Abstract
The aim of the present study was to evaluate the wound healing performance of cefazolin-loaded gelatin nanofiber mats in post-operative wound. The obtained nanofibers were smooth, non-beaded and having diameter ranging from 620–680 nm. Nanofiber mats that are prepared exhibit high drug entrapment, excellent oxygen permeability and sustained drug release behavior. Further, medicated nanofiber mats showed an accelerated wound healing as compared to plain cefazolin. Macroscopical and histological evaluations demonstrated that cefazolin-loaded gelatin nanofiber showed increased epithelialization rate and collagen deposition. The results indicated that therapeutic strategies offer new prospects in the management of post-operative wound repair.
Introduction
Although many advances have been made in the management of surgical wounds and various approaches including aseptic techniques, prophylactic antibiotics and laparoscopic surgery have been undertaken, the infection of surgical wounds and wound failure remain common complications of surgery (Sharma et al. Citation2014b, Ueno et al. Citation2006). A wound bed provides an ideal condition for the growth of microorganisms owing to its moist, warm and nutritious environment (Bowler et al. Citation2001). Thus an ideal formulation for post-operative wound management is desirable that possesses a broad spectrum of activity and is capable of controlling the bleeding, preventing of infection and reduce the scarring (Garg et al. Citation2012). A standard wound dressing material should be non-toxic, have good permeability and skin flexibility, biocompatibility and biodegradability characteristics. The traditional cotton-gauge dressings only provide a reliable initial mechanical protection, but, absorbing the wound discharge, it becomes a medium inviting the growth of pathogenic microorganism (Garg et al. Citation2014b, Gupta et al. Citation2015). Therefore, instant care of skin wound is necessary for prevention of microbial infection and trans-epidermal water loss resulting in brisk regeneration of wound tissue (Garg et al. Citation2014a, Silverman et al. Citation1989).
The latest advances in nanotechnology enabled to fabricate nanofibrous constructs that possess architectural and morphological properties resembling the natural extracellular matrix (Kaur et al. Citation2014b, Liao et al. Citation2006, Smith and Ma Citation2004). In contrast to traditional bandages which do not adequately meet the requirements of wound care, fiber mats fabricated with electrospinning have a potential to provide an excellent platform for wound healing (Chen et al. Citation2008, Rho et al. Citation2006). Consequently, the research involving the use of electrospun nanofiber mats that accelerate wound healing and prevent bacterial infections has gained momentum in the recent past (Fu et al. Citation2014, Son et al. Citation2006). Nanofibers produced by electrospinning process manifest high porosity levels (Gagandeep et al. Citation2014, Zhu et al. Citation2008), gas permeation (Singh et al. Citation2015) and possess a high surface area to volume ratio (Malik et al. Citation2015). These properties lead to an enhancement in the rate of cell respiration (Sharma et al. Citation2014a), skin regeneration (Morie et al. Citation2014), moisture regeneration (Singh et al. Citation2014), and removal of exudates (Huang et al. Citation2003).
The effectiveness of a wound dressing is influenced by the rate and manner in which the drug is released. Therefore a combination of initial burst release followed by sustained drug release is highly desirable for the treatment of initial as well as latent infection (Garg et al. Citation2013). Currently, there is no available wound dressing that combines the advantages of controlled release of antimicrobials, restricted permeability and biodegradability (Goyal et al. Citation2014). The nature of the vehicle, the physicochemical properties of the drug plays an important role to achieve the aforementioned targets (Kaur et al. Citation2014a). Gelatin and its copolymers are extensively investigated as a preferred material for polymeric implants due to their non-immunogenic, biodegradation, biocompatibility and controlled drug release profile. Gelatin (a long fibrous non-immunogenic protein) was chosen as the polymer of choice owing to its merits like biological origin, good swelling property, biocompatibility, non-immunogenic and biodegradability under normal physiological conditions and commercial availability at low cost (Kim et al. Citation2009). Gelatin is also known to hamper the loss of fluid due to exudation resulting in the enhancement of its wound healing properties (Chong et al. Citation2007, Tanaka et al. Citation2005). Moreover it can also accelerate wound healing, because of the anti-inflammatory nature of one of its amino acid glycine (Zhong et al. Citation2003). Further, electrospinning provides lot of opportunities to meet all the criteria of an ideal wound dressing required for effective wound care management such as controlled drug release behavior, oxygen permeability and water evaporation (Modgill et al. Citation2014). Cefazolin is among the drugs of choice in most of the surgical wound cases, with the mechanism of inhibiting the cell wall synthesis by binding to specific penicillin-binding proteins (PBPs).
In our application, a reinforcing gelatin nanofibrous mat affords the desired mechanical strength to the dressing, while the porous structure of the scaffold is aimed to provide necessary moisture control and controlled release of antimicrobials in order to defend the wound site from infection and promote wound healing (Chaudhary and Garg Citation2015, Garg et al. Citation2015). The controlled biodegradation of selected polymeric constituents enable easy removal of the dressing from the wound site. The present study is undertaken to evaluate the wound healing potential of cefazolin-loaded gelatin nanofiber mat in surgical wound model in rats.
Experiment section
Materials
Cefazolin sodium was obtained as a gift sample from Nectar Life Sciences (Punjab, India). Gelatin was purchased from Sigma Aldrich, Chandigarh, India. The freeze dried culture of Pseudomonas aeruginosa (MTCC No. 424) and Staphylococcus aureus (MTCC No.96) was procured from IMTECH Chandigarh, India. All other chemicals and solvents used were of analytical grade.
Fabrication of drug loaded nanofibers
Excess amount of drug (950 mg/ml) was added to 2,2,2-trifluoroethanol (1 ml) to prepare a saturated drug solution and was added to the polymeric solution prepared in 2,2,2-trifluoroethanol. The drug polymer dispersion was stirred for 30–45 min at room temperature to obtain a clear homogenous solution. Polymeric solutions were placed into a 10 ml syringe having needle with a tip diameter of 22 gauges. The syringe was placed in the electrospinning assembly (E-spin NANO, IIT Kanpur, India). A syringe pump was used to deliver the polymer solution at a rate from 0.04 ml/min. The applied potential was varied from 11 to 17 kV with a fixed tip collector distance of 15 cm. The metal collector was covered with an aluminum foil. The drug loaded nanofiber mats were collected on the surface of aluminum foil. The drug loaded nanofibers were collected and dried overnight under vacuum at room temperature for further analysis.
Nanofiber crosslinking
Drug loaded gelatin nanofiber mats were cross-linked with glutaraldehyde vapor by placing them in a sealed desiccator containing 20 ml of 50% aqueous glutaraldehyde solution in a petri dish for 48 h at room temperature. Subsequent to cross linking, samples were exposed in fuming hood for 2 h followed at 100 °C for 1 h to remove residual glutaraldehyde (Zhang et al. Citation2006).
Characterization of nanofiber mats
The morphology of all the electrospun nanofibers was characterized by optical microscopy using Motic digital microscope (DMWB series, Causeway Bay, Hong Kong) and scanning electron microscopy (SEM) Jeol (JSM-6510) (CA, USA). In each case, a small section of the fiber was placed on the SEM sample holder and sputter coated with platinum. Accelerating voltage of 20 kV was employed to take the SEM images. The average diameter of nanofibers was obtained from at least 10 measurements on typical SEM image. To investigate any possible chemical interaction between the cefazolin and carrier, infrared spectrums of drug and drug loaded nanofiber were observed using IR spectrometer (Perkin-Elmer spectrum RX1) (Massachusetts, US). The electrospun nanofibers were cut into small pieces and mixed with KBr to make sample pellets. Measurements were taken in a range between 4000 and 800 cm−1 with a resolution of 2 cm−1. Differential scanning Calorimetry (DSC) thermograms of the fabricated nanofibers were obtained using DSC 1 STAR system (Mettler Toledo, OH). DSC measurement was done with a sample weight of 3–5 mg under a nitrogen atmosphere with a scanning speed of 10 °C/min. The samples were heated from 0 to 300 °C at a rate of 10 °C/min. The physical state of the drug in the nanofibers was also studied by X-ray diffraction. X-ray diffraction (XRD) patterns were traced employing X-ray diffractometer (Philips PW 1729, CA, USA) for the samples, using Ni filtered CuK (∝) radiation, a voltage of 35 kV, a current of 20 mA and receiving slit of 0.2 in. The samples were analyzed over 2θ range of 2–40° with scan step size of 0.020° (2θ) and scan step time of 1 s.
Entrapment efficiency
The entrapment efficiency was calculated by drying the drug loaded nanofibers at 40 °C or 5 min inside a hot air oven, removing the nanofiber film of 1 × 1 cm2 from the surface of the aluminum sheet and sonicated for 30 min in PBS pH 7.4 using a probe sonicator, further stirring was continued for 60 min. The amount of drug was calculated by UV analysis of the solutions using the calibration curve at absorption maxima of 271.79 nm and was compared with the amount of drug that was loaded during the process of electrospinning of these fibers as per the following equation (Kataria et al. Citation2014).
Percentage drug loading
The percent drug loading of drug loaded nanofiber was determined by total amount of drug extracted from the polymeric nanofiber to the weight of polymer used in the carrier system. It can be expressed by the following equation:
Drug loading (%) = Mass of drug/Mass of polymer × 100.
Degree of swelling
The degree of swelling of the fabricated nanofibers patches were calculated by the following equation:
where M is the weight of swollen nanofibers sample which is wiped to dry with filter paper, Md is the dried mass of immersed sample in buffer medium, measured by drying the swollen nanofibers mats in an oven at 40 °C to get a constant weight. The test was carried out in the release medium, i.e. phosphate buffered saline (PBS), pH 7.4 at 37 °C for time period 4, 8 and 12 h (Kataria et al. Citation2014).
Water vapor transmission rate
The rate of water evaporation was determined by gravimetric technique using cup method. The permeability cup was filled with 20 ml of distilled water and the prepared nanofiber mat was tightly fixed onto its opening. Evaporation of water was determined by measuring the change in weight of the cup. An open cup was used as the control.
Bacteria penetration
Bacterial penetration was determined by disk diffusion method. Pre-sterilized nanofiber film of 5 × 5 cm2 films was inoculated with bacterial suspension (Pseudomonas aeruginosa or Staphylococcus aureus (108 bacteria/ml)). The film was fold over and the leaflet from all sides tied firmly to form the pouch and was placed on agar plates. After one week of incubation at 37 °C the agar plates were analyzed for bacterial penetration.
In-vitro release studies
The in vitro drug release behavior of prepared nanofibers was studied in phosphate buffer saline (PBS 7.4), the release studies were carried out at 37 °C at 100 rpm in a thermostatically shaking incubator (LSB- 1005RE, Daihan Labtech. Co. Ltd. Korea) (New Delhi, India). Samples were withdrawn at predetermined interval and replaced with fresh medium to maintain the sink condition. The drug content was analyzed in each sample using UV spectrophotometer. Drug release data were analyzed by various mathematical models to understand the mechanism of drug release.
In-vitro antibacterial activity
The antimicrobial activity of prepared formulations was determined against S. aureus and P. aeruginosa in Muller–Hinton broth. The nutrient broth surface was inoculated by using a swab dipped in the bacterial cell suspension. Samples (plain drug, drug free and drug loaded nanofibers) were carefully placed on the agar surface for the evaluation and comparison of antimicrobial activity. The zones of inhibition were measured after 1 and 2 days.
In-vivo evaluation of nanofiber
The studies were carried out according to the guidelines of the Council for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, government of India. 32 Wistar Rats (all weighing 180–220 g) were randomized into 4 groups consisting of eight animals in each group. These four groups were designated for control (no treatment), plain drug, drug free nanofibers and cefazolin-loaded nanofibers. The animals were anesthetized with intramuscular injection of ketamine (75 mg/kg). After anesthetizing the animals, the dorsal skin region of the animals was shaved to remove any hair present on the skin. Following this, the animals were positioned prone on a surgical table, their back skin disinfected with betadine 10%, and the hair of an area of the skin preselected using a caliper were completely shaved with a razor to make a 2 cm long incision by means of a No. 24 scalpel; the depth of incision included both dermis and hypodermis. In each group, incised wounds were embedded with respective nanofiber wound dressings and the extent of wound healing was observed over a period of 15 days by visual observation. The wounds of rats were observed at a regular interval of every 5 days in order to observe the progress of wound healing until the wounds were healed completely.
Assay of collagen
Portion of regenerated tissue sample weighing 20 mg were defatted in chloroform: methanol (2:1) and dried in acetone before use. Collagen was estimated by the method of Woessner. Briefly, the samples were autoclaved for 6 h at 15 lb pressure. The hydrolysate is diluted to 10 ml, with distilled water and filtered. Prepared hydrolysate was further diluted with 5.0 ml assay buffer, followed by the addition of 2.5 ml of chloramine-T reagent and stand for 20 min at room temperature. To this add 2.5 ml of freshly prepared dimethylaminobenzaldehyde reagent and mix thoroughly and incubate the tubes at 600 °C for 15 min and cool in tap water for 5 min and the absorbance was measured at 550 nm. The collagen fraction was determined according to the following equation:
Histological analysis
For histological studies, the wound tissue specimens obtained on the 7th day and 14th day from all the four animal groups were fixed in 10% formalin solution for 24 h and dehydrated with a sequence of ethanol-xylene series of solutions. The materials were filtered and embedded with paraffin (40%–60%). Microtome sections were processed in alcohol-xylene series and stained with haematoxylin-eosin dye. The histological changes were observed under an optical microscope (Sundaramurthi et al. Citation2012).
Statistical analysis
Each experiment in in-vitro studies was done in triplicate, and the data were analyzed by one way analysis of variance using the GraphPad Prism software (Version 3.0) (CA, USA). P-value less than 0.05 were considered statistically significant.
Results and discussion
Characterization of nanofiber mats
The nanofibers morphology was determined by optical microscopy and SEM (). The results obtained from SEM studies were investigated and the average diameter of polymeric nanofibers was predicted. The diameter of blank nanofibers was in range of 620–660 nm and 650–680 nm for drug loaded nanofibers. The values of 15 kV (applied potential), 0.4 ml h−1 (flow rate) and the fixed distance of 15 cm as process parameters and polymer concentration of 5 wt.% in 2,2,2-trifluoroethanol as formulation parameters were identified as the optimum which produce uniform, non-beaded nanofibers with a mean diameter of 650 ± 30 nm. Improved structural morphology of nanofiber could be a function of solution conductivity and high dielectric constant of 2,2,2-trifluoroethanol. These forces increases the charge density which impart a high elastic force within the jet resulting a high degree of jet stretching resulting in smooth, bead less and ultrafine nanofibers. However, there were some irregularities in the form of bundles and junctions were observed could be attributed to incomplete drying of nanofiber owing to flow rate and relate lively small distance between the jet and collector. Drug loading did not have significant impact on fiber diameter.
Figure 1. In-vitro characterization [(A) SEM, (B) DSC and (C) XRD]. The figure to the left illustrates the characterization of plain drug and right side for drug loaded nanofibers.
![Figure 1. In-vitro characterization [(A) SEM, (B) DSC and (C) XRD]. The figure to the left illustrates the characterization of plain drug and right side for drug loaded nanofibers.](/cms/asset/ce36d814-dc12-4116-8f48-987024758f47/ianb_a_1102741_f0001_c.jpg)
shows DSC thermograms of pure drug and drug-loaded nanofiber. Phase transition temperature of the cefazolin as observed by DSC thermograms was found to be 175 °C. DSC thermograms of drug loaded nanofibers did not exhibit similar endothermic transitions to those observed with native drug demonstrated that incorporated drug was no longer present in its native structure. The phase transition peaks of the drug are broader and shifted towards low temperatures in electrospun nanofiber suggests that cefazolin was found to exist in the metastable state in the fibers, which could be related to spontaneous organization of drug molecules during electrospinning thereby prevent the formation of stable drug crystal. The results of DSC harmonized with those of XRD that the original structure of cefazolin was lost and it was completely transformed into an amorphous state in nanofibers. showed a comparison of all the three thermograms. Broad diffraction peaks appearing at a diffraction angle of 2θ=28.315° corresponding to native drug suggests an amorphous state of the encapsulating material. The existence of broad peak near the diffraction angle of 2θ=28.315° can also be verified by the XRD study.
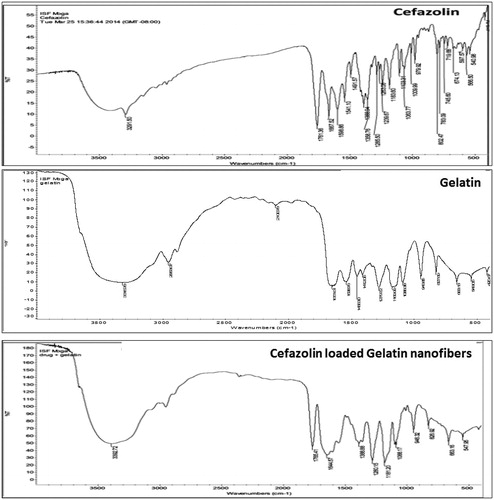
The IR spectra were studied for any kind of interactions or incompatibility between drug and excipients used in formulation. The peaks presented in 1241, 1183 and 1100 cm−1 represent the grouping CN stretch, while the peaks 1540 cm−1 are related to the stretching of the secondary amide NH grouping. The thiadiazole and tetrazole rings represent the tertiary amines and therefore do not present characteristic peaks in the infrared spectra. The IR spectra obtained for pure drug and drug loaded nanofiber () were compared, indicated no chemical interaction between drug and polymer. The presence of some integral peaks of cefazolin (1761, 1667, 1599 and 1541 cm−1) in loaded nanofiber clearly indicated the presence of drug component in the developed formulations.
FTIR study was conducted to investigate the possible structural changes in gelatin after cross-linking. The FTIR spectra of the cross linked gelatin fibers revealed similar infrared regions to plain gelatin nanofiber. The characteristics peaks at 1530–1545 cm−1 and 1630–1640 cm−1 for amide was observed which was distinguish feature of gelatin indicating no changes in chemical structure of gelatin after cross-linking. The cross-linked and plain gelatin fibers were discriminated by a change in color from white to pale yellow. The color change results due to aldimine linkage reaction during cross linking process.
Drug entrapment and drug loading efficiency
Entrapment efficiency of cefazolin in gelatin nanofibers was observed to be 93.7 ± 1.4%. The high entrapment efficiency could be related to the method of drug loading which prevent premature drug leaching, large throughput and a high mass transfer rate. The percent drug loading of the optimized batch was found to 24%–28%. Considering the experimental process employed in the fabrication of nanofiber, the drug loading capacity was found to be efficient. Higher drug pay load could be attributed to the intrinsic drug solubility where the finding suggested that cefazolin is freely soluble in TFE (≥910 mg/ml).
Swelling index
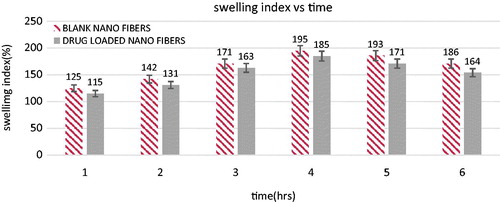
The degree of swelling of polymeric nanofibers plays a key role in the release of therapeutic molecules from the nanofiber mat. Gelatin nanofibers possess relatively high swelling efficacy than drug loaded fiber which could be related to gelatin content, fiber diameter, fiber arrangement and crosslinking time. comparing the drug loaded fiber plain gelatin fibers exhibited a smaller diameter, thereby exhibits a high absorption capacity. Further crosslinking of drug loaded gelatin nanofibers, liming the overall swelling efficiency. A little change in swelling capacity after 6 h in tested formulations suggested cross linked gelatin effectively surpassed the hydrolytic degradation of gelatin.
Bacteria penetration
The in-vitro bacterial permeability test demonstrated that drug loaded gelatin nanofiber dressing prepared by electrospinning method effectively prevent bacterial permeation. No CFU of tested strain was observed, suggesting that tested strain do not cross the membrane (). This could be attributed to pores size of the prepared nanofiber membrane. Crosslinking with glutaraldehyde vapors gives the smaller pore size (Jui-Yang Lai et al. Citation2013), which might be smaller than the size of selected strain (0.5 μm). Further impermeability for bacterial penetration would be related to the inherent antimicrobial property of drug loaded nanofiber.
Table I. Water permeability and bacterial permeability of cefazolin gelatin wound dressing.
Water vapor permeation
An ideal wound dressing would offer desired water evaporative rate to maintain the moist environment at the wound bed. As indicated in , the water loss from gelatin nanofiber was relatively higher than the drug loaded fiber. The rate of water vapor transmission from tested nanofibers consists of two steps adsorption and diffusion. In general, the rate of water permeation from nanofiber mat is inversely proportional to pore density and pore size. As crosslinking significantly reduce the pore size as well as pore density, thereby retard the rate of water transmission rate.
In vitro release studies and release kinetics
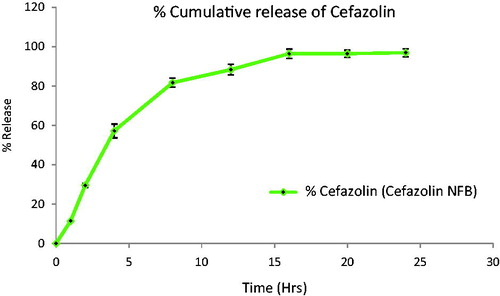
In-vitro release showed sustained and controlled release profile of cefazolin from cross linked gelatin nanofibers (). Initial burst release of cefazolin was observed due to surface adsorbed or near surface entrapped drug followed by constant pattern was seen up to 24 h with percentage cumulative release of 95.7 ± 1.5%. Shutting in a high initial burst release can be interpreted by the fact that polymeric swelling is compromised. Cross-linking of gelatin allowed the control of drug release of drug and the diffusion rate of dissolution medium into the cross linked matrix and the drug dissolution. Further, the release data were analyzed by mathematical model to understand the mechanism of drug release. The correlation coefficient (R2 value) was higher for zero order release suggested the mechanism of drug release follow the Fickian diffusion. The later part of drug release also depends on the concentration gradient and polymeric degradation.
In- vitro antibacterial activity
The antibacterial activity of prepared formulation against polymicrobial wound infections (S. aureus and P. aeruginosa) was determined by cup diffusion method. The results of antimicrobial activity are shown in . Plain gelatin nanofibers did not show any antimicrobial activity against the selected strains. Cefazolin-loaded nanofibers demonstrated excellent antimicrobial efficacy with an inhibition diameters of 2.7 ± 0.1 cm and 2.5 ± 0.1 cm for S. aureus and P. aeruginosa respectively after 48 h, whereas plain cefazolin produced zone of inhibition of 1.7 cm against tested bacterial strain. Higher antimicrobial activity of prepared nanofiber formulations than the free drug could be attributed to the pattern of drug release and higher drug solubility in nanofiber. Higher drug solubility and controlled drug release ensure high local antimicrobial concentration for an extended duration of release consequently showed higher inhibitory activity.
Table II. Zones of inhibition (antimicrobial activity) fabricated nanofibers against S. aureus and P. aeruginosa.
In vivo evaluation of nanofibers
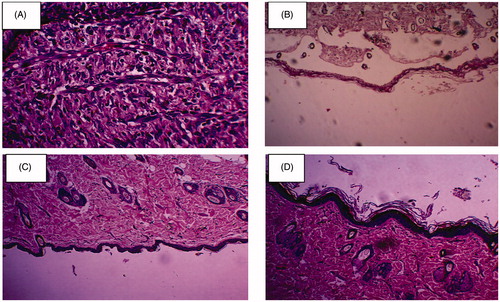
The physical estimation of wound healing can be clearly observed in . These figures depicted the process of healing of all the four animal groups. The wounds of rats were observed on every fifth day for the progress of healing. The area of the wound decreased considerably with the passage of time. The wounds observed in control group exhibited the slowest rate of wound healing. In case of unloaded gelatin mats, a descent healing potential was observed, which can be related to the integral efficiency of the biomaterial and its potential to provoke hemostasis (Miyata et al. Citation1988, Pendharkar and Gorman Citation2006). The biodegraded product of gelatin i.e. proline and glycine serve as an activator for the debriding enzymes such as metaloprotease which further facilitated the healing process (Francesko Citation2012). Highest rate of wound healing was observed in case of cefazolin-loaded nanofibers. Enhance wound healing in drug loaded nanofiber is related to controlled drug release characteristics ensured high local antimicrobial concentration at the wound bed for an extended duration. No scar formation was observed in case of nanofiber therapy, which indicated an accelerated and healthy healing. Enhanced healing process is further associated to semipermeable nature of nanofiber dressing provides a moist environment and protects the wound against infectious microorganism. Moreover, nanofiber due to its unique surface properties promotes cell attachment to accelerate the healing of cutaneous wounds. Collagen assay suggested that collagen content began to rise sharply in all experimental formulations from five days to a maximum at 15 days (). Drug loaded nanofiber exhibited higher collagen content could be related to hydrocolloid nature of gelatin, maintain moist environments that promote faster re-epithelialization by promoting wound contraction.
Figure 5. Physical estimation of wound healing at various time intervals in (A) control, (B) plain gelatin nanofibers, (C) cefazolin and (D) Cefazolin-loaded gelatin nanofibers.
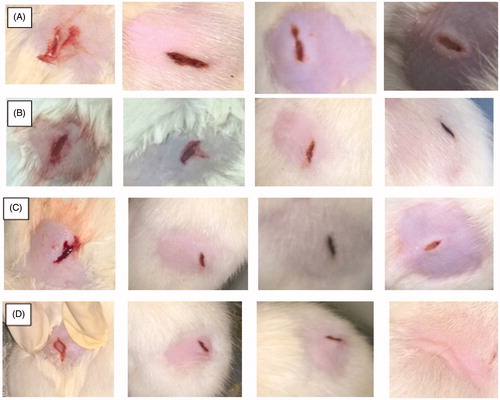
Table III. Collagen (mg) content per 100 mg of the wound bed.
Skin irritation test
None of the applied nanofiber mats showed any kind of hypersensitivity reactions on the skin. There was no sign of erythema/eschar and/or edema on the skin of rats, which indicates the biocompatibility of the fiber with the skin.
Histopathological analysis
Histological observations revealed that the bioadhesive, biodegradable cefazolin-loaded gelatin nanofibers could be a better alternative for post-operative wound care for small incisions. Results demonstrated that drug loaded nanofibers was able to provide better cell attachment between the wound edges with comparatively thicker keratin layer in comparison to plain cefazolin. showed the comparison of epithelial growth progression in control and nanofibers treated groups. Groups treated with drug loaded nanofibers showed an accelerated of epithelial generation as depicted by a rich keratinized network on the 10th day of treatment, while a shattered and incomplete epithelium was observed in other test samples. Accelerated epithelial regeneration in drug loaded gelatin nanofibers can be credited to extended antimicrobial activity and anti-inflammatory properties resulting in reduced reactive cell infiltration, which further aided in the proliferation of fibrous connective tissue and sequential regeneration of keratin layer. The numbers of reactive cells markedly reduce in groups treated with drug loaded nanofibers. This could be explained by the semipermeable nature of prepared nanofiber and the biological function of gelatin which act as a cofactor for the matrix metalloproteinase enzymes, which are thought to play a major role in the proliferation, migration (adhesion/dispersion), differentiation and angiogenesis of cells involved in wound healing (Kirsner and Eaglstein Citation1993, Knighton et al. Citation1981).
Conclusion
Gelatin nanofibers containing cefazolin were successfully fabricated by electrospinning technique. Characterization of prepared formulations displayed excellent drug entrapment efficiency with controlled drug release pattern. Nanofiber due to its unique surface properties acting as a semi-permeable barrier and helps in protecting wounds against bacterial contamination. Broad spectrum antibacterial activity of the prepared formulation could be successfully used to treat variety of infected wound types. In addition to antibacterial activity, gelatin nanofiber due to its close similarity with extra cellular matrix promotes cell adhesion and other biological useful to improve dermal wound healing. Results suggested that cefazolin-loaded gelatin nanofiber could serve as an appropriate dressing material to achieve optimal wound healing.
Declaration of interest
The authors Dr. Goutam Rath and Dr. Amit K Goyal are thankful to the Department of Biotechnology (DBT), New Delhi (F.No. 102/IFD/SAN/2577/2011-2012 dated September 28, 2011) and Punjab Technical University, Kapurthala, Punjab for providing financial assistance to carry out research.
References
- Bowler P, Duerden B, Armstrong D. 2001. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 14:244–269.
- Chaudhary C, Garg, T. 2015. Scaffolds: a novel carrier and potential wound healer. Crit Rev Ther Drug Carrier Syst. 32:277–321.
- Chen JP, Chang GY, Chen JK. 2008. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloid Surface A. 313:183–188.
- Chong EJ, Phan TT, Lim IJ, Zhang YZ, Bay BH, Ramakrishna S, Lim CT. 2007. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 3:321–330.
- Francesko A. 2012. Multifuncional biopolymer-bases materials for modulatig the activites of chronic wound enzymes. [dissertation]. [Barcelona (Spain)]: Universitat Politécnica de Catalunya.
- Fu R, Li C, Yu C, Xie H, Shi S, Li Z, Wang Q, Lu L. 2014. A novel electrospun membrane based on moxifloxacin hydrochloride/poly(vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Deliv. [Epub ahead of print]. DOI:10.3109/10717544.2014.918676.
- Gagandeep, Garg T, Malik B, Rath G, Goyal AK. 2014. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 53:10–16.
- Garg T, Kumar A, Rath G, Goyal AK. 2014a. Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit Rev Ther Drug Carrier Syst. 31:531–557.
- Garg T, Rath G, Goyal AK. 2014b. Ancient and advanced approaches for the treatment of an inflammatory autoimmune disease-psoriasis. Crit Rev Ther Drug Carrier Syst. 31:331–364.
- Garg T, Rath G, Goyal AK. 2015. Colloidal drug delivery systems: current status and future directions. Crit Rev Ther Drug Carrier Syst. 32:89–147.
- Garg T, Singh O, Arora S, Murthy R. 2012. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Goyal G, Garg T, Rath G, Goyal AK. 2014. Current nanotechno-logical strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst. 31:365–405.
- Gupta P, Garg T, Tanmay M, Arora S. 2015. Polymeric drug-delivery systems: role in p-gp efflux system inhibition. Crit Rev Ther Drug Carrier Syst. 32:247–275.
- Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. 2003. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 63:2223–2253.
- Lai JY, Ma DH, Lai MH, Li YT, Chang RJ, Chen LM. 2013. Characterization of cross-linked porous gelatin carriers and their interaction with corneal endothelium: biopolymer concentration effect. PLoS One. 8:e54058.
- Kataria K, Gupta A, Rath G, Mathur RB, Dhakate SR. 2014. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int J Pharm. 469:102–110.
- Kaur M, Garg T, Rath G, Goyal AK. 2014a. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst. 31:49–88.
- Kaur R, Garg T, Rath G, Goyal AK. 2014b. Advanced aerosol delivery devices for potential cure of acute and chronic diseases. Crit Rev Ther Drug Carrier Syst. 31:495–530.
- Kim SE, Heo DN, Lee JB, Kim JR, Park SH, Jeon SH, Kwon IK. 2009. Electrospun gelatin/polyurethane blended nanofibers for wound healing. Biomed Mater. 4:044106.
- Kirsner R, Eaglstein W. 1993. The wound healing process. Dermatologic clinics. 11:629–640.
- Knighton D, Silver I, Hunt T. 1981. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 90:262–270.
- Liao S, Li B, Ma Z, Wei H, Chan C, Ramakrishna S. 2006. Biomimetic electrospun nanofibers for tissue regeneration. Biomed Mater. 1:R45–53.
- Malik R, Garg T, Goyal AK, Rath G. 2015. Diacerein-loaded novel gastroretentive nanofiber system using PLLA: development and in vitro characterization. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI:10.3109/21691401.2014.10004921.
- Miyata T, Kodaira K, Furuse M, Noishiki Y. 1988. Hemostatic agent composed of collagen/gelatin and protamine. Google Patents.
- Modgill V, Garg T, Goyal AK, Rath G. 2014. Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI:10.3109/21691401.2014.924007.
- Morie A, Garg T, Goyal AK, Rath G. 2014. Nanofibers as novel drug carrier - an overview. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI:10.3109/21691401.2014.927879.
- Pendharkar S, Gorman A. 2006. Method of providing hemostasis to a wound. Google Patents.
- Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, et al. 2006. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 27:1452–1461.
- Sharma R, Garg T, Goyal AK, Rath G. 2014a. Development, optimization and evaluation of polymeric electrospun nanofiber: a tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI:10.3109/21691401.2014.966194
- Sharma R, Singh H, Joshi M, Sharma A, Garg T, Goyal AK, Rath G. 2014b. Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst. 31:187–217.
- Silverman RA, Lender J, Elmets CA. 1989. Effects of occlusive and semiocclusive dressings on the return of barrier function to transepidermal water loss in standardized human wounds. J Am Acad Dermatol. 20:755–760.
- Singh B, Garg T, Goyal AK, Rath G. 2015. Development, optimization, and characterization of polymeric electrospun nanofiber: a new attempt in sublingual delivery of nicorandil for the management of angina pectoris. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI:10.3109/21691401.2015.1052472.
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. 2014. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol. 43:263–269.
- Smith L, Ma P. 2004. Nano-fibrous scaffolds for tissue engineering. Colloid Surface B. 39:125–131.
- Son WK, Youk JH, Park WH. 2006. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr Polym. 65:430–434.
- Sundaramurthi D, Vasanthan KS, Kuppan P, Krishnan UM, Sethuraman S. 2012. Electrospun nanostructured chitosan–poly (vinyl alcohol) scaffolds: a biomimetic extracellular matrix as dermal substitute. Biomed Mater. 7:045005.
- Tanaka A, Nagate T, Matsuda H. 2005. Acceleration of wound healing by gelatin film dressings with epidermal growth factor. J Vet Med Sci. 67:909–913.
- Ueno C, Hunt TK, Hopf HW. 2006. Using physiology to improve surgical wound outcomes. Plast Reconstr Surg. 117:59S–71S.
- Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. 2006. Crosslinking of the electrospun gelatin nanofibers. Polymer. 47:2911–2917.
- Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ. 2003. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr, Metab Care. 6:229–240.
- Zhu X, Cui W, Li X, Jin Y. 2008. Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules. 9:1795–1801.