Abstract
In the present study, we investigated a novel green route for synthesis of zinc oxide (ZnO) nanocrystals using Prunus × yedoensis Matsumura leaf extract as a reducing agent without using any surfactant or external energy. Standard characterization studies were carried out to confirm the obtained product using UV–Vis spectra, SEM–EDS, FTIR, TEM, and XRD. In addition, the synthesized ZnO nanocrystals were coated onto fabric and leather samples to study their bacteriostatic effect against odor-causing bacteria Brevibacterium linens and Staphylococcus epidermidis. Zinc oxide nanocrystal-coated fabric and leather showed good activity against both bacteria.
Introduction
The green synthesis of metallic nanoparticles has attracted tremendous attention in recent years due to lower cost and greater eco-friendliness. Among various metal nanoparticles, zinc oxide nanocrystals (ZnO) exhibit unique characteristics such as semiconducting (CitationSangeetha et al. 2011), piezoelectric (CitationFujihara et al. 2001), and pyroelectric properties (CitationVayssieres et al. 2001), for transparent electronics (CitationVanathi et al. 2014), spin electronics (CitationWang 2004), and coating (CitationWaham et al. 2010), as ultraviolet (UV) light emitters (CitationAkiyama et al. 1998), chemical sensors (CitationMochinaga et al. 1998), and catalysts (CitationElseviers and Verelst 1999), and in space applications (CitationSingh et al. 2011). Several chemical and physical methods are employed to obtain ZnO nanocrystals, but these are generally not environmentally friendly and lead to health concerns. Biological synthesis of metallic nanoparticles with well-controlled and defined size and shape has been attained through the use of plant extracts in eco-friendly approaches with environmental concerns (CitationBar et al. 2009, CitationGnanasangeetha and Sarala Thambavani 2013, CitationRajiv et al. 2013, CitationVanathi et al. 2014, CitationYuvakkumar et al. 2014). Apart from plant material, some enzymes (CitationPrasad and Jha 2009), bacteria (CitationJayaseelan et al. 2012; CitationWang et al. 2015)), seaweeds (e.g., Sargassum muticum) (CitationAzizi et al. 2014), and gelatins (CitationDarroudi et al. 2014) have been used as green routes for the synthesis of ZnO nanocrystals. However, obtaining nanoparticles through bacteria and other sources leads to several difficulties in terms of production, cultivation, maintenance, and cost effectiveness. Thus, to overcome these limitations, economical and widely available leaf extract from the Yoshino cherry (Prunus × yedoensis Matsumura; Japanese: Somei-Yoshino) was used for the synthesis of ZnO nanocrystals. Brevibacterium linens and Staphylococcus epidermidis are responsible for body odor as well as odor on feet, shoes and/or socks, which is mediated through the breakdown of amino acids present in sweat (CitationAra et al. 2006, CitationKanlayavattanakul and Lourith 2011). CitationNazeruddin et al. (2014) summarized a table in his article, in which nanoparticles that have been synthesized by various biological routes by various researchers were discussed well.
As one of the objectives of this study, a green chemistry approach is adopted for the synthesis of ZnO nanocrystals using Prunus × yedoensis leaf extract, which is an eco-friendly route, due to its vital applications against odor-causing bacteria. The synthesized ZnO nanocrystals have been subsequently characterized by various instrumental techniques. The antibacterial activity of ZnO nanocrystal-coated cotton fabrics and tanned leather has been examined toward B. linens and S. epidermidis, Gram-positive bacteria.
Materials and methods
Leaf extract preparation and synthesis of ZnO NPs
Mature Prunus × yedoensis leaves (50 g) were collected in and around Chonbuk National University, Iksan, South Korea and washed thoroughly with distilled water, cut into small pieces, and boiled in 200 ml of sterile Nanopure water (conductivity = 18 μΩ/m, TOC < 3 ppb, Barnstead, Waltham, MA, USA) for 30 min to obtain the extract, followed by filtration (Whatman No. 42) and storage in a refrigerator for future use. One hundred milliliters of 0.1 M zinc nitrate [(Zn(NO3)2.6H2O)-, Sigma-Aldrich, St. Louis, MO, USA] was added dropwise using a burette into 20 ml of the hot leaf extract under continuous stirring at 80°C for 8 h. The ZnO nanocrystals were synthesized through reduction of zinc nitrate by the phytochemical polyphenols present in the leaf extract, which are essential for the ligation of transition metal ions to form metal oxide nanocrystals (CitationVanathi et al 2014, CitationYuvakkumar et al. 2014). During the reaction, a yellow-colored precipitate was obtained as an initial conformation. The precipitate was washed several times with ethanol, followed by calcination at 450°C for 1 h in a furnace (AJEON Heating Industrial Co., LTD, Gyeonggi-do, South Korea). Finally, a white powder was obtained and used in subsequent experiments.
Characterization of ZnO NPs
The obtained colloidal ZnO nanocrystals were scanned after color change with a UV–Vis spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) in the wavelength range of 200–800 nm using a dual beam operated at 1 nm resolution. Biological transmission electron microscopy (Bio-TEM, H-7650, Hitachi, Ltd., Tokyo, Japan,) was used to examine the surface morphology and size of the ZnO nanocrystals. For TEM measurements, a drop of solution containing the particle was deposited on a copper grid covered with amorphous carbon. After allowing the film to stand for 2 min, the extract solution was removed by means of blotting paper and the grid allowed to dry before the measurement. X-ray powder diffraction of the samples was obtained using a Rigaku X-ray diffractometer (XRD, Rigaku, Japan). Fourier transform infrared (FTIR) spectra of the ZnO nanocrystals were obtained using a Perkin-Elmer FTIR spectrophotometer (Norwalk, CT, USA) in the diffuse reflectance mode at a resolution of 4 particles cm− 1 in KBr pellets. Scanning electron microscopy (SEM) was used to examine the binding nature of the ZnO nanocrystals coated onto leather and fabric. Energy dispersive spectroscopic (EDS) (JED 2300, JEOL) analysis was carried out on the prepared samples for qualitative elemental analysis.
Treatment of fabric and leather with ZnO nanocrystals and their antibacterial activity
ZnO nanocrystals were applied to fabric and leather by employing a pad–dry–cure method (CitationThitilertdecha et al. 2010, CitationYuvakkumar et al. 2014). The antibacterial activities of the ZnO nanocrystals on treated and untreated fabric and leather were evaluated against odor-causing bacteria B. linens (KACC-14346) and S. epidermidis (KACC-13234) procured from the Korean Agriculture Culture Collection, Suwon, South Korea (KACC). The cultures were maintained on nutrient agar and the antibacterial activity was determined using the method developed by CitationVelmurugan et al. (2014). The antibacterial activity was evaluated by measuring the zone of inhibition against the test organisms. Finally, we measured diameters (mm) of zone of inhibition of the control strain and test with a ruler and caliper readings.
Results and discussion
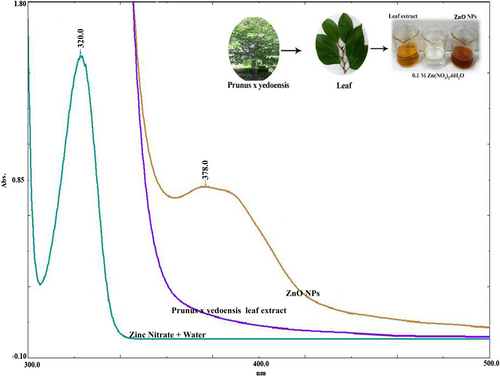
UV–visible spectroscopy of ZnO nanocrystals
In the present investigation, the synthesis of ZnO nanocrystals was carried out employing a novel, environmentally benign synthetic strategy using Prunus × yedoensis leaf extract. ZnO nanocrystals demonstrated a broad absorption peak at 378 nm, as shown in . The UV absorbance at 378 nm was in accordance with previous reports (CitationNawaz et al. 2011, CitationVanathi et al. 2014) and the band gap of the ZnO nanocrystals was calculated according to the method proposed by CitationVanathi et al. (2014). It is reported in the literature that typical ZnO nanocrystals show the characteristic surface plasmon resonance (SPR) at the wavelength in the range of 350–400 nm. The SPR absorbance is sensitive to the nature, size and shape of particles present in the solution and it also depends upon their inner particle distance and the surrounding media.
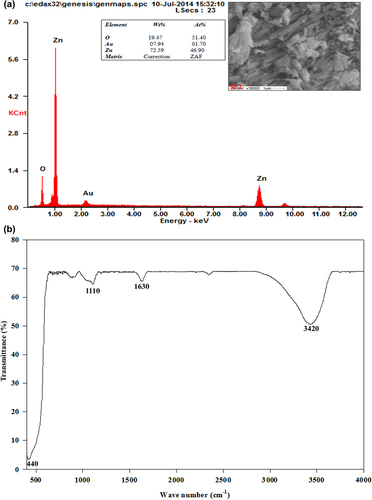
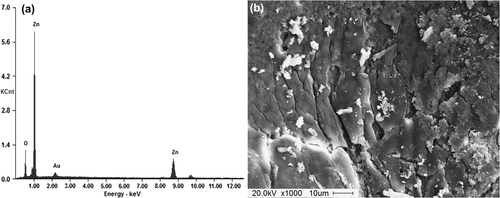
SEM–EDS spectra and the image of ZnO nanocrystals
The energy-dispersive X-ray spectroscopy (EDS) analysis results of the green synthesized ZnO nanocrystals are shown in , and the inset shows the SEM image of ZnO nanocrystals. The results demonstrate the strong signals in zinc and oxygen, further confirming the formation of ZnO nanocrystals. Our EDS results are in good accordance with earlier reports (CitationEl Ghoul et al. 2012, CitationRajiv et al. 2013, CitationVanathi et al. 2014, CitationYuvakkumar et al. 2014). The morphology of the nanocrystals revealed non-uniform distribution in the form of a needle-like structure, as shown in the inset SEM image in , which is very similar to the results of earlier studies (CitationYuvakkumar et al. 2014).
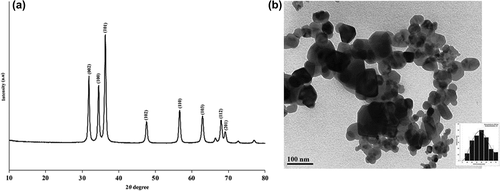
FTIR spectra and XRD graph showing ZnO nanocrystals
The significant absorption peaks in the FTIR spectra () of the prepared ZnO nanocrystals at 3420, 1630, and 1110 cm− 1 could be assigned to O–H stretching, the H–O–H bending vibration mode due to the adsorption of moisture, and Zn–O stretching vibration, respectively (CitationHong et al. 2009, CitationYuvakkumar et al. 2014). The XRD peaks at 2θ = 32.10°, 34.62°, 36.37°, 47.24°, 56.70°, 64.20°, 67.82° and 69.18° were assigned to the (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (1 1 2), and (2 0 1) crystal planes of the ZnO nanocrystals, respectively (). The diffraction peaks could be referenced as the spherical and hexagonal wurtzite structure phase of zinc oxide, which was evaluated using data from JCPDS card No. 89-7102. The strong and narrow peak denotes that the product was crystalline in nature.
TEM image of ZnO nanocrystals and particle size distribution
TEM indicated that the ZnO nanocrystals were spherical or oval while some were non-uniformly shaped and distributed in the prepared sample (), and the inset shows the particle size distribution graph. The obtained image is in accordance with the results of an earlier study (CitationVanathi et al. 2014, CitationSivaraj et al. 2014). It was observed that the nanoparticles formed were of different sizes, and particle size was found to be 10.80 nm, 26.12 nm, 41.14 nm and 16.48 and the mean size was about 20.06 nm, which lies in the nano range. The histogram of particle size distribution in the inset indicates that 10% of the particles have a size of around 40 nm with a standard deviation of 22.12% in size, while the remaining particles are around 60 nm with a standard deviation of 14.12%.
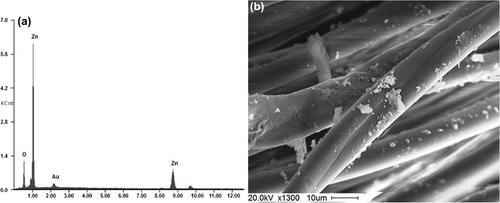
SEM–EDS and SEM image studies of ZnO nanocrystal-treated and -untreated fabrics
The morphological changes of cotton and leather samples before and after treatment with ZnO nanocrystals were clearly observed from the SEM images. and show the EDS spectra of ZnO nanocrystal-coated fabric and leather samples. and show the morphology of ZnO nanocrystal-treated cotton fabric and leather samples. SEM images clearly show the imposition of ZnO nanocrystals onto the surface of the fabric and leather with good distribution. SEM images clearly show the significant difference between the untreated and treated cotton fibers and leather due to the imposition of ZnO nanocrystals onto the surface of the fibers and leather. Both the micrographs clearly show that the ZnO nanocrystals were well distributed onto the fabric and leather.
Antibacterial studies
To test the ability of the prepared ZnO nanocrystals to inhibit bacterial (B. linens and S. epidermidis) growth, testing was performed using a disc diffusion method, using the standard antibiotic Penicillin-G (10 mcg) as a control. A zone of inhibition (ZoI) appeared around the ZnO nanocrystal-coated fabric and leather due to the bacteriostatic effect of the product. shows the antibacterial activity of the ZnO nanocrystals and standard antibiotic-coated fabric and leather. The greatest inhibitory effect was observed against B. linens with a ZoI of a diameter of 26 mm followed by inhibition against S. epidermidis with a ZoI of a diameter of 14 mm on the fabric. The ZnO NP-coated leather showed ZoI diameters of 14 and 12 mm against B. linens and S. epidermidis, respectively. Penicillin-G exhibited ZoI diameters of 52 and 38 mm against B. linens and S. epidermidis on the fabric samples and 44 and 30 mm on the leather samples, respectively.
Table I. Zone of inhibition (mm) of control, ZnO NPs, standard antibiotic-coated fabric and leather against odor-causing bacteria.
Possible mechanism of ZnO nanocrystal formation using Prunus × yedoensis leaf extract
The possible mechanism of ZnO nanocrystal formation using Prunus × yedoensis leaf extract is shown in [Note* This scheme is obtained from CitationYuvakkumar et al. (2014)]. The ester oxygen atom and the phenolic hydroxyl groups of polyphenols form a p-track conjugation effect when the hydroxyl groups bind with the metal to form metal–phenolate complex (Zinc–ellagate complex) by the chelating effect. These complexes undergo direct decomposition at 450(C and this leads to formation of ZnO nanostructures. Moreover, the Prunus × yedoensis leaf extract has significant potential due to its polyphenols and has an easy electron-losing capacity which results in the formation of green zinc–ellagate complex formation. In our synthesis, aromatic hydroxyl groups present in Prunus × yedoensis leaf extract ligate with zinc ions to form the zinc–ellagate complex at pH 5–7. The pH value varies depending upon the metal ions (CitationWang et al. 2009, CitationYuvakkumar et al. 2014). The Prunus × yedoensis leaf extract used has a rich source of active ingredients such as polyphenols, alkaloids, flavonoids, and vitamins. These active compounds are highly responsible for the reduction and capping of ZnO nanocrystals. In addition, these compounds could perform functions like involving their antibiotic, antioxidant, radical scavenging, and antiviral effects etc., (CitationYuvakkumar et al. 2014). These natural plant phytochemicals have favorable effects for the synthesis of ZnO nanocrystals and are also anticarcinogenic and antioxidant in nature (CitationPrzewloka and Shearer 2002, CitationWang et al. 2009, CitationYuvakkumar et al. 2014).
Scheme 1. Possible mechanism of ZnO nanocrystal formation (CitationYuvakkumar et al. [2014]).
![Scheme 1. Possible mechanism of ZnO nanocrystal formation (CitationYuvakkumar et al. [2014]).](/cms/asset/3551a015-786d-47b9-913f-46f1b7c96d8f/ianb_a_1059840_f0006_b.gif)
Conclusion
A green synthetic approach employing simple, inexpensive and abundant eco-friendly material for the synthesis of ZnO nanocrystals would have a major impact in nanoparticle research. The antibacterial results clearly demonstrate that the ZnO nanocrystal-coated fabric and leather samples have the ability to inhibit odor-causing bacteria. The future studies may include the use of synthesized nanoparticles along with routinely used antibiotics to see their synergistic effects. It confirms that ZnO nanocrystals are capable of rendering high antibacterial efficiency and hence have great potential in the preparation of drugs against bacteria. Therefore, green-synthesized ZnO nanocrystal-coated socks and shoes have immense potential for use by athletes and the general public.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgement
This research work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the government (MEST) (No. 2011-0020202). This research was also supported by the Korean Ministry of Education, Science and Technology, Award NRF-2011-35B-D00020.
References
- Akiyama H, Yamasaki O, Kanzaki H, Tadaa J, Arata J. 1998. Effects of zinc oxide on the attachment of Staphylococcus aureus strains. J Dermatol Sci. 17:67–74.
- Ara K, Hama M, Akiba S, Koike K, Okisaka K, Hagura T, et al. 2006. Foot odour due to microbial metabolism and its control. Can J Microbiol. 52:357–364.
- Azizi S, Ahmad MB, Namvar F, Mohamad R. 2014. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater Lett. 116:275–277.
- Bar H, Bhui DK, Sahoo GP, Sarkar P, Pyne S, Misra A. 2009. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A. 348:212–216.
- Darroudi M, Sabouri Z, Oskuee R, Zak A, Kargar H, Hamid MHN. 2014. Green chemistry approach for the synthesis of ZnO nanopowders and their cytotoxic effects. Ceram Int. 40:4827–4831.
- El Ghoul J, Barthou C, El Mir L. 2012.Synthesis by sol-gel process, structural and optical properties of nanoparticles of zinc oxide doped vanadium. Superlattice Microst. 51:942–951.
- Elseviers WF, Verelst H. 1999. Transition metal oxides for hot gas desulphurization. Fuel. 78:601–612.
- Fujihara S, Naito H, Kimura T. 2001. Visible Photoluminescence of ZnO Nanoparticles Dispersed in Highly Transparent MgF2 Thin-Films via Sol-Gel Process. Thin Solid Films. 389:227–232.
- Gnanasangeetha D, SaralaThambavani D. 2013. One Pot Synthesis of Zinc Oxide Nanoparticles via Chemical and Green Method. Res J Mater Sci. 1:1–8.
- Hong RY, Li JH, Chen LL, Liu DQ, Li HZ, Zheng Y, Ding J. 2009. Synthesis, surface modification and photocatalytic property of ZnO nanoparticles. Powder Technol. 189:426–432.
- Jayaseelan C, Abdul Rahuman A, Vishnu Kirthi A, Marimuthu S, Santhoshkumar T, Bagavan A. 2012. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydropila and their activity against pathogenic bacteria and fungi. Spectrochim Acta Part A. 90:78–84.
- Kanlayavattanakul M, Lourith N. 2011. Body malodours and their topical treatment agents. Int J Cosmetic Sci. 33:298–311.
- Mochinaga R, Yamasaki T, Arakawa T. 1998. The gas sensing of SmCoOx/MOx(M = Fe, Zn, In, and Sn) having a heterojunction. Sensor Actuat B-Chem. 52:96–99.
- Nawaz HR, Solangi BA, Zehra B, Nadeem U. 2011. Preparation of nano Zinc oxide and its Aapplication in leather as a retanning and antibacterial agent. J Sci Ind Res. 2:164–170.
- Nazeruddin GM, Prasad NR, Waghmare SR, Garadkar KM, Mulla IS. 2014. Extracellular biosynthesis of silver nanoparticle using Azadirachta indica leaf extract and its anti-microbial activity. J Alloy Comp. 583:272–277.
- Prasad K, Jha AK. 2009. ZnO nanoparticles: synthesis and adsorption study. Nat Sci. 1:129–135.
- Przewloka SR, Shearer BJ. 2002. The further chemistry of ellagic acid II Ellagic acid and water-soluble ellagates as metal precipitants. Holzforschuang. 56:13–19.
- Rajiv P, Rajeshwari S, Venckatesh R. 2013. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochemica Acta Part A. 112:384–387.
- Sangeetha G, Rajeshwari S, Venckatesh R. 2011. Green synthesis of zinc oxide nanoparticles by Aloe barbadensis miller leaf extract: Structure and optical properties. Mater Res Bull. 46:2560–2566.
- Singh N, Mehra RM, Kapoor AJ. 2011. Synthesis and characterization of ZnO nanoparticles. Nano- Electron Phys. 3:132–139.
- Sivaraj R, Pattanathu R, Rahman KSM, Rajiv P, Salam HA, Venckatesh R. 2014. Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen. Spectrochim Acta A. 133:178–181.
- Thitilertdecha N, Teerawutgulrag A, Kilburn JD, Rakariyatham N. 2010. Identification of Major Phenolic Compounds from Nephelium lappaceum L. and Their Antioxidant Activities. Molecules. 15: 1453–1465.
- Vanathi P, Rajiv P, Narendhran S, Rajeshwari S, Rahman PKSM. 2014. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach. Mater Lett. 134:13–15.
- Vayssieres L, Keis K, Hagfeldt A, Lindquist SE. 2001. Three-dimensional array of highly oriented crystalline ZnO Microtubes. Chem Mater. 13:4395–4398.
- Velmurugan P, Cho M, Lee SM, Park JH, Bae S, Oh BT. 2014.Antimicrobial fabrication of cotton fabric and leather using green-synthesized nanosilver. Carbohydr Polym. 106:319–325.
- Waham R, Kimm YS, Lee DS, Seo JM, Shin HS. 2010. Controlled synthesis of zinc oxide nanoneedles and their transformation to micro flowers. Sci Adv Mater. 2:35–42.
- Wang XB, Huo KF, Zhang F, Hu Z, Chu PK, Tao HS, et al. 2009. Structural regulation and optical properties of one-dimensional ZnO nanomaterials in situ grown from and on brass substrates. J Phys Chem C. 113:170–173.
- Wang ZL. 2004. Nanostructures of zinc oxide. Mater Today. 7:26–33.
- Wang C, Kim JY, Singh P, Mathiyalagan R, Jin Y, Yang DC. 2015. Green synthesis of silver nanoparticles by Bacillius methylotrophicus, and their antibacterial activity. Artif Cells Nanomed Biotechnol. 1–6.
- Yuvakkumar J, Suresh A, Nathanael J, Sundrarajan M, Hong SI. 2014. Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications. Mater Sci Eng C. 41:17–27.