Abstract
The multiple properties of zinc sulphide nanoparticles (ZnS-NPs) are attracting great attention in the field of chemical and biological research. ZnS-NPs also find their application in biosensor and photocatalysis. Zinc is an important metal ion in retina and its deficiency leads to age-related macular degeneration. As of now, not much research is available on bio-interaction of ZnS as nanoform with retinal pigment epithelial (RPE) cells. RPE cells in the retina help in maintaining normal photoreceptor function and vision. To begin with, ZnS-NPs were synthesized and characterized using UV-visible spectra, X-ray diffraction, Fourier transform infrared spectrum, transmission electron microscopy and dynamic light scattering. Followed by the confirmation of nanoparticles, our study extended to investigate the impact of ZnS-NPs in primary mouse RPE (MRPE) cells at different concentrations. ZnS-NPs showed dose-dependent cytotoxicity in MRPE cells and no changes were observed in cells’ tight intactness at minimal concentration. In addition, exposure to ZnS-NPs increased cellular permeability in dose- and time-dependent manner in MRPE cells. The findings from DCFH-DA analysis revealed that ZnS-NPs-treated cells had elevated level of reactive oxygen species and partial activation of cell apoptosis was identified after exposure to ZnS-NPs at higher concentration. Furthermore, pre-treatment of the primary MRPE cells with ZnS-NPs led to phosphorylation of Akt (Ser 473), which indicates the crucial role of ZnS-NPs in regulating cell survival at minimal concentration. Altogether, this study enumerates requisite dose of using ZnS-NPs to maintain healthy RPE cells and contributes to future studies in development of therapeutic drug and drug carrier for ocular-related disorders.
Introduction
Nanotechnology corresponds to a new platform that assures to provide a broad range of improved technologies for biological and biomedical applications. Nanostructure and nanoparticles of zinc sulphide (ZnS-NPs) were explored as they have unique chemical and physical properties, such as high electrochemical coupling coefficient, UV detection, catalysis, high photo-stability and broad range of radiation absorption (Fang et al. Citation2011, Hoffmann et al. Citation1995, Ni et al. Citation2004). These multifunctional properties of ZnS-NPs have made them useful in various applications which include manufacturing biosensors, cell imaging and electrical materials (Bahadir and Sezgintürk Citation2014, Elghanian et al. Citation1997, Fang et al. Citation2011). In human body, zinc is the second most abundant transition metal ion (Wills et al. Citation2008). Particularly zinc is highly available in retinal pigmented ocular tissues (Ugarte et al. Citation2012). Zinc, as a prominent metal, participates in multiple functions such as gene expression, quenching of free radicals, cell metabolism, cell proliferation and retinal development (Otteson et al. Citation2004, Ricchelli et al. Citation2011, Watabe et al. Citation2011). Zinc deficiency is also a principal cause for visual impairments such as abnormal dark adaption and night blindness (Erie et al. Citation2009).
In retina, retinal pigment epithelial (RPE) cell plays vital role in photoreceptor cell survival and normal functioning of photoreceptors which includes synthesis and transportation of many substances, such as vitamin A and metabolites and phagocytosis of moulted outer segments of rods and cones (Bok Citation1993, Marmorstein Citation2001, Rizzolo Citation1997). In order to have proper vision, RPE cells and differentiated melanocytes produce a light-absorbing pigment called melanin (Strauss Citation2005). RPE cells that produce melanin contain zinc which is expected to be involved in regulatory function of retinal photoreceptors that in turn is likely to decrease with ageing (Sarna Citation1992). Several recent studies also witnessed that inadequate level of zinc in the RPE cell induces caspase-dependent apoptosis which may lead to progression of macular degeneration (Hyun et al. Citation2001, Nakajima et al. Citation2014, Wood and Osborne Citation2001). On the other hand, overload of zinc may cause dysfunction of the immune system, oxidative stress involved cell toxicity, mitochondrial injury (Ugarte and Osborne Citation2014) and zinc-induced copper deficiency (Willis et al. Citation2005). As a matter of fact the RPE contains photoreceptors and retinal neurons that are highly sensitive and induce apoptosis in the condition of “loosely-bound” zinc in excess concentration (Redenti et al. Citation2007). Furthermore, earlier studies have reported that zinc deficiency which results in elevation of free radicals called reactive oxygen species (ROS), in turn leads to apoptosis through hypo-phosphorylation of Akt and extracellular signal-regulated kinase pathway (Clegg et al. Citation2005, Lefebvre et al. Citation1999). Akt, a serine/threonine protein kinase, regulates survival, proliferation, protein translation and metabolism. So, maintaining homeostasis of zinc in RPE cells is very important for normal retinal function. In consideration of all the above facts instead of using zinc as bulk material, nano-size particles can be used to have a better retinal zinc metabolism. Since nano-size particles characteristically possess a larger percentage of atoms at the material’s surface, it can lead to increased surface reactivity (Nel et al. Citation2006). Moreover, the nanoparticles and quantum dots have tendency to interact with many proteins and macromolecules that enable them to be used as therapeutic agents as well as drug carrier to target cells (Bajwa et al. Citation2015). As far as bio-distribution is concerned, there is no evidence provided for ZnS-NPs reaching the retina, but there are reports that show other nanoparticles like yttrium and ceria particles reaching retina (Chen et al. Citation2006, Mitra et al. Citation2014). Compared to other nano-size metal ions, zinc is naturally available in retina and using it in nanoform will make it an ideal available source of nanoparticle that can effectively control and regulate retinal cell viability.
In the present study, we look into the cytotoxicity effect of ZnS-NPs on RPE cells in dose-dependent manner. We further extended to identify the possible biological function of ZnS-NPs in regulating ROS elevation, cell permeability and to explore the involvement of Akt phosphorylation in RPE cells at various concentrations of ZnS-NPs. Outcome of our work will be more useful to evaluate the future perspective of using ZnS-NPs as drug carrier and to treat retinal diseases.
Materials and methods
ZnS nanoparticles biosynthesis and characterization
ZnS-NPs were synthesized using the biomass of bacterium Brevibacterium casei which was cultured in nutrient broth media (Karthikeyan et al. Citation2015). The harvested biomass was incubated with 5 mM ZnSO4 for 96 h. After incubation, the presence of ZnS-NPs inside the cells was confirmed by visual colour change. The biomass was separated by centrifugation and washed thrice with 50 mM phosphate buffer (pH 7.0). The ZnS-NP was separated from biomass using sonicator (Sonics Vibra Cell, Newtown, USA) and purified using Millipore filter (0.22 μm pore size). The purified nanoparticles were characterized using UV-visible spectrophotometer, X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectrum, transmission electron microscopy (TEM) and dynamic light scattering (DLS) in order to determine the nature of chemical, shape and size of the ZnS-NPs (Bai et al. Citation2006, Luo et al Citation2010, Malarkodi and Annadurai Citation2013). Optical absorbance spectra were analysed by UV-visible spectrophotometer in the range of 200–550 nm. The ZnS nanoparticles’ XRD patterns were obtained by D8 Advance ECO XRD Systems with SSD160 1 D Detector, Bruker, USA. FTIR measurements were analysed by a Shimadzu spectrometer in the range of 4000–300 cm−1 in transmission mode. The nanoparticle powder was prepared and added to 100 mg of KBr for FTIR measurement. Nanoparticles dispersion was determined by TEM, zeta potentials and size measurement. One or two drops of nanoparticle solution with ethanol were loaded on copper grid coated an amorphous carbon film. A Carl Zeiss microscope operated at 100 kV was used for TEM measurement. Zetasizer Nano ZS90 instrument (Malvern Instruments, Worcestershire, UK) was used to determine the size and zeta potential of nanoparticles by DLS.
Isolation of primary mouse RPE cells
Two-week-old CBA/J mouse were used in the experiments. The mouse eyes were enucleated and primary RPE cells were isolated and cultured as reported previously (Karthikeyan et al. Citation2010). Primary RPE cells were cultured in Iscove’s modified Dulbecco’s medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin and incubated with 5% CO2 at 37 °C. Normally after nine days, the mouse RPE (MRPE) cells get 70–80% confluence with consistent pigments. Third and fourth passage MRPE cells were used for the entire experiments.
Cell viability assay
The mitochondrial reductase activity was analysed using MTT (3-(4, 5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide) assay according to the manufacturer instructions (Roche Diagnostics GmbH, Mannheim, Germany). MRPE cells were seeded at a density of 2 × 103 cells per well grown in 96-well microtiter plates and starved in IMDM with 0.5% FBS for 6 h. To examine the effect ZnS-NPs on RPE cell viability, cells were treated with various concentrations of ZnS-NPs and incubated for 24, 48 and 72 h at 37 °C. Ten μL of MTT was added to each well and incubated for 4 h (at 37 °C). The added MTT gets reduced to insoluble formazan by the mitochondrial dehydrogenases from viable cells. 100 μL of the dissolving buffer was added to the well to dissolve the reduced product and the absorbance of purple formazan was read at 595 nm in a multi well spectrophotometer (Bio-Rad, Hercules, CA, USA).
Morphological analysis of MRPE cells
Confluent MRPE cells were starved with 0.5% serum for 6 h and incubated with various concentrations of ZnS-NPs (200 nM, 400 nM, 600 nM, 800 nM and 1000 nM), for 24 h at 37 °C. The ZnS-NPs-treated and -untreated MRPE cells were observed under inverted phase-contrast microscope (Zeiss, Oberkochen, Germany) to study the morphological changes.
Transwell monolayer permeability assay
MRPE cells were seeded on transwell plate and after their reach of required confluency, the cells were examined under microscope for integrity of epithelial morphology. The MRPE cells were starved with 0.5% serum for 6 h and then treated with various concentrations of ZnS-NPs. To identify the permeability of fluorescent-dye Rhodamine B isothiocyanate (RITC)-Dextran (Sigma-Aldrich, St Louis, MO, USA), it was loaded on apical side of the chamber and if the treatment caused any damages to epithelial tight junction then RITC molecule penetrates into the basolateral chamber. The amount of dextran in the basolateral chamber was measured at 540 nm excitation and 580 nm emission in fluorescence microplate reader FLx800 (Biotek, Winooski, VT, USA).
Intracellular ROS measurement
MRPE cells were incubated with ZnS-NPs (different concentrations) and without ZnS-NPs, labelled with 2′,7′-dichlorofluoresceindiacetate (2,7-DCFH-DA; Sigma-Aldrich) for 30 min at 37 °C. The effect of ZnS-NPs on ROS production in MRPE cells was analysed under fluorescence microscope. For measuring ROS production, the DCFH-DA-labelled cell fluorescence was measured at 495 nm excitation and 525 nm emission wavelength in fluorescence microplate reader FLx800 (Biotek). Quantitative analysis was performed by measuring the fluorescence intensity relative to the control.
Measurement of apoptosis by acridine orange/ethidium bromide (AO/EtBr)
AO/EtBr double staining was used to study the apoptotic effect of ZnS-NPs on MRPE cells. Acridine orange (AO) stains both live and dead cells and EtBr stains only membrane integrity lost cells. Using this double staining technique live and dead cells were differentiated, live cell with intact membrane will stain green in colour and dead cell will stain red. Primary MRPE cells were trypsinized and pelleted after 24 h of incubation with ZnS-NPs (200, 400, 600, 800 and 1000 nM concentrations). Again the cell pellets were resuspended in 1 mL PBS. 1 μL each from AO (5 μg/mL) and EtBr (3 μg/mL) preparation were added to the cell suspension and observed immediately under fluorescent microscope (Axio Scope.A1, Carl Zeiss, Oberkochen, Germany) using green and red filter. From ten randomly selected fields, numbers of live and dead cells were counted and represented in percentage.
Flow cytometric analysis
The percentage of MRPE cell apoptosis was measured using the propidium iodide (PI) (Sigma-Aldrich) staining. Live/dead cell assay with PI was used to monitor the percentage of cells which undergoes apoptosis induced by ZnS-NPs. Cells were grown in 6-well plate till confluence and treated with ZnS-NPs for 24 h. After treatment, the live and dead cells were collected respectively and stained with 10 μg of PI and analysed in Beckman coulter FC500.
Western blot analysis
To study the effect of ZnS-NPs on protein expression of MRPE cells, cells were treated with ZnS-NPs and harvested after 8 h. The cells were trypsinized and cell suspension was centrifuged at 4000 rpm for 2 min. 100 μL of lysis buffer was added to the pellet and the cell pellet was disrupted by using Sonicator for three pulses with 5-s intervals. The lysate was centrifuged at 12,000g, 4 °C for 20 min and the supernatant was collected separately. The protein concentrations were quantified by rapid protein quantification kit (Sigma-Aldrich) as per manufacturer’s instruction. About 50 μg of protein was separated on a SDS-PAGE gel and transferred to nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA) using semi-dry western blot transfer apparatus (TE 70 PWR, Amersham Biosciences). After blocking, the membranes were incubated for 1 h at room temperature in primary anti-phospho Akt (Ser 473) and anti-total Akt antibody at ratio (1:1000) recommended by Cell Signalling Technology kit (Danver, MA, USA). The respective HRP-conjugated secondary antibodies (1:2000) were incubated at room temperature for 1 h. After washing the blot, immune reactive bands were developed using enhanced chemiluminescence (Amersham Biosciences).
Statistical analysis
All results were analysed as the mean ± standard error of the mean (SEM) values. Statistical calculations were carried out using Graph pad prism 5 (Graph pad software, San Diego, CA, USA). A significance level of P<0.05 was considered to be statistically significant.
Results and discussion
Biosynthesis and characterization of ZnS-NPs
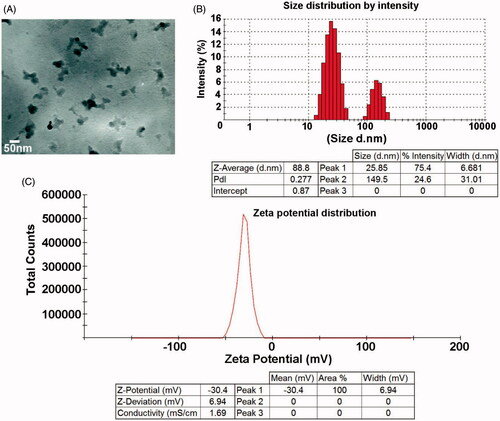
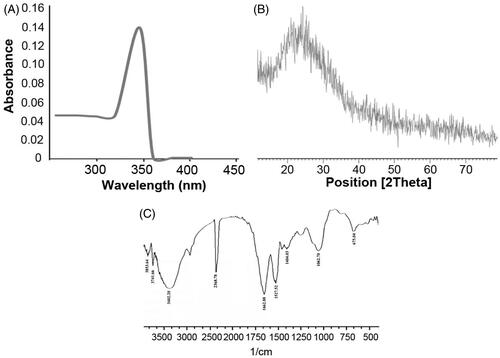
Zinc, commonly used as bulk material, when studied at nano-scale has significant reactive surface area compared to its bulk form. The characteristics of ZnS-NPs were confirmed by UV-visible spectra, XRD, FTIR spectrum, TEM and DLS. The purified ZnS-NPs, on visual observation, showed white colour formation and on further characterization by UV-visible spectrum, showed optimum absorption in the wavelength range of 330–350 nm () (Senapati et al. Citation2013). The crystal formation and size distribution of ZnS-NPs was analysed by XRD. The broadening of the XRD peak, as seen in the image, indicates the formation of ZnS nanoparticles. Apart from the single diffraction, peak there were no other diffraction peaks from 2θ=10–70°, indicating that ZnS-NPs exist in an amorphous phase (). The diffraction peak was observed at 26.6° which was in a good agreement with earlier reports and the standard Zn metal (Bai et al. Citation2006, Ghosh et al. Citation2006) (Joint Committee on Powder Diffraction Standards – File No. 5-0566, JCPDS, USA). Secreted metabolic products from bacteria might interact with crystal surface and the reduction mechanism involved must have resulted in nanoparticle synthesis (Bai et al. Citation2006, Joerger et al. Citation2000). Interestingly, our results showed FTIR peak corresponding to protein or peptide molecules which were bound to the surface of ZnS nanoparticles. shows that the FTIR spectrum ranged from 300 cm−1 to 4000 cm−1 and the major peaks were obtained at 3402.20 cm−1, 2368.78 cm−1, 1527.52 cm−1, 1662.88 cm−1, 1062.70 cm−1 and 675.04 cm−1 and they were recognized as that of ZnS-NPs. The peaks identified in the present work were same as the absorption peaks of ZnS-NPs reported earlier (Hudlikar et al. Citation2012). Further, TEM analysis helps to determine the structure of the nanoparticles. The morphological features of these nanoparticles, which fall within the range of various shapes viz. spherical, hexagonal and triangle with size in 50–200 nm range, were investigated by TEM (). The size distribution of the ZnS-NPs, obtained from DLS experiment, was shown in . The average particle size was identified as 88 nm which coincided with TEM analysis. Zeta potential is a unique identifier of dispersion stability of nanoparticles. As shown in , the zeta potentials of biologically synthesized ZnS-NPs were measured at −30 mV. Our results were consistent with previous reports and low values of zeta potentials are as a result of low amount of adsorbed sulphide ions in the diluted solutions (Luo et al. Citation2010, Praus et al. Citation2014).
Figure 1. ZnS nanoparticles were biologically synthesized using bacterial biomass and it was characterized by various techniques. (A) The absorbance of ZnS-NPs was analysed by UV visible spectrophotometer and the peak was observed at 346 nm. (B) The crystal formation of nanoparticles was analysed by XRD. (C) The major peaks (3402.20 cm−1, 2368.78 cm−1, 1527.52 cm−1, 1662.88 cm−1, 1062.70 cm−1 and 675.04 cm−1) attained from the FTIR spectrum represents the functional groups of ZnS nanoparticles.
Dose-dependent effect of ZnS-NPs in MRPE cells
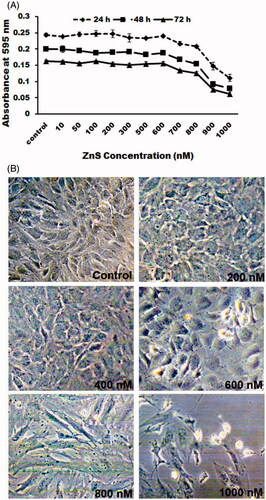
Zinc is an essential micronutrient and the most important trace element in the eye (Ugarte and Osborne Citation2014) and its deficiency leads to neuronal apoptosis (Lee et al. Citation2015). At the same time, zinc cannot be supplemented in high concentration which may lead to dysfunction of immune system and cause copper deficiency (Willis et al. Citation2005). Since the surface of nanoparticles is more reactive, they secured their place as a more active participant in controlling cell survival and were used in different biomedical applications. Therefore, identifying the cytotoxic effect of nano-size materials in biological system will be the path to study its scope as new therapeutic molecule and drug carrier. Here we identified the dose-dependent and time-dependent effect of ZnS-NPs on MRPE cells. The optimal non-inhibitory concentrations of ZnS-NPs on MRPE cells were found using MTT assay (). There was no significant loss of cell viability in control cells (0.5% FBS) and below 600 nM (ZnS-NP) at 24, 48 and 72 h, but as the concentration increased, the viability of MRPE cells was reduced. Recent report has elaborately demonstrated the role of various form of zinc chemicals such as ZnO-NPs, ZnSO4 and ZnO in controlling H2O2-induced cell apoptosis (Mao et al. Citation2013), and stated that cytotoxicity of ZnSO4 and ZnO was higher than nano-ZnO in IEC-6 cells. In our study we took up novel approach, investigated other form of Zn nanoparticles, ZnS-NPs and its role in primary MRPE cells. For morphological analysis, primary MRPE cells were treated with various concentrations of ZnS-NPs for 24 h. Control cells (0.5% FBS) and lower concentrations (i.e., 200, 400 and 600 nM) of ZnS-NPs-treated MRPE cells had hexagonal shape and no morphological changes were found (). At the same time, high concentrations of ZnS-NPs-induced (800 and 1000 nM) morphological alterations such as shrinkage, rounding up and loss of cell adhesion. These findings suggested that minimal concentration of ZnS-NPs maintain cell viability and normal structure of RPE cells.
Figure 3. Cytotoxic effect of ZnS-NPs on MRPE cells. (A) MRPE cells were treated with various concentrations of ZnS-NPs and cell viability was measured after 24, 48 and 72 h. (B) MRPE cells were treated with various concentrations of ZnS-NPs and morphological changes were observed by phase contrast microscope. Higher doses of ZnS-NPs-induced (800 nM and 1000 nM) morphological alteration at 24 h.
ZnS-NPs maintain tight integrity and permeability of MRPE cells
Studies have reported that the oxidative stress mediates damages in the blood retinal barrier (BRB) and disrupts tight junctional integrity (Bailey et al. Citation2004, Miura and Roider Citation2009). RPE cells are more prone to oxidative stress due to ageing and environmental factors. Accumulating evidences suggest that zinc deficiency in retinal cells leads to oxidative stress and macular degeneration. To evaluate the ability of ZnS-NPs in maintaining tight junctional integrity in epithelial cells, MRPE cells were grown to confluence on transwell filters and then treated with ZnS-NPs at various concentrations. ZnS-NPs induced a permeability of RITC-labelled dextran from apical side of MRPE cell monolayer to basal side of chamber in dose- and time-dependent manner. Maximal dextran permeability was observed at concentrations of 800 nM and 1000 nM at 24 h (). These data prove that ZnS-NPs maintain tight junction of cell monolayer intact at lower concentration and MRPE cell permeability was induced at the higher concentration of ZnS-NPs availed to the cells. Furthermore, studies have identified that without showing much toxicity, gold nanoparticles and other nanoparticles can cross the BRB to deliver therapeutics in cases of retinal disorders (Kim et al. Citation2009, Singh et al. Citation2009). Interestingly, our results also showed that the presence of minimal level of ZnS-NPs in MRPE cells have no toxicity and significantly controlled the epithelial cell permeability.
Figure 4. Dose- and time-dependent effect of ZnS-NPs on MRPE cell permeability. MRPE cells were grown to confluent monolayers on porous membranes (12-well transwell insert) and treated with ZnS-NPs and the flux of RITC-dextran from the upper to the lower chamber was measured 6, 12, 18 and 24 h after treatment. Values are expressed in relative fluorescence units (RFUs) as mean ± SEM, with each condition performed at least in triplicate.
Identification of ROS elevation at maximal ZnS-NPs pre-treated cells
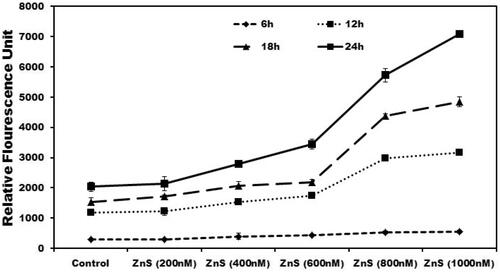
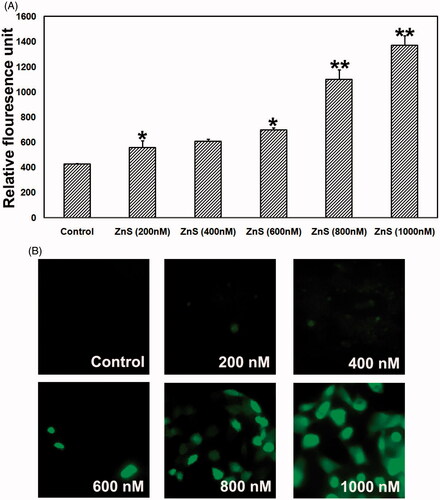
Moderate level of ROS induces cell proliferation whereas extreme amount of ROS generation induces apoptosis (Das et al. Citation2014). To evaluate the mechanism of ROS generation using ZnS-NPs in MRPE cells, we analysed the intracellular ROS production using oxidation of non-fluorescent DCFH-DA to cell permeable fluorescent derivative DCF. showed that ZnS-NPs stimulated ROS elevation in MRPE cells following a concentration dependent manner. Less fluorescence intensity was observed in control and minimal level of ZnS-NPs-treated cells, whereas strong green fluorescence was observed in 800 and 1000 nM ZnS-NPs-treated cells. Results showed that the relative fluorescence value (RFU) of the oxidized DCF was progressively increased in higher concentrations of ZnS-NPs-treated cells when compared with control and lower concentration (). In connection to the earlier reports that ZnO nanoparticles were involved in ROS elevation in various cells (Guo et al. Citation2013, Wang et al. Citation2014) and optimal concentration of zinc carnosine (ZnC) reduced H2O2-induced ROS production in human lymphoblastoid cell line (Ooi et al. Citation2014), our results also represented that minimal level of ZnS-NPs maintain normal ROS rise and increased the generation of ROS at higher concentrations treatment (i.e., 800 and 1000 nM). Cerium and yttrium oxide nanoparticles were proved to be neuro-protective through anti-oxidative property (Schubert et al. Citation2006) and the latter has the ability to protect photoreceptor cells from light-induced cell death (Mitra et al. Citation2014) under ROS elevation. Moreover, intravitreously injected nanoceria have effective role in inhibiting ROS generation and photoreceptor damage (Chen et al. Citation2006). Our results clearly showed that minimal concentration of ZnS-NPs has significant impact in maintaining normal ROS production. Since minimal concentration of ZnS-NPs can maintain normal ROS production, developing ZnS-NPs are most efficient therapeutic molecule for retinal cells compared with other nanoparticles.
Figure 5. Dose-dependent effect of ZnS-NPs on ROS elevation in MRPE cells. (A) There was no fluorescence indication of ROS generation at minimal concentrations (200, 400 and 600 nM) of ZnS-NPs in treated MRPE cells. (B) Relative fluorescence unit shows that the ROS generation was increased in higher concentrations (800 and 1000 nM) of ZnS-NPs-treated MRPE cells when compared with control and lower concentrations. Values are expressed in relative fluorescence unit (RFU) as mean ± SEM, with each condition performed in triplicate (n=3, *P<0.05 V control, **P<0.01 V control).
Evaluation of apoptotic role of ZnS-NPs in MRPE cells
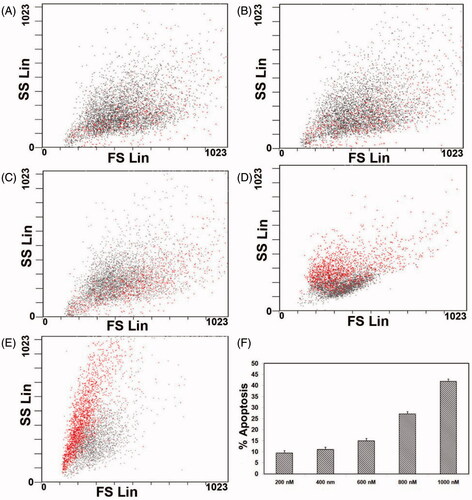
To study the effect of ZnS-NPs on MRPE cell apoptosis, we investigated using AO/EtBr staining and flow cytometry analysis with PI. Initially, we analysed through AO/EtBr staining to verify whether MRPE cells maintain normal morphological features or induce apoptosis in the presence of ZnS-NPs at various concentrations. shows normal viable control cells and up to concentration of 600 nM ZnS-NPs-treated cells which were observed as green fluorescence. At the same time, 800 nM ZnS-NPs-treated cells showed green to yellow–green coloration and 1000 nM ZnS-NPs availed MRPE cells displayed an intense red or yellow-red fluorescence which indicate the apoptotic cells (). Furthermore, the flow cytometry data using PI show a concentration-dependent increase in apoptosis of cells. PI enters the cell through damage in cell membrane and binds to DNA. The result of treatment with various concentrations (i.e., 200, 400, 600, 800 and 1000 nM) of ZnS-NPs on MRPE cells was that the apoptotic rate was increased from 9% to 28.6% and 44.62% respectively (). The flow cytometry results also suggested that 24-h exposure to ZnS-NPs apparently elevated the MRPE cell death in a dose-dependent manner. Furthermore, we also identified that the ZnS-NPs-induced cell death was mainly late apoptosis. Altogether, our results represent that ZnS-NPs induce apoptosis in MRPE cells through increased generation of ROS in concentration-dependent manner, consistent with previous findings of other form of zinc nanoparticles, as well as various in vivo and in vitro tests of a wide range of nanoparticles (Akhtar et al. Citation2013, Donaldson et al. Citation2001). Loads of recent researches were focusing nano-ophthalmology and its approach to current therapeutic challenges. Many recent studies also had identified that oxidant injured rat retinal cell apoptosis was successfully controlled by various nanoparticles such as nanoceria and platinum nanoparticles (Chen et al. Citation2006, Clark et al. Citation2011). In our study, we identified the effect of ZnS-NPs on primary MRPE cells and observed that only 50% of cells underwent apoptosis even in higher dose. So it will be a step ahead using zinc nanoparticle not only for ocular related diseases, but it can also be used as a better therapeutic carrier molecule for other diseases and future therapeutic molecule of interest.
Figure 6. The patterns of cell damage were assessed using an acridine orange/ethidium bromide differential staining method. Images show AO/EtBr staining of control and MRPE cells treated with various doses of ZnS-NPs. Green cells indicate live cells and red or yellow–red indicate apoptotic cells. All experiments were repeated three times with similar results.
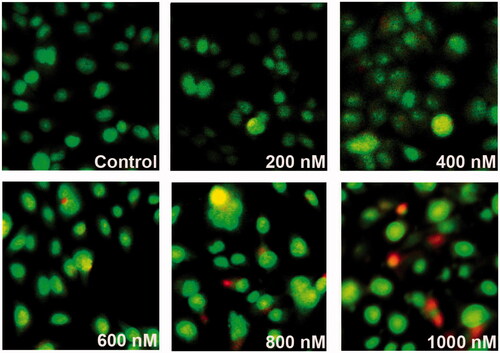
Figure 7. FACS analysis of primary MRPE cells after exposure to different concentrations of ZnS-NPs for 24 h. (A) Cells treated with 200 nM. (B) Cells treated with 400 nM. (C) Cells treated with 600 nM. (D) Cells treated with 800 nM. (E) Cells treated with 1000 nM. (F) Graph representing the percentage of apoptotic cells. Results are representative from three independent experiments. The standard deviations are shown.
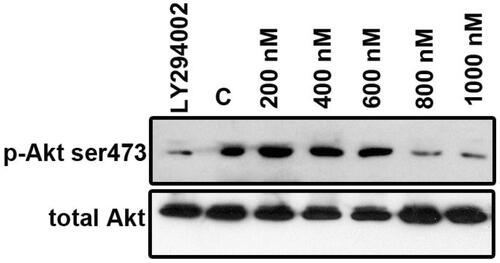
ZnS-NPs induced Akt phosphorylation in MRPE cells
To examine whether ZnS-NPs were involved in altering PI3K/Akt phosphorylation, we performed western blotting to explore the status of Akt phosphorylation at the Ser473 residue using anti-phospho-Akt (Serine 473) and total Akt using anti-Akt antibody in MRPE cells. Minimal amount of ZnS-NPs-treated (i.e., 200, 400 and 600 nM) cell extracts’ protein level of phosphorylated Akt was significantly increased at 8 h compared with that of the Akt inhibitor (LY294002) and maximal dose of ZnS-NPs-treated (i.e., 800 and 1000 nM) cells (). These results represent that ZnS-NPs, at lower concentration, have effective role in regulating Akt phosphorylation. Total Akt was used as loading control which had no significant changes in any of the samples. These data strongly suggest that ZnS-NPs enhance Akt activity in a dose-dependent manner. Our report was consistent with Lee et al. (Citation2009) who suggested that exogenous zinc activates Akt signaling. Clegg et al. (Citation2005) have reported that zinc deficiency causes hypoactivation of Akt which leads to cell death. In addition, zinc also protects the cell damage caused by H2O2 through Akt (Liang et al. Citation2012). Zinc oxide nanoparticles mediate their cytotoxic effect through mitochondria mediated ROS pathway. In addition to this, in HepG2 cells, ZnO nanoparticles were also shown to induce p53 phosphorylation, activate Jun N-terminal kinase (JNK) and p38 pathways which can lead to apoptosis (Sharma et al. Citation2012). Wang et al. (Citation2014) also demonstrated that ZnO-NPs induce oxidative stress and mediate apoptosis in primary astrocytes through JNK signalling pathway. Comparing these to our result it can be inferred that P-Akt level increases with optimized treatment of ZnS-NPs and it may be possible that other pathways might have prominent role to enact apoptosis in response to overdose of ZnS-NPs. Further studies need to be carried out in this aspect.
Figure 8. ZnS-NPs regulate Akt phosphorylation (Ser473) in MRPE cells. MRPE cells were pre-treated with various concentrations of ZnS-NPs and Akt phosphorylation inhibitor LY294002 (10 μM) and analysed by western blotting using phospho-specific Akt antibody. 0.5% FBS-availed cells were used as control. As a loading control, same samples were analysed using total Akt antibody. These experiments were performed thrice with similar results and significant differences from control group were observed.
Conclusion
Most of the nanoparticles implicated as therapeutics for ocular diseases have proven toxic effects. In the present study, we investigated the cytotoxic effect of biologically synthesized ZnS-NPs on primary MRPE cells. Our investigation showed that the maximum concentration of ZnS-NPs that can maintain viability, integrity and cell permeability of MRPE cells is 600 nM. Above this maximum concentration, ZnS-NPs trigger intracellular ROS elevation and activated partial apoptosis of MRPE cells; however, ZnS-NPs (below 600 nM) can be exploited as a medicine for ocular diseases. We have also found that Akt kinase activity was enhanced by ZnS-NPs (up to 600 nM) in MRPE cells. The mechanism underlying ZnS-NPs-induced Akt activation is open for investigation. Altogether, it can be concluded that the ZnS-NPs exert dose-dependent cytotoxic effect on primary MRPE cells.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported in part by a Grant-in-Aid from Department of Science and Technology (SERB) of India (SB/FT/LS-204/2012) and Kalasalingam academy of research and education (KARE) to T.K. B.K. is the recipient of senior research fellowship (09/1012 (0005) 2K11 – EMR – I) from the Council of Scientific and Industrial Research (CSIR), India.
References
- Akhtar MJ, Kumar S, Alhadlaq HA, Alrokayan SA, Abu-Salah KM, Ahamed M. 2013. Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. Toxicol Ind Health. (in press) 0748233713511512.
- Bai HJ, Zhang ZM, Gong J. 2006. Biological synthesis of semiconductor zinc sulfide nanoparticles by immobilized Rhodobactersphaeroides. Biotechnol Lett. 28:1135–1139.
- Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. 2004. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Visual Sci. 45:675–684.
- Bajwa N, Mehra NK, Jain K, Jain NK. 2015. Pharmaceutical and biomedical applications of quantum dots. Artif Cells Nanomed Biotechnol. 10:1–11.
- Bahadir EB, Sezgintürk MK. 2014. A review on impedimetric biosensors. Artif Cells Nanomed Biotechnol. 11:1–15.
- Bok D. 1993. The retinal pigment epithelium a versatile partner in vision. J Cell Sci.17:189–195.
- Chen J, Patil S, Seal S, McGinnis JF. 2006. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 1:142–150.
- Clark A, Zhu A, Sun K, Petty HR. 2011. Cerium oxide and platinum nanoparticles protect cells from oxidant-mediated apoptosis. J Nanopart Res. 13:5547–5555.
- Clegg MS, Hanna LA, Niles BJ, Momma TY, Keen CL. 2005. Zinc deficiency‐induced cell death. IUBMB Life. 57:661–669.
- Das TP, Suman S, Damodaran C. 2014. Induction of reactive oxygen species generation inhibits epithelial–mesenchymal transition and promotes growth arrest in prostate cancer cells. Mol Carcinog. 53:537–547.
- Donaldson K, Stone V, Seaton A, MacNee W. 2001. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Persp. 109:523.
- Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. 1997. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 277:1078–1081.
- Erie, JC, Good JA, Butz, JA, Pulido JS. 2009. Reduced zinc and copper in the retinal pigment epithelium and choroid in age-related macular degeneration. Am J Ophthalmol. 147:276–282.
- Fang X, Zhai T, Gautam UK, Li L, Wu L, Bando Y, Golberg D. 2011. ZnS nanostructures from synthesis to applications. Prog Mat Sci. 56:175–287.
- Ghosh G, Naskar MK, Patra A, Chatterjee M. 2006. Synthesis and characterization of PVP-encapsulated ZnS nanoparticles. Opt Materials. 28:1047–1053.
- Guo D, Bi H, Liu B, Wu Q, Wang D, Cui Y. 2013. Reactive oxygen species-induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol in Vitro. 27:731–738.
- Hoffmann MR, Martin ST, Choi WY, Bahnemann DW. 1995. Environmental applications of semiconductor photocatalysis. Chem Rev. 95:69–96.
- Hudlikar M, Joglekar S, Dhaygude M, Kodam K. 2012. Latex-mediated synthesis of ZnS nanoparticles: green synthesis approach. J Nanopart Res. 14:1–6.
- Hyun HJ, Sohn JH, Ha DW, Ahn YH, Koh JY, Yoon YH. 2001. Depletion of intracellular zinc and copper with TPEN results in apoptosis of cultured human retinal pigment epithelial cells. Invest Ophthalmol Visual Sci. 42:460–465.
- Joerger R, Klaus T, Granqvist CG. 2000. Biologically produced silver–carbon composite materials for optically functional thin film coatings. Adv Mater. 12:407–409.
- Karthikeyan B, Arun A, Harini L, Sundar K, Kathiresan T. 2015. Role of ZnS nanoparticles on endoplasmic reticulum stress-mediated apoptosis in retinal pigment epithelial cells. Biol Trace Elem Res. (in press) DOI https://doi.org/10.1007/s12011-015-0493-2
- Karthikeyan B, Kalishwaralal K, Sheikpranbabu S, Deepak V, Haribalaganesh R, Gurunathan S. 2010. Gold nanoparticles down regulate VEGF-and IL-1β-induced cell proliferation through Src kinase in Retinal pigment epithelial cells. Exp Eye Res. 91:769–778.
- Kim JH, Kim JH, Kim KW, Kim MH, Yu YS. 2009. Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 20:505101.
- Lee JM, Lee JM, Kim KR, Im H, Kim YH. 2015. Zinc preconditioning protects against neuronal apoptosis through the mitogen-activated protein kinase-mediated induction of heat shock protein 70. Biochem Biophys Res Commun. 459:220–226.
- Lee S, Chanoit G, McIntosh R, Zvara DA, Xu Z. 2009. Molecular mechanism underlying Akt activation in zinc-induced cardioprotection. Am J Physiol Heart Circ Physiol. 297:H569–H575.
- Lefebvre D, Boney CM, Ketelslegers JM, Thissen JP. 1999. Inhibition of insulin-like growth factor-I mitogenic action by zinc chelation is associated with decreased mitogen-activated protein kinase activation in RAT-1 fibroblasts. FEBS Lett. 449:284–288.
- Liang D, Yang M, Guo B, Cao J, Yang L, Guo X, Gao Z. 2012. Zinc inhibits H2O2-induced MC3T3-E1 cells apoptosis via MAPK and PI3K/AKT pathways. Biol Trac Elem Res. 148:420–429.
- Luo J, Qu D, Tikhonov A, Bohn J, Asher SA. 2010. Monodisperse, high refractive index, highly charged ZnS colloids self assemble into crystalline colloidal arrays. J Colloid Interface Sci. 345:131–137.
- Malarkodi C, Annadurai G. 2013. A novel biological approach on extracellular synthesis and characterization of semiconductor zinc sulfide nanoparticles. Appl Nanosci. 3:389–395.
- Mao L, Chen J, Peng Q, Zhou A, Wang Z. 2013. Effects of different sources and levels of zinc on H2O2-induced apoptosis in IEC-6 cells. Biol Trac Elem Res. 155:132–141.
- Marmorstein AD. 2001. The polarity of the retinal pigment epithelium. Traffic. 2:867–872.
- Mitra RN, Merwin MJ, Han Z. 2014.Yttrium oxide nanoparticles prevent photoreceptor death in a light-damage model of retinal degeneration. Free RadicBiol Med. 75:140–148.
- Miura Y, Roider J. 2009. Triamcinolone acetonide prevents oxidative stress-induced tight junction disruption of retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol. 247:641–649.
- Nakajima E, Hammond KB, Shearer TR, Azuma M. 2014. Activation of the mitochondrial caspase pathway and subsequent calpain activation in monkey RPE cells cultured under zinc depletion. Eye. 28:85–92.
- Nel A, Xia T, Mädler L, Li N. 2006. Toxic potential of materials at the nanolevel. Science. 311:622–627.
- Ni Y, Yin G, Hong J, Xu Z. 2004. Rapid fabrication and optical properties of zinc sulfide nanocrystallines in a heterogeneous system. Mater Res Bull. 39:1967–1972.
- Ooi TC, Mohammad NH, Sharif R. 2014. Zinc carnosine protects against hydrogen peroxide-induced DNA damage in WIL2-NS lymphoblastoid cell line independent of poly (ADP-Ribose) polymerase expression. Biol Trac Elem Res. 162:8–17.
- Otteson DC, LiuY, Lai H, Wang C, Gray S, Jain MK, Zack DJ. 2004. Krüppel-like factor 15, a zinc-finger transcriptional regulator, represses the rhodopsin and interphotoreceptor retinoid-binding protein promoters. Invest Ophthalmol Visual Sci. 45:2522–2530.
- Praus P, Dvorský R, Kovář P, Svoboda L. 2014. Agglomeration of ZnS nanoparticles without capping additives at different temperatures. Open Chem. 12:312–317.
- Redenti S, Ripps H, Chappell RL. 2007. Zinc release at the synaptic terminals of rod photoreceptors. Exp Eye Res. 85:580–584.
- Ricchelli F, Sileikyte J, Bernardi P. 2011. Shedding light on the mitochondrial permeability transition. Biochim Biophys Acta. 1807:482–490.
- Rizzolo LJ. 1997. Polarity and the development of the outer blood retinal barrier. Histol Histopathol. 12:1057–1067.
- Sarna T. 1992. New trends in photobiology: properties and function of the ocular melanin—a photobiophysical view. J Photochem Photobiol B. 12:215–258.
- Schubert D, Dargusch R, Raitano J, Chan SW. 2006. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun. 342:86–91.
- Senapati US, Jha DK, Sarkar D. 2013. Green synthesis and characterization of ZnS nanoparticles. Res J Physical Sci. 1:1–6.
- Sharma V, Diana A, Alok D. 2012. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis. 8:852–870.
- Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. 2009. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 16:645–659.
- Strauss O. 2005. The retinal pigment epithelium in visual function. Physiol Rev. 85:845–881.
- Ugarte M, Grime GW, Lord G, Geraki K, Collingwood JF, Finnegan ME, et al. 2012. Concentration of various trace elements in the rat retina and their distribution in different structures. Metallomics. 4:1245–1254.
- Ugarte M, Osborne NN. 2014. Recent advances in the understanding of the role of zinc in ocular tissues. Metallomics. 6:189–200.
- Wang J, Deng X, Zhang F, Chen D, Ding W. 2014. ZnO nanoparticle-induced oxidative stress triggers apoptosis by activating JNK signaling pathway in cultured primary astrocytes. Nanoscale Res Lett. 9:1–12.
- Watabe Y, Baba Y, Nakauchi H, Mizota A, Watanabe S. 2011. The role of zinc family zinc finger transcription factors in the proliferation and differentiation of retinal progenitor cells. Biochem Biophys Res Commun. 415:42–47.
- Willis MS, Monaghan SA, Miller ML, Mc Kenna RW, Perkins WD, Levinson BS, Bhusan V, Kroft SH. 2005. Zinc-induced copper deficiency a report of three cases initially recognized on bone marrow examination. Am J Clin Pathol. 123:125–131.
- Wills NK, Ramanujam VS, Kalariya N, Lewis JR, van Kuijk FJGM. 2008. Copper and zinc distribution in the human retina: relationship to cadmium accumulation, age, and gender. Exp Eye Res. 87:80–88.
- Wood JP, Osborne NN. 2001. The influence of zinc on caspase-3 and DNA breakdown in cultured human retinal pigment epithelial cells. Arch Ophthalmol. 119:81–88.