Abstract
The ability to generate lung and airway epithelial cells from human bone marrow mesenchymal stem cells (hBMSCs) would have applications in regenerative medicine, modeling of lung disease, drug screening, and studies of human lung development. In this research, hBMSCs were cultured in specialized airway epithelial cell growth media for differentiation of airway epithelial cells, including keratinocyte growth factor transferrin, bovine pituitary extract, epinephrine, triiodothyronine and retinoic acid. The surfactant protein C, a specific marker of type II pneumocytes, and its corresponding protein were demonstrated by immunofluorescence and western blotting after differentiation of airway epithelial cells, respectively. These cells were then transferred into an induced acute lung injury model. The results showed that the hBMSCs could induce differentiation in airway epithelial cells under the special conditions of the medium, the result for surfactant protein C was positive in differentiated airway epithelial cells using immunofluorescence and western blotting, and these cells were successfully colonized in the injured lung airway. In conclusion, our research shows that a population of airway epithelial cells can be specifically generated from hBMSCs and that induced cells may be allowed to participate in tissue repair.
Introduction
Lung transplantation is an acceptable means of treating several end-stage lung diseases, but donor shortage is a major problem. Over the past few years, the number of lung transplantations performed annually has reached a plateau because the donor pool is approaching its limits and cannot be expanded further (CitationSamadikuchaksaraei et al. 2006). Thus, other sources of a replacement gas-exchange unit are being sought. Alveoli are the basic functional units of the lungs, and gas exchange occurs across their membranes. Therefore, the first step in the tissue engineering of a lung lobe needs to be aimed at the generation of alveolar tissue. The alveoli are located in the respiratory zone of the lungs, at the distal termination of the alveolar ducts and atria. There are three major cell types in the alveolar wall, including type-pneumocytes, type-pneumocytes, and macrophages. Type II pneumocytes are those that secrete a pulmonary surfactant to lower the surface tension of water and allow the membrane to separate, thereby increasing its capability to exchange gases. Without this surfactant, the alveoli would collapse and very large forces would be required to re-expand them. Moreover, it is known that if type I pneumocytes are lost after a peripheral lung injury, type II pneumocytes undergo proliferation and differentiation to the type I phenotype (CitationBishop 2004). Thus, type II pneumocytes are crucial to the natural regenerative process and gas exchange in the alveoli.
Recent studies have suggested that both embryonic stem cells and adult bone marrow stem cells can participate in the regeneration and repair of diseased adult organs, including the lungs (CitationAliotta et al. 2005, CitationWeiss et al. 2006). Mesenchymal stem cells (MSCs) are multi-potent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, myocytes, adipocytes, and other cells (CitationKavanagh et al. 2015, CitationDeng et al. 2015). MSCs are promising candidates for use in cell therapies and tissue engineering and repair. They are an adult stem cell population and derive their name from an intrinsic ability to differentiate into multiple cell lineages of mesodermal origin, such as bone marrow. Bone marrow stromal cells (BMSCs) are easy to culture and manipulate in vitro and have great plasticity. Thus, they have become an important tool in cell replacement therapy and are presently considered as candidates for different clinical applications. However, it is unclear whether BMSCs will be clinically useful for the treatment of lung injury. In this research, Type II pneumocytes were obtained from human BMSCs using a factor cocktail, and applied to repair acute lung injury model of mouse.
Materials and methods
Samples
The collection of human bone marrow tissue for research purposes was approved by the Research Ethics Committee of the Military General Hospital in Beijing of PLA. The human samples were obtained from volunteers with informed consent and according to a protocol approved by the Research Ethics Committee.
Isolation and culture of hBMSCs
Bone marrow aspirates drawn from the iliac crest of donors were obtained, and human MSCs were isolated using previously described methods (CitationPittenger et al. 1999, CitationAggarwal and Pittenger 2005). Briefly, 5 mL of bone marrow aspirate was combined with 15 mL of PBS buffer and centrifuged at 900 g for 5 min at room temperature. The cells were then re-suspended and gently layered onto a Percoll cushion (density, 1.073 g/mL) at 5 × 108 nucleated cells/20 mL. The low-density hBMSC-enriched mononuclear fraction was collected, washed three times using 5 mL of PBS buffer, and centrifuged to collect the cells. Cells were re-suspended in hBMSC complete culture medium (containing L-DMEM, 10% fetal bovine serum, antibiotic/antimycotic, and glutamine) and plated at 1 × 104 cells/well in 6-well cell culture plates. Cells were subcultured into new plates and incubated at 37°C with 5% CO2.
Induction of airway epithelial phenotypic differentiation
Airway epithelial cells were induced according to the previous research, with some improvements (CitationWang et al. 2005, CitationSueblinvong et al. 2008). Briefly, growth medium was removed from hBMSC cultures and replaced with induced medium supplemented with 10 ng/mL keratinocyte growth factor, 10 μg/mL transferrin, 30 μg/mL bovine pituitary extract, 0.5 μg/mL epinephrine, 6.5 ng/mL triiodothyronine, 0.5 μg/mL hydrocortisone, and 0.1 ng/mL retinoic acid. Cells were incubated for 3 weeks under these conditions, with fresh medium changed every 2 days.
Immunofluorescence
The hBMSCs were washed three times with PBS buffer, then fixed with 4% paraformaldehyde for 15 min at room temperature, and washed again three times with PBS buffer. Cells were permeabilized by incubating with 0.125% Triton X-100 for 10 min. The hBMSCs were then washed three times with PBS buffer and incubated with 4% BSA for 30 min. Cells were then incubated with primary antibodies in a humidified chamber at 4°C overnight. After three washes with PBS, the cells were incubated with FITC-labeled secondary antibodies at room temperature for 1 h, the cells were rinsed three times with PBS buffer for 5 min each. Finally, nuclei were labeled by incubation with 4,6 diamidino-2-phenylindole (DAPI) (Sigma, USA). The cells were examined by a phase contrast fluorescence microscope (Olympus, Japan).
Western blotting
The surfactant protein C, a specific marker of type II pneumocytes, was detected by western blotting. Cells were lysed using M-PER Protein Extraction Reagent (Pierce, USA) supplemented with protease inhibitor cocktail (DMSF). Protein concentrations of the extracts were measured with the BCA assay (Pierce, USA) and equalized with the extraction reagent. Equal amounts of the extracts were loaded and subjected to SDS-PAGE, transferred onto nitrocellulose membranes, and then blotted. Specific antibodies and horseradish peroxidase-coupled secondary antibodies were purchased from Santa Cruz, USA. Membranes were probed using ultra-enhanced chemiluminescence western blotting detection reagents. GAPDH was used as internal control.
Transplantation of airway epithelial cells into the model of acute lung injury
The mouse model of acute lung injury was made using previously reported methods (CitationSilliman et al. 1998, CitationCakar et al. 2000, CitationRojas et al. 2005). Animals received cyclosporin A (10 mg/kg/d), beginning 1 d before transplantation and administered until the end of the study. Transplantation surgeries occurred 7 d after lung injury, and each experimental mouse received 2–3 × 106 airway epithelial cells or hBMSCs in 2 ml of PBS buffer intravenously. Control animals received an equal volume of PBS. Two weeks after transplantation, the mice were sacrificed and then perfused with saline and 4% paraformaldehyde (Sigma, USA). A total of 50 cross-sections with a thickness of 10 μm were made from all lung tissues. Because of special human nuclear protein, we use immunohistochemistry to identify transplanted human airway epithelial cells or hBMSCs. To distinguish between the injected airway epithelial cells, hBMSCs, and the control group, DAPI staining was performed as counterstaining and stained cells were observed at a wavelength of 488 nm under a fluorescence microscope.
Results
Culture of hBMSCs in vitro
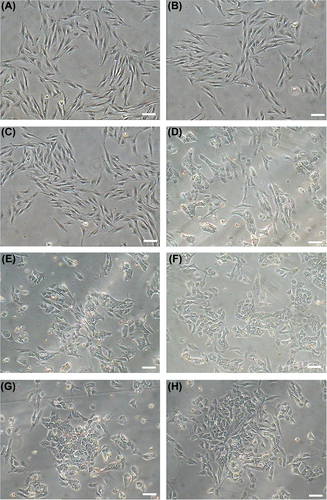
The hBMSCs were large, lucent, and with strong refraction. Nuclei were oval and mixed with some blood cells. The non-adherent cells were removed on the third day and hBMSCs were fusiform and showed cell-like clones, with growth being slower. Four days later, MSCs proliferated rapidly. We observed a delayed outgrowth of exponentially growing populations of cells with spindle-shaped morphology, indicative of hBMSCs ().
Figure 1. hBMSCs were differentiated into airway epithelial cells by cocktail factors induction. hBMSCs metamorphosed from spindle- to round- or irregularly-shaped at fifteen day after inducement, until the cells were very closely located to each other and developed a cobblestone pattern. (A) Day 0 after inducement, (B) Day 5 after inducement, (C) Day 10 after inducement, (D) Day 14 after inducement, (E) Day 15 after inducement, (F) Day 17 after inducement, (G) Day 19 after inducement, (H) Day21 after inducement. (bar = 100 μm).
Differentiation of the hBMSCs into airway epithelial cells
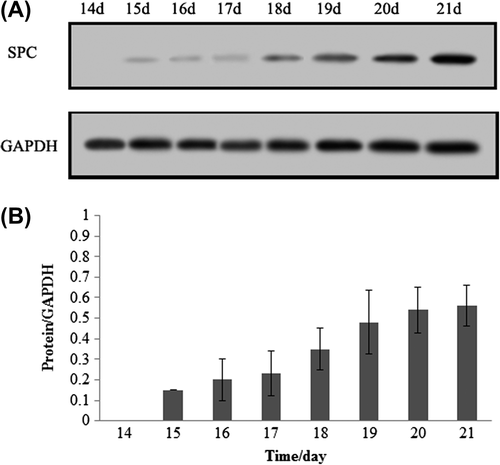
The capacity for hBMSCs to differentiate into airway endothelial cells was tested by induction with a factor cocktail and subsequent morphological and phenotypic analysis. Fifteen days after induction of cells with the factor cocktail, the cell morphology changed from spindle to round or irregularly shaped, until the cells were very closely located to each other and developed a cobblestone pattern (). To confirm that differentiation had occurred, the expression of airway endothelial cell markers was examined in the induced hBMSCs by immunofluorescent staining and western blotting. Induced cells were positive for surfactant protein C, a specific marker of type II pneumocytes ( and ).
Figure 2. Identification surface markers of airway epithelial cells. The surfactant protein C, a specific marker of type II pneumocytes was detected using immunofluorescence.
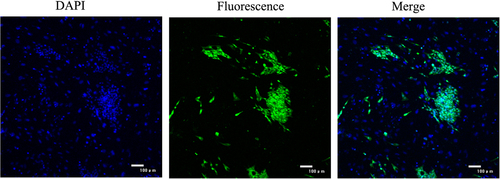
Figure 3. Western Blot analysis of airway epithelial cells -specific genes. (A) the specific marker of type II pneumocytes, surfactant protein C was detected using western blot, the results showed that surfactant protein C were positive at fifteen day after inducement, and showed a time-lapse increase with induced time. (B) Quantification of surfactant protein C in different induced times.
Transplantation of airway epithelial cells into the model of acute lung injury
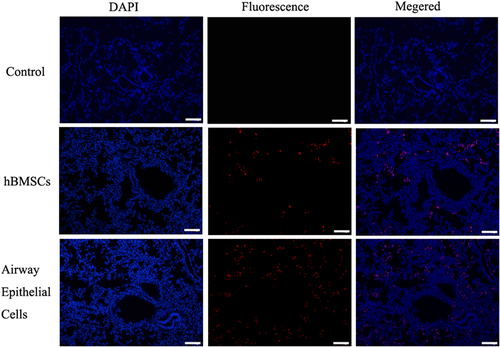
The hBMSC-derived airway epithelial cells and hBMSCs were transplanted respectively to take part in tissue regeneration in acute lung injury. To test whether the transplanted airway epithelial cells and hBMSCs participated in the recovery of pulmonary alveoli, a paraffin section was used to observe the location for transplantation of airway epithelial cells in scathing lung tissue. Human nuclear protein was used as a label to identify the hBMSCs or hBMSC-derived airway epithelial cells in the tissue of pulmonary alveoli. The transplanted hBMSCs or hBMSC-derived airway epithelial cells were counted from 15 and 18 serial section slides out of a total of 50 sections respectively. The results demonstrated that hBMSC-derived airway epithelial cells and hBMSCs could take part in the recovery of acute lung injury, but the group of type II pneumocytes was significantly higher than the group of hBMSCs. Human type II pneumocytes were predominantly found in the pulmonary alveoli, but a small number were detected in the group of hBMSCs ().
Figure 4. Transplantation of hBMMSCs, hBMMSCs-derived n airway epithelial cells resulted in mouse acute lung injury. To test whether transplantation of airway epithelial cells and hBMSCs taken part in recovery of pulmonary alveolus, paraffin section was used to observed the location for transplantation of airway epithelial cells in scathing lung tissue. Human nuclei protein was used as a label to discover the hBMSCs or hBMSCs-derived airway epithelial cells in tissue of pulmonary alveolus. The results showed that the human cells were found in mouse lung tissue through tail vein injection, but the group of type II pneumocytes was significantly higher than group of hBMSCs. (bar = 100 μm).
Discussion
Recent studies challenge the view that tissues are maintained solely by organ-specific stem cells. There is evidence that adult stem cells from a variety of sources can generate not only their own lineages, but those of other tissues, sometimes crossing barriers of embryonic derivation previously thought impenetrable (CitationNeuringer and Randell 2004, CitationKorbling and Estrov 2003). MSCs have several properties that make them attractive as a potential treatment for lung injury. MSCs avoid allo-recognition, home-in to sites of injury, and suppress inflammation as well as immune responses (CitationNemeth et al. 2009, CitationCurley et al. 2012). In this study, we have shown that, by manipulation of the culture conditions, hBMSCs can be encouraged to differentiate into lung-specific type II pneumocytes in vitro. Most of these lung-specific type II pneumocytes express surfactant protein C. Surfactant protein C has been known for many years to be a specific marker for alveolar type II cells (CitationGlasser et al. 1990, CitationGlasser et al. 2000). Identification of this protein confirmed the presence of alveolar type II pneumocytes in the hBMSC-derived cultures. Growth-factor-directed differentiation is the most common method by which derivatives of all three germ layers have been obtained from both mouse and human BMSCs. For example, differentiating MSC cultures have been enriched in this way for neurons (CitationLu et al. 2004), cardiomyocytes (CitationRangappa et al. 2003), and hepatocyte-like cells (CitationCampard et al. 2008). The cocktail of factors was successfully used to induce the differentiation of type II pneumocytes from hBMSCs in this research. Type II epithelial cells of lung generated from stem cells in vitro would have multiple applications, including re-cellularization of decellularized lung scaffolds to provide an autologous graft for transplantation, study of human lung development, modeling of diseases that primarily affect airway epithelial cells, and drug screening (CitationHuang et al. 2014, CitationGreen et al. 2013). However, the cell transplantation of alveolar type II pneumocytes was the main prospect of allotransplantation in the current research. In our research, the type II pneumocyte-derived hBMSCs and hBMSCs were transplanted into a mouse model of acute lung injury respectively, and our result showed that human cells were identified in recipient mouse lungs by immunofluorescent staining for special human nuclear protein. Although the human cells could locate lung tissue and take part in tissue repair using markers of special human nuclear protein, there were distinctions between the type II pneumocyte-derived hBMSCs and hBMSCs in the numbers of cells located in injured tissue; the group of type II pneumocytes was significantly higher than the group of hBMSCs.
In summary, the results of our research demonstrated that hBMSCs have the capacity to generate a functional airway epithelium. In light of their potential to repair the injured pulmonary alveoli, hBMSCs could theoretically and virtually provide an unlimited supply of cells for transplantation. Their great promise provides hope that airway epithelial tissue derivatives of hBMSCs could be grafted to reconstitute the airway epithelium in a variety of airway diseases, including bronchopulmonary dysplasia, cystic fibrosis, or bronchiolitis obliterans.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aggarwal S, Pittenger MF. 2005. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 105:1815–1822.
- Aliotta JM, Passero M, Meharg J, Klinger J, Dooner MS, Pimentel J, Quesenberry PJ. 2005. Stem cells and pulmonary metamorphosis: new concepts in repair and regeneration. J Cell Physiol. 204:725–741.
- Bishop AE. 2004. Pulmonary epithelial stem cells. Cell proliferat. 37:89–96.
- Cakar N, der Kloot TV, Youngblood M, Adams A, Nahum A. 2000. Oxygenation response to a recruitment maneuver during supine and prone positions in an oleic acid-induced lung injury model. Am J Respir Crit Care Med. 161:1949–1956.
- Campard D, Lysy PA, Najimi M, Sokal EM. 2008. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 134:833–848.
- Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, et al. 2012. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 67:496–501.
- Deng F, Lei H, Hu Y, He L, Fu H, Feng R, et al. 2015. Combination of retinoic acid, dimethyl sulfoxide and 5-azacytidine promotes cardiac differentiation of human fetal liver-derived mesenchymal stem cells. Cell Tissue Bank.
- Glasser SW, Korfhagen TR, Bruno MD, Dey C, Whitsett JA. 1990. Structure and expression of the pulmonary surfactant protein SP-C gene in the mouse. Journal Biol Chem. 265:21986–21991.
- Glasser SW, Burhans MS, Eszterhas SK, Bruno MD, Korfhagen TR. 2000. Human SP-C gene sequences that confer lung epithelium-specific expression in transgenic mice. Am J Physiol Lung Cell Mol Physiol. 278:L933–945.
- Green MD, Huang SX, Snoeck HW. 2013. Stem cells of the respiratory system: from identification to differentiation into functional epithelium. BioEssays: News Rev Mol Cell Devlop Biol. 35:261–270.
- Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, et al. 2014. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 32:84–91.
- Kavanagh DP, Suresh S, Newsome PN, Frampton J, Kalia N. 2015. Pretreatment of Mesenchymal Stem Cells Manipulates Their Vasculoprotective Potential While Not Altering Their Homing Within the Injured Gut. Stem Cells.
- Korbling M, Estrov Z. 2003. Adult stem cells for tissue repair - a new therapeutic concept? The New England J Med. 349:570–582.
- Lu P, Blesch A, Tuszynski MH. 2004. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 77:174–191.
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. 2009. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 15:42–49.
- Neuringer IP, Randell SH. 2004. Stem cells and repair of lung injuries. Respir Res. 5:6.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. 1999. Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147.
- Rangappa S, Fen C, Lee EH, Bongso A, Sim EK. 2003. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 75:775–779.
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. 2005. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 33:145–152.
- Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, Bishop AE, et al. 2006. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 12:867–875.
- Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. 1998. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J clin Invest. 101:1458–1467.
- Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. 2008. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 177:701–711.
- Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA, Jr., et al. 2005. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA. 102:186–191.
- Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. 2006. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc. 3:193–207.
