Abstract
Unrestricted somatic stem cells (USSCs) loaded in nanofibrous polycaprolactone (PCL) scaffolds can be used for skin regeneration when grafted onto full-thickness skin defects of rats. Nanofibrous PCL scaffolds were designed by the electrospinning method and crosslinked with laminin protein. Afterwards, the scaffolds were evaluated by scanning electron microscopy, and physical and mechanical assays. In this study, nanofibrous PCL scaffolds loaded with USSCs were grafted onto the skin defects. The wounds were subsequently investigated 21 days after grafting. Results of mechanical and physical analyses showed good resilience and compliance to movement as a skin graft. In animal models; study samples exhibited the most pronounced effect on wound closure, with statistically significant improvement in wound healing being seen at 21 days post-operatively. Histological examinations of healed wounds from all samples showed a thin epidermis plus recovered skin appendages in the dermal layer for samples with cell. Thus, the graft of nanofibrous PCL scaffolds loaded with USSC showed better results during the healing process of skin defects in rat models.
Introduction
The final goal of tissue engineering should be the functional recovery of damaged tissue in vivo, and the in vitro reconstruction of tissue architecture, while realizing delicate tissue-specific functions (CitationRaf and Singer 2000). The reconstruction of skin defects remains a major concern when the defective area is widespread, severely contaminated by microorganisms, or poorly vascularized, as can be the case with irradiation defects, congenital skin disorders, or extensive burns. Numerous materials for skin regeneration, including temporary substitutes, such as porcine xenografts, synthetic membranes, and allogeneic substitutes or permanent skin substitutes, such as cultured epidermis and dermal substitutes, have been investigated (CitationSheridan and Tompkins 1990). Among these materials, artificial dermal substitutes are structurally optimized to be incorporated into the surrounding tissue and to allow cell invasion by fibroblasts and capillaries for subsequent dermal remodeling (CitationSuzuki et al. 1990). Mesenchymal stem cells (MSCs) or epidermal cells have been demonstrated playing an effective role in promoting wound healing when injected into the skin defect, either alone or in combination with human amniotic membrane (HAM) (CitationKim et al. 2008). Sung has shown that HAM loaded with MSCs improves wound healing (CitationKim 2009). Umbilical cord blood contains hematopoietic as well as non-hematopoietic MSCs, the latter also known as CBEs (Cord Blood Embryonic-like stem cells) (CitationBranski et al. 2009). CBEs have been shown to differentiate into neural, hepatobiliary, pancreatic-like precursors, and other potential types of cells (CitationBranski et al. 2009). It was also suggested that stem cells from umbilical cord blood are able to differentiate into epithelial cells under in vitro conditions and could therefore be used as a starting material for isolation and expansion of cells in large skin defects (CitationKamolz et al. 2006). Human umbilical cord blood is a rich source of hemopoietic stem cells for clinical application, and may be one of the largest sources of stem cells with naive immune status (CitationWilson et al. 2011). Cord-lining-MSCs express CD23, CD14 and low amounts of CD34 and CD35; they do not express endothelial marker CD31; they have greater in vitro expansion than Wharton's jelly-derived MSCs (CitationIshige et al. 2009). CD14 inhibits T cells. Wharton's-jelly derived MSCs do not express CD14 or CD23. Despite those descriptions, the cell markers of umbilical cord-derived MSCs are under great debate (CitationKita et al. 2010, CitationWu et al. 2007). One of the key factors of tissue engineering is to create a three-dimensional scaffold with suitable properties as well, such as degradation rate, high porosity, interconnected pores, etc. Typically, biodegradable polymeric scaffolds are fabricated using different methods (CitationMikos et al. 1993, CitationNam et al 2000). In natural tissues, cells are surrounded by extracellular matrix, which has physical structural features ranging from the nanometer scale to the micrometer scale. Hence, a nano-structured porous and large surface area is needed as an alternate to natural ECM. To mimic the natural ECM, many research groups have tried to fabricate nanofibrous scaffolds by different methods, including electrospinning (CitationBiazar 2013 and Citation2014, CitationBiazar and Heidari 2013a, Citation2013b, Citation2014a and Citation2014b, CitationSahebalzamani et al. 2014 and Citation2015, CitationZeinali et al. 2014, CitationBiazar et al. 2014, CitationHeidari et al. 2014). A wide variety of natural materials, such as collagen [Biobrane™, Integra®, Alloderm™], fibrin [BioSeed™], HA [Laserskin™], GAGs [Integra®], etc. have been used in commercialized skin grafts (CitationMetcalfe and Ferguson 2007, CitationYannas 2004). Nylon [TransCyte™] and biodegradable polymers such as polyglactin [Dermagraft®] and polycaprolactone (PCL) (CitationRuszczak 2003) were used for fabricating skin substitutes. The combined application of both cell sources (USSCs and scaffold) is expected to have wide clinical use for the treatment of skin lacerations, and warrants further analysis. PCL is a degradable aliphatic ester that the US Food and Drug Administration (FDA) has approved for human clinical use. Extensive research has been conducted on PCL, considering its advantages such as biocompatibility, low cost, ease of use with controlled pore size and shape, and appropriate mechanical strength. PCL is a hydrophobic polyester without bioactive fragments, which might limit its in vivo application. Electrospinning is one of the most important methods for fabrication of nanofibrous scaffolds (CitationBiazar and Heidari 2014b). In this study, nanofibrous PCL scaffolds were fabricated by electrospinning. The samples were evaluated by scanning electron microscope (SEM), physical and mechanical analysis, and in vitro assays. They were then loaded with USSCs, implanted in rats with skin damage, and investigated by different analyses.
Materials and methods
PCL with molecular weight of 80,000 (g mol − 1) was purchased from Sigma-Aldrich (St Louis, MO); acetic acid and formic acid (AA/FA) solvent system for PCL was purchased from Sigma-Aldrich and used as received without further purification. Electrospinning apparatus used in this study was procured from Asia Nano Meghyas Company (Iran). PCL was dissolved at a predetermined concentration in AA/FA, 70/30 vol. %. The PCL solution (12%w/v) was contained in a glass syringe controlled by a syringe pump. A positive high voltage source was applied through a wire, at the tip of a syringe needle. In this situation, a strong electric field was generated between the PCL solution and a collector. When the electric field reached a critical value with increasing voltage, mutual charge repulsion overcame the surface tension of the polymer solution and an electrically charged jet was ejected from the tip, with a conical shape, as the Taylor cone. The solution was electrospun from a 5 ml syringe with a needle diameter of 15 mm and mass flow rate of 1 ml/h. A high voltage (20 kV) was applied to the tip of the needle attached to the syringe when the fluid jet was ejected. The linear rate of the rotating disk was set to 1000 rpm. The resulting fibers were collected on 15 mm cover slips placed on the respective collectors. Ultrafine fibers were formed by narrowing the ejected jet fluid as it gathered increasing surface charge density due to evaporation of the solvent. An electrospun PCL nanofibrous mat was carefully detached from the collector and dried in vacuum for 2 days at room temperature to remove solvent molecules completely.
Electrospun fiber modification
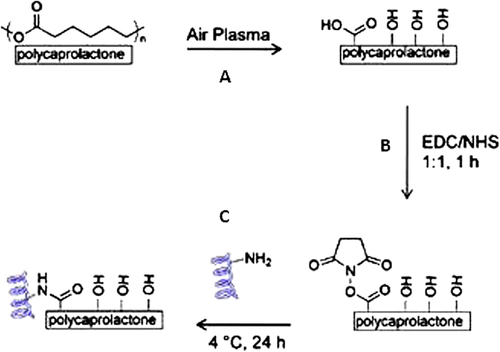
Vacuum-dried PCL fibers were cut to fit into 24-well plates and plasma-treated in air using an inductively coupled radio frequency (RF) plasma cleaner (DIENER Electronic GmbH, Germany) for 1 min at RF power of 100 W, to introduce carboxylic functionalities to the surface of the fibers (). The plasma-treated fibers were then immersed in a MES buffer containing 5 mg/ml of EDC and 5 mg/ml of NHS for 1 h at RT. Fiber mats were then rinsed with MES buffer and incubated in a 50 μg/ml laminin solution overnight at 4°C. The protein solution was removed and the mats were washed in a 0.05% Tween 20 solution in PBS, with gentle shaking for 30 min to remove physically absorbed proteins (). Samples were kept sterile for cell culture studies or rinsed thoroughly with deionized water and dried for characterization.
In vivo study
Cell culture and surgical procedures
The culture and isolation protocol of the USSC from fresh umbilical cord blood was previously described by Kogler (CitationKogler et al. 2004). Forty male Wistar white rats aged approximately 4–8 weeks and weighing 180–220 g at the beginning of the experiment, were divided into four groups. The protocol for the experiment was approved by the Institutional Animal Care and Use Committee of Beheshti University. Animals were handled according to the guidelines established for animal care at the center. Each rat had free access to both sterile water and standard rodent soft chow, ad libitum. Animals were anesthetized using 80 mg/kg ketamine and an injection of 10 mg/kg xylazine, and their backs were shaved and swabbed with providone iodine followed by 70% ethanol. The scrubbing was then repeated two more times. Then a sterile template measuring 1 × 1 cm was placed on their skin and the outline was traced using a sterile fine felt-tipped pen. The medial border of the template was oriented parallel to the sagittal axis of the animal. Full-thickness wounds of 1 cm × 1 cm were made by excising the skin within the confines of the square down to the level of subcutaneous panniculus carnosus. The wound was covered with the designated scaffold and sutured by using 10–0 nylon at intervals of 0.75 cm. Among 40 of the skin defects, 10 defects were grafted with nanofibrous scaffolds, with about 2 × 106 USSCs. The wounds were covered with a standard wet compress to prevent scaffold detachment and desiccation, and then each animal was housed in its own cage to avoid damage to the wound. Post-surgical care included analgesic and antibiotic (cyclosporine 10 mg/kg) injections. The immunosuppressive drug, cyclosporine, was given to animals every day at a dose of 10 mg/kg by subcutaneous injection. Checking for any post-surgical pain, distress, or complications was done 24 h after surgery and daily afterwards.
Histological assessment
The wounds were harvested 21 days after grafting, then stained and investigated for histological assessment. The reconstituted skin was cut to the control depth determined as the excision down to the level of panniculus carnosus (10 mm wide, 10 mm long, and 5 mm deep), and fixed in 10% formalin at 4°C for 5 days, dehydrated, and then paraffin-embedded. Serial 2 μm paraffin sections were cut with a Senior Precision Rotary Microtome (Model-RMT-30, Radical Instruments, Haryana, India) and stained with hematoxylin and eosin according to the routine histology protocol. The wounds were evaluated for the presence of smooth muscle, number of sebaceous units and hair follicles, epithelialization, vascular hyperplasia, etc., using an optical microscope (Nikon, Mellville, NY). The results of each group were statistically analyzed using analysis of variance. The quantitative analyses of data were carried out with the statistical functions of MS Excel.
Results and discussion
Characterization
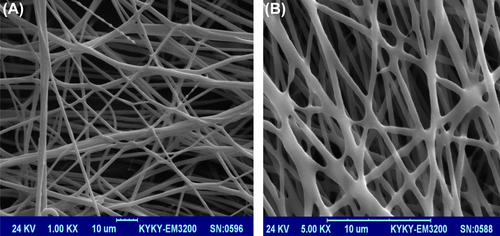
Nanofibrous PCL scaffolds were designed by the electrospinning method. shows the SEM images of the nanofibrous mats at different magnifications. The smooth and homologous nanofibers have clearly been shown in this figure. The average size for the unmodified nanofibers and the nanofibers modified with laminin were about 150 and 200 nm, respectively.
shows the contact angle and tensile strength obtained for the unmodified and the modified nanofibers. The contact angles of 112 and 58 were obtained for the unmodified nanofibrous mat and the nanofibrous mat modified with laminin, respectively. The 54°difference in the contact angle, obtained for the laminin-coated nanofibrous mat when compared to the unmodified nanofibrous mat, shows better hydrophilicity of the coated mat than the unmodified ones. Tensile strength of the nanofibrous scaffold modified with laminin protein was increased (4.6 MPa) when compared to that of the unmodified nanofibrous scaffold (4.1 MPa).
Table I. Mechanical and physical properties of nanofibrous PCL scaffolds. (The data are presented as the mean values S.D., P < 0.05).
In vivo results
Measurement of wound size
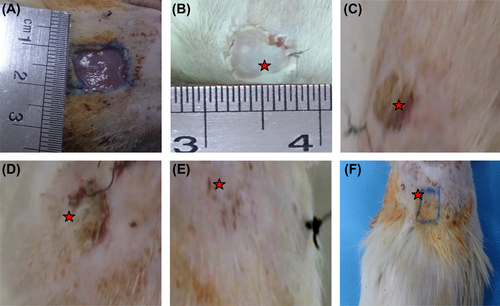
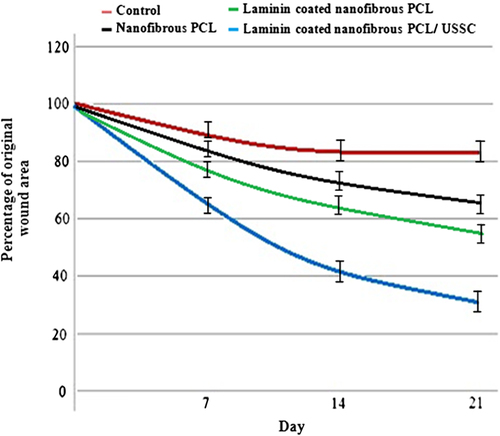
The wound surface of study samples did not show any signs of inflammation. The study samples showed the most pronounced effect on wound closure, with statistically significant improvements in wound healing (≥ 70%) being seen at 21 days after grafting with the laminin-coated nanofibrous PCL scaffold with USSCs ( and ). By contrast, the study control group, demonstrated ≤ 20% wound closure and was still poorly healed ().
Histological assessment
Histological images related to the control, grafted, and normal skin groups have been displayed in . Histological examination of wounds in the nanofibrous group, 21 days after grafting, exhibited the well-recovered epidermal and dermal layers. Formation of epidermal and dermal layers, formation of sebaceous gland was clearly seen in the nanofibrous group under study, similar to the histological features of the normal group. The formation of hair bulbs was clearly seen in the samples, especially with cells. The control group showed slightly thickened epidermis with the fibrotic tissue. The nanofibrous group demonstrated a reduced amount of fibrotic tissue and also an increased number of sebaceous units, hair follicles, and vascularization. Skin appendages were partially recovered in nanofibrous groups.
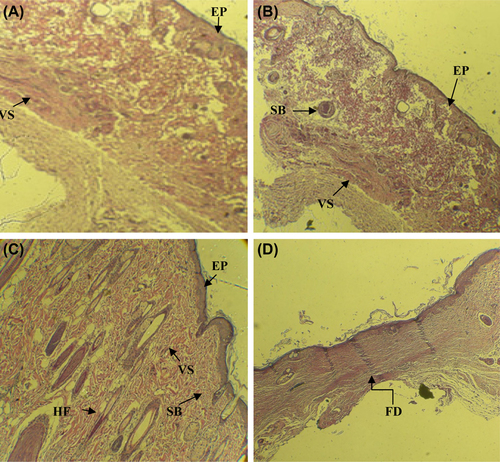
Conclusion
This study reveals the great efficacy of the laminin-modified nanofibrous PCL scaffold loaded with unrestricted somatic stem cells (USSCs) as skin grafts for treating the skin wounds in a rat model. The combined use of nanofibers and USSCs for repairing the acute 1 cm × 1 cm wound resulted in successful wound healing and skin regeneration in rats. On post-operative day 21, the reconstructed skin in the nanofibrous scaffolds loaded with USSCs, demonstrated an intact epithelium together with the formation of new hair follicles and sebaceous glands, which resembled the structures of natural skin. The USSCs exhibited a specific effect on wound healing, especially on regeneration of skin appendages. Considered together, these findings suggest a great potential of the nanofibrous scaffolds loaded with USSCs as efficient skin grafts for the treatment of acute full-thickness skin wounds.
Declaration of interest
The authors state no conflict of interest and have received no payment in the preparation of this manuscript.
References
- Biazar E. 2013. Use of umbilical cord and cord blood-derived stem cells for tissue repair and regeneration. Expert Opin Biol Ther. 13:1653–1662.
- Biazar E. 2014. Polyhydroxyalkanoates as potential biomaterials for neural tissue regeneration. Int J Polym Mater Polym Biomat. 63: 898–908.
- Biazar E, Heidari S. 2013a. Effects of chitosan cross linked nanofibrous PHBV scaffold combined with mesenchymal stem cells on healing of full-thickness skin defects. J Biomed Nanotech. 9:1471–1482.
- Biazar E, Heidari S. 2013b. Chitosan-cross linked nanofibrous PHBV nerve guide for rat sciatic nerve regeneration across a defect bridge. ASAIO Journal. 59:651–659.
- Biazar E, Heidari S. 2014a. Unrestricted Somatic Stem Cells Loaded in Nanofibrous Scaffolds as Potential candidate for Skin Regeneration. Int J Polym Mater Polym Biomat. 63:741–752.
- Biazar E, Heidari S. 2014b. Development of chitosan-crosslinked nanofibrous PHBV guide for repair of nerve defects. Artif Cells Nanomed Biotechnol. 42:385–391
- Biazar E, Heidari S, Sahebalzamani A, Hamidi M, Ebrahimi M. 2014. The healing effect of unrestricted somatic stem cells loaded in nanofibrous Polyhydroxybutyrate-co-hydroxyvalerate scaffold on full-thickness skin defects. J Biomater Tiss Eng. 4:20–27.
- Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. 2009. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 35:171–180.
- Heidari S, Biazar E, Rezaei M, Rahmati M, Ronaghi A, Ebrahimi M, et al. 2014. The healing effect of unrestricted somatic stem cells loaded in collagen-modified nanofibrous PHBV scaffold on full-thickness skin defects. Artif Cells Nanomed Biotechnol. 42:210–216.
- Ishige I, Nagamura-Inoue T, Honda MJ, Harnprasopwat R, Kido M, Sugimoto M, et al. 2009. Comparison of mesenchymal stem cells derived from arterial, venous,and Wharton's jelly explants of human umbilical cord. Int J Hematol. 90:261–269.
- Kamolz LP, Kolbus A, Wick N, Mazal PR, Eisenbock B, Burjak S, et al. 2006. Cultured human epithelium: human umbilical cord blood stem cells differentiate into keratinocytes under invitro conditions. Burns. 32:16–19.
- Kim CH, Kim SS, Sohn SK, Kim DH, Song CG, Kim HJ. 2008. The effect of human amniotic membrane, epidermal cells and marrow mesenchymal stem cells in healing a skin defect. J Korean Orthop Assoc. 43:276–286.
- Kim SS, Song CK, Shon SK, Lee KY, Kim CH, Lee MJ, Wang L. 2009. Effects of human amniotic membrane grafts combined with marrow mesenchymal stem cells on healing of full-thickness skin defects in rabbits. Cell Tissue Res. 336:59–66.
- Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. 2010. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev. 19:491–502.
- Kogler G, Sensken SJ. Airey A. 2004. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 200:123–135.
- Metcalfe AD, Ferguson MWJ. 2007. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 4:413–437.
- Mikos AG, Sarakinos G, Lyman MD, Ingber DE, Vacanti JP, Langer R. 1993. Prevascularization of Porous Biodegradable Polymers. Biotechnol Bioeng. 42:716–723.
- Nam YS, Yoon JJ, Park TG. 2000. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J Biomed Mater Res. 53:1–7.
- Raf C, Singer AJ. 2000. Wound repair: basic biology to tissue engineering. In: Lanza RP, Langer R, Vacanti J, Eds. Principles of Tissue Engineering, 2nd ed. Academic Press, pp. 857–878.
- Ruszczak Z. 2003. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev. 55:1595–1611.
- Sahebalzamani A, Biazar E. 2015. Surface modification of PHBV nanofibrous mat by Laminin protein and its cellular study. Int J Polym Mater Polym Biomat. 64:149–154.
- Sahebalzamani A, Biazar E, Shahrezaei M, Hosseinkazemi H, Rahimnavaei H. 2014. Modification of Poly Caprolactone Nanofibrous Mat by Laminin Protein and Its Cellular Study. J Biomater Tiss Eng. 4:423–429.
- Sheridan RL, Tompkins RG. 1990. Skin substitutes in burns. Burns. 25:97–103.
- Suzuki S, Matsuda K, Isshiki N, Tamada Y, Ikada Y. 1990. Experimental study of a newly developed bilayer artificial skin. Biomaterials. 11:356–360.
- Wilson A, Butler PE, Seifalian AM. 2011. Adipose-derived stem cells for clinical applications: A review. Cell Prolif. 44:86–98.
- Wu KH, Zhou B, Lu SH, Feng B, Yang SG, Du WT, et al. 2007. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 100:608–616.
- Yannas IV. 2004. Synthesis of tissues and organs. Chembiochem. 5:26–39.
- Zeinali R, Biazar E, Heidari S, Rezaei Tavirani M, Asadipour K. 2014. Regeneration of Full-Thickness Skin Defects Using Umbilical Cord Blood Stem Cells Loaded into Modified Porous Scaffolds. ASAIO Journal. 60:106–114.