].
- Wu XM, Wang HY, Li GF, Zang B, Chen WM. 2009. Dobutamine enhances alveolar fluid clearance in a rat model of acute lung injury. Lung. 187:225–231. doi: 10.1007/s00408-009-9155-5. [Published online first: Epub date].
- Wyncoll DL, Evans TW. 1999. Acute respiratory distress syndrome. Lancet. 354:497–501. doi: 10.1016/S0140-6736(98)08129-X. [Published online first: Epub date].