Abstract
Nanoparticles and nanomaterials are at the prominent edge of the rapidly developing field of nanotechnology. Recently, nanoparticle synthesis using biological resources has been found to be a new area with considerable prospects for development. Biological systems are the masters of ambient condition chemistry and are able to synthesize nanoparticles by utilizing metal salts. In the perspective of the current initiative to develop green technologies for the synthesis of nanoparticles, microorganisms are of considerable interest. Thus, the present study describes a bacterial strain—Weissella oryzae DC6—isolated from mountain ginseng, for the green and facile synthesis of silver nanoparticles. The particles were synthesized effectively without the need for any supplementary modification to maintain stability. The synthesized nanoparticles were evaluated by several instrumental techniques, comprising ultraviolet–visible spectrophotometry, field emission transmission electron microscopy, energy dispersive X-ray spectroscopy, elemental mapping, X-ray diffraction, and dynamic light scattering. In addition, the biosynthesized silver nanoparticles were explored for their antimicrobial activity against clinical pathogens including Vibrio parahaemolyticus, Bacillus cereus, Bacillus anthracis, Staphylococcus aureus, Escherichia coli, and Candida albicans. Furthermore, the potential of nanoparticles has been observed for biofilm inhibition against Staphylococcus aureus and Pseudomonas aeruginosa. Thus, the synthesis of silver nanoparticles by the strain W. oryzae DC6 may serve as a simple, green, cost-effective, consistent, and harmless method to produce antimicrobial silver nanoparticles.
Introduction
In the field of nanotechnology, research regarding nanoparticles has been developing rapidly due to their exceptional catalytic, magnetic, optical, electronic, other physiochemical properties that are relatively dissimilar from the bulk material of the same composition (CitationAustin et al. 2014). Nanoparticles are gaining importance and are being sufficiently applied in areas such as mechanics, biomedical sciences, magnetics, catalysis, optics, and energy science. An important and exciting matter is the development of a consistent approach for the synthesis of nanoparticles over a range of shapes, sizes, and chemical compositions, and with high monodispersity.
Numerous techniques have been developed that usually employ molecular, atomistic, and particulate processing in a vacuum or in a liquid medium (CitationKumar et al. 2013). Most of the techniques are capital-demanding, as well as inefficient for many medicinal and biological applications. The techniques, for instance, photochemical reduction, electrochemical reduction, chemical reduction, and heat evaporation, have formerly shown results in the absorption of some toxic chemicals on the surface of nanoparticles that may have adverse effects in medical applications (CitationGutierrez-Wing et al. 2012, CitationKumar et al. 2013, CitationKheybari et al. 2010). In addition, for the stability of nanoparticles, additional reagents are needed to prevent nanoparticles from aggregation, for instance, mercapto acetate, thiourea, thiophenol, etc., which are very noxious and can contaminate the environment if technology leads to the large scale-up level. Hence, there is an ever-growing necessity to develop low-cost, simple, nontoxic, hygienic, and environmentally benign techniques for synthesis of nanoparticles.
Consequently, researchers have turned to biological systems for the synthesis and applicability of nanoparticles (CitationSadeghi and Gholamhoseinpoor 2015, CitationElumalai et al. 2014). The green synthesis has received considerable attention due to the growing need to develop eco-friendly techniques for nanoparticle synthesis (CitationSingh et al. 2015c). For instance, a great deal of effort has been put into the biosynthesis of inorganic materials, especially metal nanoparticles using microorganisms, plants, yeast, fungi, etc. Bioinspired synthesis of nanoparticles provides advancement over chemical and physical methods, as it is cost effective and avoids the use of high pressure, temperature, energy consumption, and toxic chemicals. The use of environmentally benign materials like plant leaf and root extract, bacteria, yeast, actinomycetes, and fungi offers additional benefits of compatibility for pharmaceutical and biomedical applications. These biological organisms are able to provide an environment for the synthesis of metal nanoparticles either intracellularly or extracellularly (CitationMishra et al. 2011). Basically, these organisms produce biominerals, which are composite materials and consist of an inorganic component and a special organic matrix like proteins, polysaccharides, or lipids, that controls the morphology of the inorganic compound (CitationSadeghi and Gholamhoseinpoor 2015, CitationElumalai et al. 2014). Thus, the biological routes for the synthesis of nanoparticles have contributed to the expansion of a relatively new and fundamentally unexplored area of research. Recently, silver nanoparticles have been synthesized using various natural products, for instance, fresh Panax ginseng root extract, red ginseng powder, and many bacteria including B. indica, B. methylotrophicus, B. frigoritolerans, etc. (CitationSingh et al. 2015b, CitationSingh et al. 2015d, CitationSingh et al. 2015c, CitationSingh et al. 2015e, CitationWang et al. 2015).
The mountain ginseng comprises several families of P. ginseng growing more than 30–40 years in the mountains, under shaded soil, and composed of many microorganisms (CitationKim et al. 2014). Many microorganisms from ginseng microflora have been previously explored for ginsenoside bioconversion (CitationKim et al. 2005), plant growth-promoting activity (CitationSingh et al. 2015a, CitationSukweenadhi et al. 2014), antimicrobial, and antagonistic activity (CitationFarh Mel et al. 2015). In the present study, a bacterial strain, W. oryzae DC6, isolated from the rhizospheric soil of mountain ginseng, has been demonstrated for the synthesis of silver nanoparticles without using any toxic chemicals, solvents, or reagents. The Weissella genus is described as belonging to the Leuconostocaceae family and comprises Gram-positive bacteria. To date, 19 species of the Weissella genus have been reported (www.straininfo.net).
The study focused on silver nanoparticles, due to their utmost applicability in photonics, micro-electronics, photocatalysis, lithography, etc. In addition, from a biological perspective, silver has been known to have a disinfecting effect and has found applications in traditional medicines and culinary items (CitationJain et al. 2009). Silver and its derivatives are commercially employed as antimicrobial agents against many pathogenic microorganisms (CitationKheybari et al. 2010). Recently, silver nanoparticles were demonstrated as having enhanced antimicrobial and biofilm inhibition activity as compared to that of silver metal salt (CitationNaqvi et al. 2013, CitationSingh et al. 2015b). Thus, in the present study, we explored the synthesized silver nanoparticles for their antimicrobial and biofilm inhibition action against pathogenic microorganisms.
Materials and methods
Materials
All the media were purchased from Difco, MB Cell, Seoul, Korea. Analytical grade silver nitrate (AgNO3) was purchased from Sigma-Aldrich Chemicals, USA. The pathogenic bacterial strains Vibrio parahaemolyticus [ATCC 33844], Bacillus cereus [ATCC 14579], Bacillus anthracis [NCTC 10340], Staphylococcus aureus [ATCC 6538], Escherichia coli [ATCC 10798], Pseudomonas aeruginosa [ATCC 27853], and Candida albicans [KACC 30062] were used. The bacterial strains were cultured on nutrient agar media at 37°C and preserved at − 70°C in glycerol stock vials for further study. C. albicans was cultured on Sabouraud dextrose agar (SDA) at 28°C and preserved at − 70°C in glucose yeast peptone (GYP) broth glycerol stock vials.
Isolation and molecular identification of bacteria
A sample of the soil from the mountain ginseng was obtained in a sterile bag from Hwacheon, South Korea. To obtain isolated colonies, the soil sample was serially diluted in sterile 0.8% NaCl and then spread onto Tryptic soy agar (TSA). For the screening of isolated strains, the individual colonies were further streaked in a TSA plate supplemented with 1 mM filter-sterilized silver nitrate (AgNO3). The plate was further incubated at room temperature for 48 h and observed for bacterial growth. The isolated colonies were subcultured and obtained in the pure form. Molecular identification of the isolated strain was carried out using a 16S rRNA sequencing-based method. The genomic DNA was extracted using a commercial genomic DNA extraction kit (Core Bio System). The 16S rRNA gene was amplified from the chromosomal DNA of the isolated strain using the universal bacterial primer set 27F, 518F, 800R, and 1512R. The purified PCR products were sequenced by Genotech (Daejeon, Korea). The nearly complete sequence (1485 bp) of the 16S rRNA was compiled by using SeqMan software (version 4.1). The 16S rRNA gene sequences of related taxa were obtained from the GenBank database and EzTaxon-e server (CitationKim et al. 2012).
Biosynthesis of silver nanoparticles
For the synthesis of silver nanoparticles, the selected bacterial isolate was inoculated into 100 ml of sterile tryptic soy broth (TSB). The cultured flasks were incubated in a rotating shaker set at 120 rpm for 24 h, at 37°C. After the incubation time, the bacterial pellet was collected by centrifugation at 10,000 rpm for 10 min. The collected pellet was washed thoroughly with sterile water and finally dissolved in 20 ml of sterile water, with a final concentration of 1 mM of filter-sterilized solution of AgNO3. The reaction mixture was incubated for 1–2 days in an orbital shaker at 200 rpm and 25°C. The synthesis was monitored for color change by visual inspection. At the end of the incubation period, the mixture was first centrifuged at low speed for 5–10 min to remove the cell debris. The cell pellets were also removed by repeated cycles of ultrasonication, washing with sterile water, and centrifugation. Then, the metal nanoparticles were collected by high speed centrifugation at 20,000 rpm for 10 min. The obtained product was washed thoroughly with methanol and water to remove the undesired components. Lastly, the nanoparticles were collected in the pellet form.
Characterization of silver nanoparticles
To verify reduction of metal ions, the solution was scanned in the range of 300–800 nm in a UV–Vis spectrophotometer (UV–Vis) (Ultrospec 2100 Pro, Amersham Biosciences). The nanoparticles were also analyzed using Field emission transmission electron microscopy (FE-TEM), energy dispersive X-ray spectroscopy (EDX), and elemental mapping with a JEM-2100F (JEOL) instrument operated at 200 kV. The sample for FE-TEM was prepared by placing a drop of collected silver nanoparticles on a carbon-coated copper grid and subsequently drying in an oven at 60°C before transferring to the microscope. The X-ray diffraction (XRD) analyses were performed on an X-ray diffractometer (D8 Advance, Bruker, Germany), operated at 40 kV and 40 mA, with CuKα radiation, at a scanning rate of 6°/min and a step size of 0.02, over the 2θ range of 20–80°. The size distribution profile of the nanoparticles was studied using dynamic light scattering (DLS-Photal, Otsuka Electronics, Japan). The hydrodynamic diameters and polydispersity index (PDI) were analyzed at 25°C. As a reference, a dispersive medium of pure water with a refractive index of 1.3328, viscosity of 0.8878, and dielectric constant of 78.3 was used.
The stability of nanoparticles was observed by keeping the nanoparticle solution for different time intervals at room temperature and by changing the pH over a range of 4–10. Sodium hydroxide (NaOH) was used to change the pH, and the absorbance was then observed by UV–Vis spectroscopy.
Analysis of antimicrobial activity
The antimicrobial activity of the silver nanoparticles was measured against pathogenic microorganisms such as V. parahaemolyticus, B. cereus, B. anthracis, S. aureus, E. coli, and C. albicans on Mueller-Hinton agar (MHA) plates using the disc diffusion method. An overnight log culture of each pathogenic strain (100 μL) was spread evenly on the MHA plate using a glass spreader. Then, 40 μL (100 mg/L) of the purified silver nanoparticle solution was added over each disk and kept for incubation at 37°C for 24 h. After incubation, the zones of inhibition were measured around each disc. The study was done in duplicate to check the reproducibility.
Analysis of biofilm inhibition
The biofilm inhibition activity against S. aureus and P. aeruginosa was determined as mentioned previously (CitationSingh et al. 2015c). Briefly, the 96-well micro-titer plates were filled with 100 μL of overnight-grown log phase of S. aureus and P. aeruginosa. After culturing for 24 h, different concentrations of silver nanoparticles ranging from 1–6 μg were added. The cell culture plates were then incubated for 4 h at 37°C. After incubation, the media were removed and the wells were washed 3 times with 200 μL of sterile water. Then, the microtiter plate was left to air dry, for 45 min. Next, 200 μL of a 0.1% (v/v) crystal violet solution in water was added in each well and kept for 45 min. The wells were then washed 3 times with 300 μL of sterile water to remove excess stain. The dye incorporated by the adherent cells was solubilized with 200 μL of 95% (v/v) ethanol. The absorbance of each well was measured at 595 nm using a microtiter ELISA reader. The absorbance was measured and the percentage inhibition of biofilm activity was calculated using the following equation: [1-(A595 of test/A595 of control)]X100] (CitationGurunathan et al. 2014). The study was done in duplicate to check the reproducibility, and the results were interpreted in terms of mean ± standard deviation.
Results and discussion
Screening and identification of strain
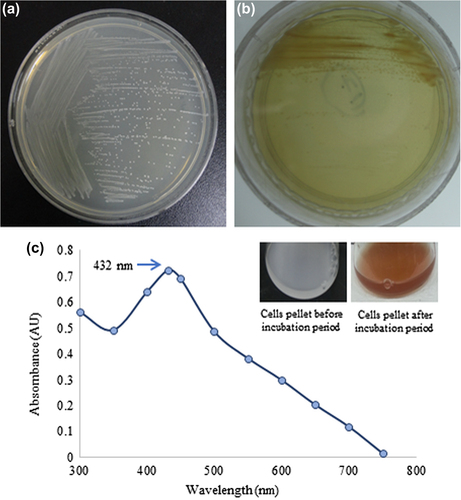
The results of screening after the incubation period showed the growth of bacterial strain DC6 on the TSA plate supplemented with 1 mM of silver nitrate, suggesting that the stain DC6 is capable of tolerating silver salt ( and ). On the basis of molecular characterization, the bacterial isolate DC6 showed 99% similarity with Weissella oryzae. Previously, the strain Weissella oryzae sp. nov. was isolated from fermented rice grains and reported as Gram-positive, irregular, and rod shaped (CitationTohno et al. 2013). Based on the percentage of similarity, the isolated potential strain was named Weissella oryzae DC6, and the 16S rRNA sequence has been submitted to NCBI with accession number KR338997.
Synthesis and characterization of nanoparticles
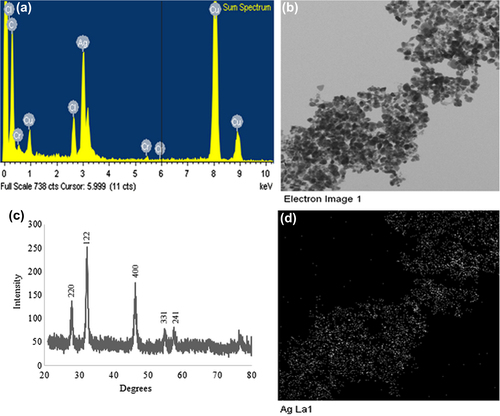
Nanoparticles synthesized by W. oryzae DC6 were confirmed by visual observation, with the appearance of color change in the reaction mixture. Before the incubation period, the color of the pellet dissolved in water was white, which subsequently changed to deep brown with increasing incubation time (). The change in the color of the reaction mixture indicates the synthesis of silver nanoparticles, as the silver nanoparticles cause surface plasmon resonance due to which the color brown appears (CitationSingh et al. 2015c, CitationNaqvi et al. 2013). This methodology for nanoparticle synthesis was found to be simple, green, and more cost-effective. The exact mechanism of synthesis remains to be elucidated. However, the reports suggest that the proteins and enzymes secreted by the microorganisms are responsible for the reduction. For the characterization of the nanoparticles, the reaction mixture was monitored by UV–Vis spectral analysis. After the incubation period, the reaction mixture was scanned in the range of 300–800 nm. In the UV–Vis absorption spectrum, a strong peak at about 432 nm was observed, which can be attributed to the surface plasmon resonance band of silver nanoparticles () (CitationWang et al. 2015, CitationSingh et al. 2015b).
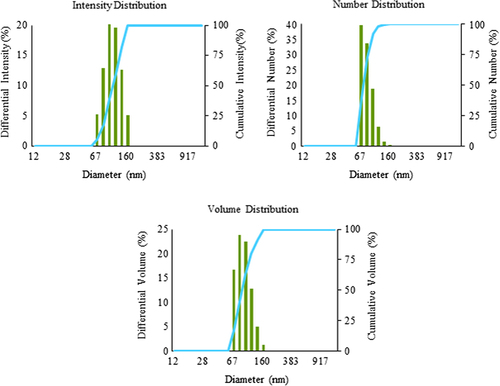
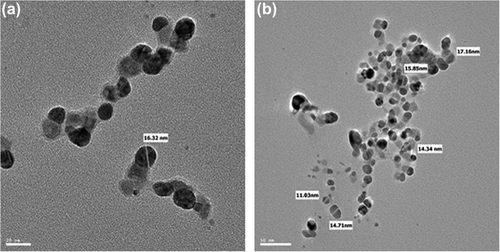
Various studies characterize the nanoparticles by FE-TEM and demonstrate their shapes and size (CitationWang et al. 2015, CitationSingh et al. 2015b). Here, the W. oryzae DC6-mediated silver nanoparticles were further observed by FE-TEM, which revealed the spherical shape of silver nanoparticles with the size range of 10–30 nm ( and ). The EDX spectrum of the nanoparticles showed the highest peak at 3 keV for silver nanoparticles () (CitationSingh et al. 2015d). The other group of metal ions also appeared in the EDX spectrum, which corresponds to the TEM grid utilized for study. showed the X-ray diffraction pattern of nanoparticles, and exhibited extreme peaks in the whole spectrum of the 2θ value ranging from 20–80, and this pattern was analogous to Braggs's reflection of silver nanocrystals (CitationGurunathan et al. 2014, CitationSingh et al. 2015b). The XRD results obtained were similar to those of a previous study which showed biogenic silver nanoparticles by Gelidiella acerosa extract (CitationVivek et al. 2011). The elemental mapping results of the W. oryzae DC6-mediated silver nanoparticles suggested that the silver was the predominant element in the product ( and ) (CitationWang et al. 2015, CitationSingh et al. 2015b). The results of particle size analysis clearly demonstrated the distribution of silver nanoparticles in the reaction mixture according to intensity, number, and volume of silver nanoparticles, respectively (). The average particle size observed was 150.2 nm, with a polydispersity index of 0.176. Thus, the characterization results indicated that the strain W. oryzae DC6 is capable of synthesizing silver nanoparticles.
For the stability of nanoparticles, after keeping the nanoparticle solution at room temperature for intervals of different durations (number of days), there was no observable variation in the UV–Vis spectrum, even after 1 month, which indicated the stable nature of nanoparticles. The results evidenced that nanoparticles were stable for more than 1 month. In addition, the effect of the change in pH in the range of 4–10 was studied. The solution of silver nanoparticles was observed before and after the addition of NaOH, and showed no major change in the wavelength, further confirming the stability of the nanoparticles (CitationSingh et al. 2015b).
Antimicrobial and biofilm inhibition activity
The multidrug-resistant microorganisms are one of the major concerns in the medical field, as the mechanism of development is difficult to control by currently available antibiotics. The silver nanoparticles have been well demonstrated for their antimicrobial efficacy against many multidrug-resistant microorganisms (CitationNaqvi et al. 2013). In our study, biosynthesized silver nanoparticles were analyzed against pathogenic microorganisms, and the results demonstrated that the nanoparticles exhibit remarkable antimicrobial activity against a range of pathogenic microorganisms including V. parahaemolyticus, C. albicans, B. anthracis, B. cereus, S. aureus, and E. coli. Antimicrobial activity against the microorganisms tested was measured by measuring the diameter of the zone of inhibition of each disc (CitationSingh et al. 2015b). Based on the zone of inhibition, the results demonstrate that the silver nanoparticles exhibit maximum antimicrobial activity against the tested microorganisms in the following manner: S. aureus, followed by C. albicans, B. cereus, V. parahaemolyticus, E. coli, and lastly, B. anthracis (), respectively. The results of zone of inhibition are interpreted in . The antimicrobial activity of sliver nanoparticles has been previously studied, and the present results were comparable with those results (CitationChen et al. 2011, CitationSingh et al. 2015d). The particular mechanism of antimicrobial action is not evidently recognized, but studies suggest that silver nanoparticles have the ability to form free radicals and damage the functions of cell membrane permeability and respiration (CitationLee et al. 2014). These factors cause the antimicrobial effect of silver nanoparticles.
![Figure 5. Antimicrobial activity of silver nanoparticles against Staphylococcus aureus [ATCC 6538] (a), Candida albicans [KACC 30062] (b), Bacillus cereus [ATCC 14579] (c), Vibrio parahaemolyticus [ATCC 33844] (d), Escherichia coli [ATCC 10798] (e) and, Bacillus anthracis [NCTC 10340] (f), respectively.](/cms/asset/e57f8ae5-2e8b-4d70-a308-71b5d276dc9c/ianb_a_1064937_f0005_oc.jpg)
Table I. Antimicrobial activity of silver nanoparticles against pathogenic microorganism.
Biofilm formation is one of the most important areas of disquiet in many medical devices, for instance, catheters and water filtration devices, which are constantly in contact with water (CitationSingh et al. 2015c). Silver nanoparticles have been reported for their biofilm inhibition activity against many pathogenic microorganisms (CitationGurunathan et al. 2014). In our study, we explored the synthesized silver nanoparticles for the inhibition of biofilm formation by S. aureus and P. aeruginosa. The results showed that 5–6 μg concentrations of silver nanoparticles are enough to inhibit the biofilm formation by S. aureus and P. aeruginosa (). Our results were in the line of previous studies, which demonstrated the biofilm inhibition potential of silver nanoparticles (CitationTaraszkiewicz et al. 2013). Thus, the synthesized silver nanoparticles have clearly shown good antimicrobial and biofilm inhibition potential, which can further be applied for many therapeutic applications as an antimicrobial agent (CitationJain et al. 2009).
Conclusion
The study highlights the facile biological synthesis of silver nanoparticles in an ecofriendly manner by the strain W. oryzae DC6, isolated from mountain ginseng soil. The silver nanoparticles produced were spherical, mono-disperse, and stable. Moreover, the silver nanoparticles have shown antimicrobial effect against V. parahaemolyticus, B. cereus, B. anthracis, S. aureus, E. coli, and C. albicans, and biofilm inhibition activity against S. aureus and P. aeruginosa. Further, they could be applied for antimicrobial application on the clinical platform. This green approach toward the synthesis of silver nanoparticles has several benefits such as ease of scale-up, economic viability, eco-approachability etc. Applications of such eco-friendly nanoparticles in bactericidal, wound-healing and other medical and electronic applications, makes this technique possibly exciting for large-scale nanoparticle synthesis.
Acknowledgments
This research was supported by the Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry & Fisheries (KIPET NO: 313038-03-2-SB010), Republic of Korea.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Austin LA, Mackey MA, Dreaden EC, El-sayed MA. 2014. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch Toxicol. 88:1391–1417.
- Chen M, Yang Z, Wu H, Pan X, Xie X, Wu C. 2011. Antimicrobial activity and the mechanism of silver nanoparticle thermosensitive gel. Int J Nanomedicine. 6:2873–2877.
- Elumalai EK, Kayalvizhi K, Silvan S. 2014. Coconut water assisted green synthesis of silver nanoparticles. J Pharm Bioallied Sci. 6:241–245.
- Farh Mel-A, Kim YJ, Van an H, Sukweenadhi J, Singh P, Huq MA, Yang DC. 2015. Burkholderia ginsengiterrae sp. nov. and Burkholderia panaciterrae sp. nov., antagonistic bacteria against root rot pathogen Cylindrocarpon destructans, isolated from ginseng soil. Arch Microbiol. 197:439–447.
- Gurunathan S, Han JW, Kwon DN, Kim JH. 2014. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 9:373.
- Gutierrez-Wing C, Velazquez-Salazar JJ, Jose-Yacaman M. 2012. Procedures for the synthesis and capping of metal nanoparticles. Methods Mol Biol. 906:3–19.
- Jain J, Arora S, Rajwade JM, Omray P, Khandelwal S, Paknikar KM. 2009. Silver nanoparticles in therapeutics: development of an antimicrobial gel formulation for topical use. Mol Pharm. 6:1388–1401.
- Kheybari S, Samadi N, Hosseini SV, Fazeli A, Fazeli MR. 2010. Synthesis and antimicrobial effects of silver nanoparticles produced by chemical reduction method. Daru. 18:168–172.
- Kim MK, Lee JW, Lee KY, Yang DC. 2005. Microbial conversion of major ginsenoside rb(1) to pharmaceutically active minor ginsenoside rd. J Microbiol. 43:456–462.
- Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, NA H, et al. 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 62:716–721.
- Kim YJ, Jeon JN, Jang MG, OH JY Kwon WS, Jung SK, Yang DC. 2014. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res. 38:66–72.
- Kumar V, Jolivalt C, Pulpytel J, Jafari R, Arefi-khonsari F. 2013. Development of silver nanoparticle loaded antibacterial polymer mesh using plasma polymerization process. J Biomed Mater Res A. 101:1121–1132.
- Lee W, Kim KJ, Lee DG. 2014. A novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli. Biometals. 27:1191–1201.
- Mishra A, Tripathy SK, Yun SI. 2011. Bio-synthesis of gold and silver nanoparticles from Candida guilliermondii and their antimicrobial effect against pathogenic bacteria. J Nanosci Nanotechnol. 11:243–248.
- Naqvi SZ, Kiran U, Ali MI, Jamal A, Hameed A, Ahmed S, Ali N. 2013. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanomedicine. 8:3187–3195.
- Sadeghi B, Gholamhoseinpoor F. 2015. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc. 134:310–315.
- Singh P, Kim YJ, Nguyen NL, Hoang VA, Sukweenadhi J, Farh Mel-A, Yang DC. 2015a. Cupriavidus yeoncheonense sp. nov., isolated from soil of ginseng. Antonie Van Leeuwenhoek. 107:749–758.
- Singh P, Kim YJ, Singh H, Wang C, Hwang KH, Farh Mel-A, Yang DC. 2015b. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int J Nanomedicine. 10:2567–2577.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, EL-agamy Farh M, Yang DC. 2015c. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol.1–6.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2015d. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol.1–8.
- Singh P, Kim YJ, Singh H, Wang C, Mathiyalagan R, Yang DC. 2015e. Biosynthesis of anisotropic silver nanoparticles by Bhargavaea indica and their synergistic effect with antibiotics against pathogenic microorganisms. J Nanomat. 2015:234741
- Sukweenadhi J, Kim YJ, Lee KJ, Koh SC, Hoang VA, Nguyen NL, Yang DC. 2014. Paenibacillus yonginensis sp. nov., a potential plant growth promoting bacterium isolated from humus soil of Yongin forest. Antonie Van Leeuwenhoek. 106:935–945.
- Taraszkiewicz A, Fila G, Grinholc M, Nakonieczna J. 2013. Innovative strategies to overcome biofilm resistance. Biomed Res Int. 2013:150653.
- Tohno M, Kitahara M, Inoue H, Uegaki R, Irisawa T, Ohkuma M, Tajima K. 2013. Weissella oryzae sp. nov., isolated from fermented rice grains. Int J Syst Evol Microbiol. 63:1417–1420.
- Vivek M, Kumar PS, Steffi S, Sudha S. 2011. Biogenic Silver Nanoparticles by Gelidiella acerosa Extract and their Antifungal Effects Avicenna. J Med Biotech. 3:143–148.
- Wang C, Kim YJ, Singh P, Mathiyalagan R, Jin Y, Yang DC. 2015. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif Cells Nanomed Biotechnol. 6:1–6.

![Figure 6. Biofilm inhibition activity of silver nanoparticles against Staphylococcus aureus [ATCC 6538] and Pseudomonas aeruginosa [ATCC 27853].](/cms/asset/6111d913-af9b-4d5b-8d3e-44cd5cb6e295/ianb_a_1064937_f0006_b.gif)