Abstract
To study the effects of keratinocyte growth factor (KGF) modified umbilical cord mesenchymal stem cells (MSC) on chronic liver injury in rats, adenovirus carrying human KGF gene (Ad-KGF) was used. Rat liver injury model was established by subcutaneous injection of CCl4 - olive oil solution; 56 male Wistar rats were randomly divided into normal control, model, MSC, KGF, and KGF/MSC groups. Of all three treatments, KGF/MSC had the most obvious therapeutic effects on liver injury, and cell injections did not cause adverse reaction. The experiment provides new data for clinical research of KGF/MSC and possible methods for liver injury treatment.
Introduction
Viral infection, alcoholism, drugs, and other factors can cause liver injury, which often leads to chronic liver diseases with cell degeneration, necrosis, and fibrosis as the main pathological changes, and is eventually developed into irreversible liver cirrhosis or even liver cancer in many patients, seriously damaging their life and health. Early liver injury and fibrosis can be reversed, so it is of great significance to promote liver cell regeneration and to resist fibrosis. Hepatocyte transplantation (Pietrosi et al. Citation2009) brings hope for the treatment of liver diseases, but the transplanted hepatocytes cannot proliferate well due to graft rejection and apoptosis. In addition, the limited sources of donor liver cells also need effective methods for in vitro amplification.
Keratinocyte growth factor (KGF) regulates mesenchymal-epithelial interaction. Binding its receptor KGFR, KGF plays an important role in formation, development, differentiation and repair of a variety of tissues and organs, including skin, intestines, stomach, lung, prostate, breast, cornea, thymus, and so on (Choate and Khavari Citation1997, Epstein et al. Citation2001, Jeschke et al. Citation2002, Levy et al. Citation1996). At present, KGF has shown significant therapeutic effects on various diseases, and new KGF-based treatments are constantly emerging. However, the role of KGF in liver injury treatment and its mechanism remain unknown. Therefore, we herein used KGF to treat liver injury based on laboratory research.
Keratinocyte growth factor protein is restricted from exerting desired therapeutic effects due to short biological half-life, poor tissue specificity, and instability because of considerable protein hydrolases in injured tissues (Guo et al. Citation1996, Werner et al. Citation1994). Mesenchymal stem cells (MSCs) are a group of pluripotent stem cells with self-renewal and multi-directional differentiation potential derived from mesoderm, which are widely distributed in various tissues, such as bone marrow, umbilical cord (UC), fat, embryo lung, and embryo kidney (Chien et al. Citation2006, Huang Citation2007). Umbilical cord, as a medical waste, is abundant in sources. It can be simply collected without damaging the donor. Moreover, it is less prone to contamination of microorganisms and tumor cells, and it does not involve social, ethical and legal debates also. Therefore, UC-MSC is expected to become the focus of cell therapy and tissue engineering. We have successfully isolated and cultured MSC from UC, and identified its biological characteristics.
In this study, we observed the therapeutic effects of KGF gene-modified human UC-MSC on liver injury by establishing a rat model of carbon tetrachloride (CCl4)-induced chronic liver injury, in order to provide experimental basis for treatment of liver injury with gene-modified stem cells.
Experimental
Materials
Male Wistar rats were provide by the animal center of our hospital. The experimental animals were level 2 (cleaning grade). The animals had pellet feed and drank water freely. The disposal of animals in experiments was in accordance with the animal ethics standards. Human UC-MSCs were separated and stored in our laboratory; adenovirus carrying green fluorescent protein (Ad-GFP) and adenovirus carrying human KGF gene (Ad-KGF) were established and preserved in our laboratory.
The main reagents and apparatus used in this study included high sugar α-MEM medium and pancreatin (Gibco, NY, USA); Fetal bovine serum (Beijing Siji Qingqiao Company, Beijing, China); CD73-PE, CD105-PE, CD45-PE, CD34-FITC, CD19-FITC, HLA-DR-PE, CD90-PE, and CD11b-PE (BD Bioscience, CA, USA); KGF detection kit (R&D Company); CCl4 and olive oil (Shanghai Haohong Chemical Science and Technology Co., Ltd, Shanghai, China); Malondialdehyde (MDA) detection kit and hydroxyproline (HYP) detection kit (Sigma-Aldrich, Seelze, Germnay). ELISA kit (BioTek, Luzern, Switzerland); Flow cytometer (Becton-Dickinson, MD, USA); CO2 incubator (Thermo Scientific, MD, USA); clean bench (Beijing First Transistor Equipment Plant, Beijing, China); −80 °C ultra-low Temperature Freezer (Sanyo, Tokyo, Japan); Inverted optical microscope (Olympus, Tokyo, Japan).
MSC culture and cell phenotypic characterization
Frozen human UC-MSCs were taken and added into MSC culture medium after recovery. Then the cells were cultured in an incubator with 5% CO2 atmosphere at 37 °C. The cells were digested, counted, and moved into 1.5 mL EP tubes with 2 × 105/tube. PBS buffer (1 mL) was used to wash the cells. Then the solution was centrifuged at 1500 r/min for 5 min and the supernatant was discarded, which was repeated twice. PBS (100 μL) was added into each tube for re-suspension. Mouse anti-human antibody (CD19, CD73, CD105, HLA-DR, CD11b, CD34, CD45 or CD90) (2 μL) was added, respectively, and then the tube was kept in dark for 30 min. dPBS buffer (1 mL) was added, and then the solution was centrifuged at 1500 r/min for 5 min and the supernatant was discarded, which was repeated twice. The cells were re-suspended in 500 μL of PBS and flow cytometry was used to detect cell surface markers.
KGF gene transfection and detection
Mesenchymal stem cells were inoculated into 6-well culture plate with a density of 1.0 × 105/well. After 24 h of culture, the medium was decreased to 1 mL. Then Ad-KGF with multiplicity of infection (MOI) of 0, 50, 100, 150, 200, and 300 were used to infect MSC. Four hours later, 1 mL of culture medium was added and the cells were cultured in the incubator with 5% CO2 atmosphere at 37 °C. After 48 h of culture, positive cells were observed, counted, and photographed under a fluorescence microscope.
Variation of gene-modified KGF expression with time identified by ELISA
Mesenchymal stem cells were inoculated into 6-well culture plate with a density of 1.0 × 105/well. After 24 h of culture, 1 mL of Ad-KGF (150 MOI) medium was added. Four hours later, 1 mL of culture medium was added and the cells were cultured in the incubator with 5% CO2 atmosphere at 37 °C. The supernatant was collected every 48 h and frozen at -80 °C, and then the medium was changed. This step was continued till the 8th day. KGF ELISA kit was used to detect the expression of KGF in the culture supernatant of MSC and its variation with time was analyzed.
Establishment and identification of animal models
Fifty-six male Wistar rats (body weight of about 200 g) were randomly divided into normal control group and experimental groups. The rat liver injury model was established with CCl4. On Day 1, each rat was given 40% CCl4-olive oil (5 mL/kg) through subcutaneous injection. Then the rats were weighed and given 40% CCl4-olive oil (3 mL/kg) through subcutaneous injection twice every week till the end of the treatment. The animals had high-fat, low-protein feed (pure corn flour mixed with 20% lard and 0.5% cholesterol) and 0.5% ethanol as drinking water. Four weeks after modeling, 4 rats from the experiment groups and the normal control group were killed. The gross morphology of the liver was observed, and the serum biochemical indices were detected to evaluate the modeling effects.
Grouping and treatment of animals
After success modeling, the model rats were grouped randomly. The group and treatment information is shown in . The rats were administrated through the caudal vein. Virus injected in Ad-KGF (1.5 × 108 pfu) was equal to Ad-KGF in MSC (1 × 106) infected by 150 MOI.
Table I. Experimental groups and administration.
Determination on survival rate and liver index of rats
At the end of the 8th week, namely the 5th week after the treatment, GraphPad Prism 5 (CA, USA) was used to calculate rat survival rate and to plot survival rate curve. The animals were weighed and anesthetized by 1% sodium pentobarbital (30 mg/kg) through intraperitoneal injection. Then the rats were taken for blood from the femoral artery. The rats were sacrificed, and the livers were removed immediately and weighed. Rat liver index (LI) in each group was calculated according to the following formula: LI = Liver weight (g)/body weight (g) × 100%.
Detection on serum liver biochemical indices
At the end of the 8th week, namely the 5th week after the treatment, 0.5 mL of blood was taken from the rat femoral artery and centrifuged at 1500 r/min for 5 min. Serum (200 μL) was taken and sent to Beijing Zhongtong Lanbo Clinical Inspection Institute. Automatic biochemical analyzer was used to detect the levels of alanine aminotransferase (AST), aspartate aminotransferase (ALT), albumin (ALB), and total bilirubin (TBILI) in the serum of each group.
Determination on MDA and HYB in liver tissues
Rat liver tissue (0.5 g) was taken and added with 4.5 mL of saline. Then it was blended in a glass homogenizer and prepared to a 10% tissues homogenate. After centrifugation at 3000 r/min and 4 °C for 10 min, the supernatant was taken to detect MDA and HYP contents in liver tissues according to the kit instructions.
Observation on morphology of liver tissue
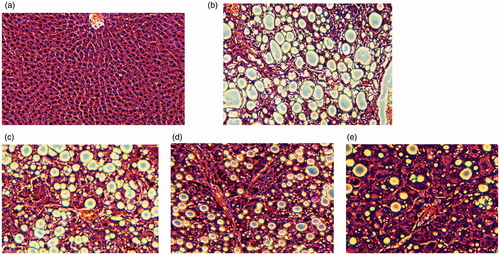
Rat liver tissue was fixed with 10% formalin, embedded with paraffin, sectioned at 4∼6 mm thickness and stained by HE. Morphological changes of liver tissue were observed under an optical microscope (×100) according to the number of fat vesicle and the changes of hepatic lobule structure after the fat pathological changes of cells in liver tissue.
Statistical analysis
Experimental data were represented by x±s. With GraphPad Prism 5, one-way analysis of variance was adopted for statistical analysis. Duncan’s t-test was used for comparison between groups.
Results
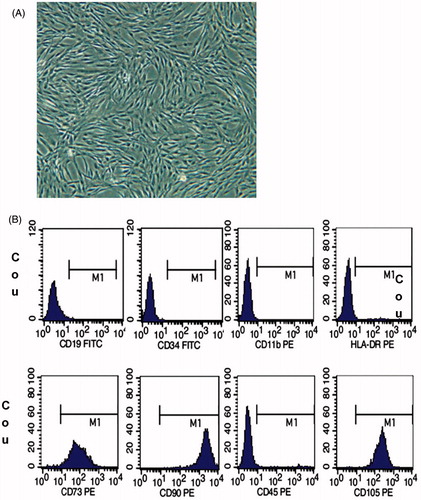
Results of UC-MSC morphology and cell phenotypic characterization
As shown in , the cultured cells grow adhering to the wall, in spindle shapes. Flow cytometry showed that CD73, CD90, and CD105 were positive, while CD19, CD34, CD45, CD11b, and HLA-DR were negative (). Cell morphology and surface markers indicated that the cultured cells were MSCs.
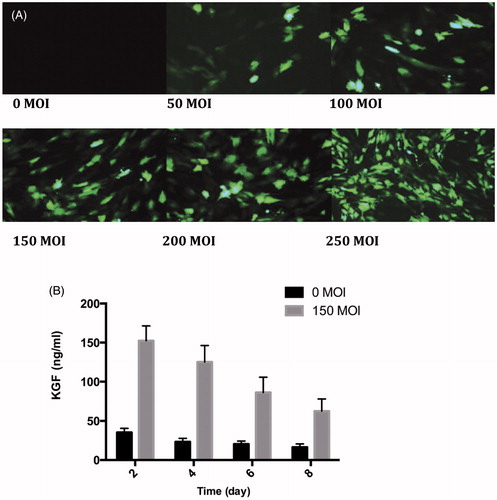
Infection efficiency of Ad-GFP to MSC
Infectious effects of Ad-GFP with different MOIs on MSC were observed under the fluorescence microscope. As shown in , there is no green fluorescence in the control group. With increasing concentration of Ad-GFP, more MSCs expressed GFP. When the infection concentration was 150 MOI, MSC infection rate reached more than 90%. When the concentration was further elevated, the positive rate only mildly increased. Ad-GFP-MSC expressed GFP and the positive rate reached over 90% at 150 MOI, hence the best infection concentration of Ad-KGF was 150 MOI.
KGF expressions of gene-modified MSCs and its variation with time
ELISA kit was used to detect KGF expression in culture supernatant in Ad-GFP-MSC (). After transfection of Ad-KGF, the amount of KGF secreted by MSC was significantly increased. The expression level, which was highest (152.35 ± 19.12 ng/mL) 48 h after infection, decreased and remained thereafter for more than 8 d. Transfection of Ad-KGF allowed short-term and efficient over-expression of KGF in MSC.
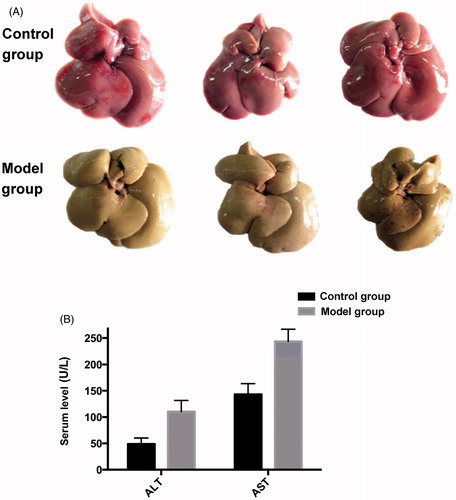
Establishment of the hepatic fibrosis damage model
During modeling, when the rats were injected with 40% CCl4 - olive oil solution, the body weight was gradually decreased and the fur was shaggy without luster; 4 weeks after modeling, the liver volume was increased with fine sandy surface, and the color was yellowish brown (). The liver index was elevated and serum ALT and AST activities were enhanced significantly (p<0.01) (), indicating the rat liver injury model was successfully established.
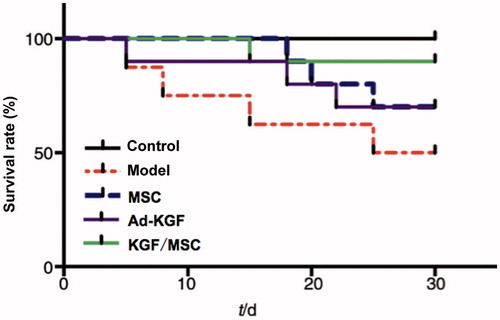
Rat survival rate, body weight and liver index
After 4 weeks of treatment, the results are shown in . Compared with the model group, body weights of animals in three treatment groups were significantly increased and the liver index was significantly decreased (p<0.01). The survival rate was only 50% in the model group at the end of the experiment, while survival rates of three treatment groups were obviously increased, 70% in MSC and Ad-KGF group and 90% in KGF/MSC group (). The three treatments all mitigated the liver damage caused by CCl4 and augmented the survival rate of animals, of which KGF/MSC showed the best therapeutic effects.
Table II. Rat body weights and liver indices after treatment (x±s).
Rat serum liver function indices
Liver function index determination results are shown in . Compared with the model group, AST, ALT, and TBILI were significantly decreased in the KGF/MSC group, while ALB was significantly increased; in the Ad-GF group, only AST was obviously decreased (p<0.05), ALT and TBILI were slightly decreased while ALB was increased, but there were no statistically significant differences; in the MSC group, serum liver function indices were also improved to different extents without statistical significant differences. Individual MSC and Ad-KGF treatments did not evidently improve the liver function after liver injury, while KGF/MSC treatment significantly did so.
Table III. Rat serum liver functions after 4 weeks of treatment (x±s).
Rat liver MDA and HYP results
Liver MDA and HYP results are shown in . Compared with the model group, liver MDAs of the treatment groups were significantly reduced (p<0.01), suggesting that all the three treatments reduced the peroxidation of liver tissue. After treatment, liver HYP was significantly decreased in KGF/MSC and MSC groups compared with that in the model group (p<0.01), but there were no statistically significant differences between Ad-KGF and model groups. Therefore, KGF/MSC and MSC therapy also prevented fibrotic reaction caused by liver injury.
Table IV. Rat liver MDA and Hyp contents (x±s).
HE staining results of pathological sections of rat liver tissue
Liver cells in the normal group were arranged evenly and compactly and the liver lobule structure was normal; animal liver sections of the model group () showed that the liver lobule was damaged with severe fat pathological change and a large amount of fat vacuoles. After treatment, liver fat pathological changes were reduced in MSC () and Ad-KGF groups () and the number of fat vacuoles was decreased; liver fat pathological changes were significantly reduced in the KGF/MSC group. The number of fat vacuoles was decreased significantly and lobular structures were partly recovered. Thus, all the three treatments relieved the pathological injury and fatty degeneration of liver, which contributed to the recovery of liver structure. Similarly, the therapeutic effects of KGF/MSC were best.
Discussion
Currently, chronic liver injury is mainly treated through prevention of degeneration and necrosis of liver cells by eliminating pathogenic factors, controlling diet and applying some traditional Chinese medicine ingredients with hepatoprotective function (Jang et al. Citation2004, Zhao et al. Citation2005), but these treatments lack the specificity. Chronic liver injury, if not treated timely, can be developed into chronic liver fibrosis and cirrhosis of the liver and even cancer. KGF, which is a growth factor secreted by mesenchymal cells including fibroblasts and endothelial cells, is a single-stranded protein molecule with the molecular weight of about 28 kD. One of the physiological functions of KGF discovered earliest is promoting proliferation of epidermal keratinocytes. Subsequently, KGF has been reported to play important roles in many physiological processes including proliferation, differentiation, migration, and injury repair of skin cells (Froget et al. Citation2003, Geer et al. Citation2005). KGF can promote proliferation of pulmonary epithelial cells during in vivo and in vitro experiments, as well as protect the lung tissue from various acute and chronic lung injuries caused by bleomycin, Bernstein test, high oxygen content, bacterial infection, and radiation. Following unclear mechanisms, KGF functions mainly by regulating cell proliferation and differentiation, synthesis and secretion of surface active substances, ion exchange and DNA repair, by maintaining epithelial barrier function and by reducing inflammation (Widera et al. Citation2003, Wu et al. Citation2011, Xu et al. Citation2006). mRNAs are expressed abundantly in KGF and KGFR of the intestinal tract, and KGF participates in regulation of a variety of physiological functions in intestinal tissues (Jonas et al. Citation2003, Visco et al. Citation2009). Egger et al. (Citation1998) found in a rat colectomy model that injection of KGF alleviated inflammatory reaction in the process of intestinal wound healing, increased the amount of acid mucin, and accelerated the healing process.
In addition to the above tissues and organs, KGF has certain effects on ovary, pancreas, urinary tract, breast, prostate, and tumors. KGF can, for example, promote the transformation of primordial follicles to primary follicles and induce the ovarian development and maturation (Kezele et al. Citation2005); promote pancreatic duct mitosis, and accelerate islet beta cell regeneration in diabetic rats (Movassat and Portha Citation2007); and induce proliferation of mouse urothelial cells and maintain the integrity of the urothelium (Bassuk et al. Citation2003). The role of KGF in tumor is not clear at present. KGF does not have obvious effect on colorectal cancer, prostate cancer or bladder cancer cells, but it can promote proliferation, migration, and adhesion of some stomach cancer and breast cancer cells (Finch and Rubin Citation2006).
Keratinocyte growth factor has never been employed to treat liver injury hitherto due to short biological half-life, poor tissue specificity, and instability because of copious protein hydrolases in the injured tissue. In recent years, stem cell research brings hope for the treatment of chronic liver injury. MSC implantation into animals with liver injury can reduce liver cell damage, elevate survival rate, and inhibit fibrosis formation (Cho et al. Citation2011, Tsai et al. Citation2009). In addition, most of the MSCs administrated via vein enter liver tissue, and MSCs have the chemotaxis of tissue damage. Therefore, MSCs can repair tissue injury and carry gene-based drugs to injury site to play dual roles (Deuse et al. Citation2009). This study showed that implantation of MSC, KGF, and KGF/MSC into animals with liver injury improved the survival rate and liver function of animals, reduced liver tissue damage significantly, and inhibited the formation of liver fibrosis. The therapeutic effects of KGF-MSCs were most obvious. In other words, KGF was herein successfully applied to treat liver disease.
Molecular indices of KGF treatment for liver injury are not comprehensive, and the relevant molecular mechanisms are not clear. KGF may treat and repair CCl4-induced rat liver injury following the processes: KGF inhibits the formation and development of inflammation by regulating inflammation; KGF promotes liver cell proliferation and repair of DNA damage, thus promoting the repair of injured liver cells and liver tissue; KGF induces vascular endothelial growth factor. At the same time, the experimental results provide experimental basis for liver injury treatment by using KGF-MSC, and inspire combination of gene therapy with cell therapy in the treatment of liver disease.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bassuk JA, Cochrane K, Mitchell ME. 2003. Induction of urothelial cell proliferation by fibroblast growth factor-7 in RAG1-deficient mice. Adv Exp Med Biol. 539:623–633.
- Chien CC, Yen BL, Lee FK, Lai TH, Chen YC, Chan SH, Huang HI. 2006. In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells. 24:1759–1768.
- Cho KA, Lim GW, Joo SY, Woo SY, Seoh JY, Cho SJ, Han HS, Ryu KH. 2011. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Liver Int. 31:932–939.
- Choate KA, Khavari PA. 1997. Direct cutaneous gene delivery in a human genetic skin disease. Human Gene Ther. 8:1659–1665.
- Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH, et al. 2009. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 120:S247–S254.
- Egger B, Tolmos J, Friess H, Sarosi I, Eysselein VE, Büchler MW. 1998. [Keratinocyte growth factor significantly improves healing of left-sided colon anastomoses]. Langenbecks Arch Chir Suppl Kongressbd. 115:95–99.
- Epstein SE, Fuchs S, Zhou YF, Baffour R, Kornowski R. 2001. Therapeutic interventions for enhancing collateral development by administration of growth factors: basic principles, early results and potential hazards. Cardiovasc Res. 49:532–542.
- Finch PW, Rubin JS. 2006. Keratinocyte growth factor expression and activity in cancer: implications for use in patients with solid tumors. J Natl Cancer Inst. 98:812–824.
- Froget S, Barthelemy E, Guillot F, Soler C, Coudert MC, Benbunan M, Dosquet C. 2003. Wound healing mediator production by human dermal fibroblasts grown within a collagen-GAG matrix for skin repair in humans. Eur Cytokine Netw. 14:60–64.
- Geer DJ, Swartz DD, Andreadis ST. 2005. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am J Pathol. 167:1575–1586.
- Guo L, Degenstein L, Fuchs E. 1996. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev 10:165–175.
- Huang HI. 2007. Isolation of human placenta-derived multipotent cells and in vitro differentiation into hepatocyte-like cells. Curr Protoc Stem Cell Biol. Chapter 1, Unit 1E 1.
- Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. 2004. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 6:532–539.
- Jeschke MG, Richter G, Höfstädter F, Herndon DN, Perez-Polo JR, Jauch KW. 2002. Non-viral liposomal keratinocyte growth factor (KGF) cDNA gene transfer improves dermal and epidermal regeneration through stimulation of epithelial and mesenchymal factors. Gene Ther. 9:1065–1074.
- Jonas CR, Gu LH, Nkabyo YS, Mannery YO, Avissar NE, Sax HC, Jones DP, Ziegler TR. 2003. Glutamine and KGF each regulate extracellular thiol/disulfide redox and enhance proliferation in Caco-2 cells. Am J Physiol Regul Integr Comp Physiol. 285:R1421–R1429.
- Kezele P, Nilsson EE, Skinner MK. 2005. Keratinocyte growth factor acts as a mesenchymal factor that promotes ovarian primordial to primary follicle transition. Biol Reprod. 73:967–973.
- Levy MY, Barron LG, Meyer KB, Szoka FC. Jr 1996. Characterization of plasmid DNA transfer into mouse skeletal muscle: evaluation of uptake mechanism, expression and secretion of gene products into blood. Gene Ther. 3:201–211.
- Movassat J, Portha B. 2007. Early administration of keratinocyte growth factor improves {beta}-cell regeneration in rat with streptozotocin-induced diabetes. J Endocrinol. 195:333–340.
- Pietrosi G, Vizzini GB, Gruttadauria S, Gridelli B. 2009. Clinical applications of hepatocyte transplantation. World J Gastroenterol. 15:2074–2077.
- Tsai PC, Fu TW, Chen YM, Ko TL, Chen TH, Shih YH, Hung SC, Fu YS. 2009. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transplant. 15:484–495.
- Visco V, Bava FA, d'Alessandro F, Cavallini M, Ziparo V, Torrisi MR. 2009. Human colon fibroblasts induce differentiation and proliferation of intestinal epithelial cells through the direct paracrine action of keratinocyte growth factor. J Cell Physiol. 220:204–213.
- Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, Williams LT. 1994. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 266:819–822.
- Widera A, Beloussow K, Kim KJ, Crandall ED, Shen WC. 2003. Phenotype-dependent synthesis of transferrin receptor in rat alveolar epithelial cell monolayers. Cell Tissue Res. 312:313–318.
- Wu H, Suzuki T, Carey B, Trapnell BC, McCormack FX. 2011. Keratinocyte growth factor augments pulmonary innate immunity through epithelium-driven, GM-CSF-dependent paracrine activation of alveolar macrophages. J Biol Chem. 286:14932–14940.
- Xu JF, Qu JM, He LX, Ou ZL. 2006. Impaired upregulation of keratinocyte growth factor in injured lungs induced by Pseudomonas aeruginosa in immunosuppressed rats. Chin Med J. 119:1421–1429.
- Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. 2005. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 11:3431–3440.