Abstract
The present study reports the simple, inexpensive, eco-friendly synthesis of silver nanoparticles (AgNPs) using coconut oil cake extract. Scanning electron microscopy–energy dispersive spectroscopy peak at 3 keV confirmed the presence of silver. Transmission electron micrograph showed that nanoparticles are mostly circular with an average size of 10–70 nm. The results of the X-ray powder diffraction analysis (2θ = 46.2, 67.4 and 76.8) indicated the crystal nature of the AgNPs. Fourier transform infrared spectroscopy analysis indicates that proteins present in the oilcake extract could be responsible for the reduction of silver ions. The synthesized AgNPs (1–4 mm) reduced the growth rate of multi-antibiotic-resistant bacteria such as Aeromonas sp., Acinetobacter sp. and Citrobacter sp. isolated from livestock wastewater.
Introduction
Silver nanoparticles (AgNPs) have wide application in different scientific fields, including environmental sciences. Numerous physical and chemical methods have been developed for the large-scale synthesis of AgNPs (Velmurugan et al. Citation2011). Unfortunately, these conventional methods are expensive and some of the chemicals used in AgNP synthesis may pollute the environment (Sastry et al. Citation2003). Thus, there is a growing need for an eco-friendly synthesis of AgNPs.
With advances in nanobiotechnology, biological synthesis of AgNPs has been proposed as an alternative to physical and chemical processes and offers budding opportunities for eco-friendly synthesis of AgNPs. Several biological materials, such as microorganisms, plant extracts and milk have been used for the synthesis of AgNPs (Ahmad et al. Citation2003, Ananda Babu and Gurumallesh Prabu Citation2011, Lee et al. Citation2013, Otari et al. Citation2012, Sengottaiyan et al. Citation2015, Venkatpurwar and Pokharkar Citation2011). Numerous studies reported the potential application of these biologically synthesized AgNPs in different scientific fields (Kim et al. Citation2007, Sun et al. Citation2011). However, several disadvantages, such as mass cultivation of microorganisms, preparation and purification of plant extracts and the methodological procedures had reduced the application in large scale. Hence, to overcome these limitations, economic and easily available agro-industrial waste, oil cake was used for the synthesis of AgNPs.
In India, various types of oil cakes are produced on a large scale, as a by-product of oil manufacturing industry (Singh et al. Citation2003). Coconut oil cake (COC) is a byproduct obtained after oil extraction from dried copra. It contains starch, soluble sugars, proteins, lipids and a trace amount of nitrogen (Ramachandran et al. Citation2004). Apart from animal fed it is generally used as a substrate for the production of several extracellular microbial enzymes (Benjamin and Pandey Citation1996, Sabu et al. Citation2002, Selvakumar et al. Citation1998), a biostimulating agent for the heavy metal removal (Govarthanan et al. Citation2014a). The byproducts of Cocos nucifera has been used for the synthesis of gold nanoparticle (Roopan and Elango Citation2015), lead nanoparticles (Elango and Roopan Citation2015) and AgNPs (Roopan et al. Citation2013). However, there is no report for the synthesis of AgNPs using COC.
Bacterial resistance to antibiotics is considered as a most important public health problem because of their potential effect on the ecosystem and human health (Kummerer Citation2009). Several disinfection processes have been developed to inactivate the antibiotic resistant bacteria (ARB) present in the wastewaters (Panacek et al. Citation2006). A great deal of research reported that AgNPs had both antibacterial and antifungal activity. However, only limited studies reported the antibacterial activity of AgNPs against ARB. AgNPs exhibited bactericidal activity against multidrug-resistant Pseudomonas aeruginosa, Escherichia coli and Streptococcus pyogenes. A better understanding of antibacterial activity is a critical prerequisite for the development for AgNPs-based wastewater disinfection. Hence, the objectives of the present study were: (i) to synthesize AgNPs using COC extract, (ii) characterization of synthesized AgNPs and (iii) to assess the antibacterial activity of biologically synthesized AgNPs toward ARB isolated from livestock wastewaters.
Materials and methods
Oil cake extraction
COC was procured from a local market in Mallasamudram, Namakkal, Tamil Nadu, India. The chemical composition of the COC is presented in (Gohl Citation1970). The oil cake was suspended in sterile ultrapure water (conductivity = 18 μΩ/m, TOC < 3 ppb) (Barnstead, Waltham, MA), and the flask was shaken at a constant speed of 180 rpm for 2 h. Later, the mixture was filtered through Whatman No. 1 filter paper followed by 0.2 μm membrane filter. The filtered extract was used for the synthesis of AgNPs.
Table 1. Chemical composition of COC.
Synthesis of AgNPs
Silver nitrate (AgNO3) was purchased from Sigma-Aldrich (St. Louis, MO), and the nanoparticle synthesis was carried out according to Lee et al. (Citation2013). Briefly, 4 ml of COC extract was mixed with 96 ml of 1 mm AgNO3 solution and the resulting milky white mixture was incubated for 8 h in a rotary shaker (180 rpm) at 26 °C. Reduction of Ag+ ions to Ag nanocrystals was monitored by the change in color of the reaction mixture from milky white to dark brown.
Characterization of AgNPs
The morphology of the synthesized AgNPs was examined using biological transmission electron microscopy (Bio-TEM; H-7650, Hitachi, Tokyo, Japan). The X-ray powder diffraction (XRD) was carried out using Rigaku X-ray diffractometer (Rigaku, Tokyo, Japan). The scanning was performed in the region of 2θ = 30–80° at 0.041°/min with a time constant of 2 s. The elemental composition of the synthesized AgNPs was confirmed by scanning electron microscopy–energy dispersive spectra (SEM–EDS; JEOL-64000, Tokyo, Japan). The Fourier transform infrared spectra (FTIR) of the AgNPs was obtained on a PerkinElmer FTIR spectrophotometer (Waltham, MA) in the diffuse reflectance mode at a resolution of 4 cm−1 in KBr pellets.
Antibacterial activity
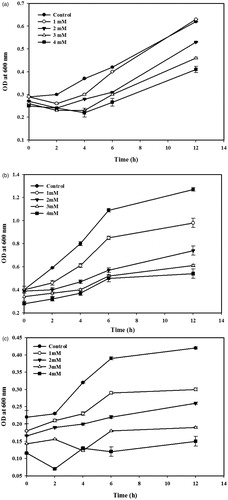
The multi-ARB (Tetracycline, Sulfathiazole, Ampicillin) (Acinetobacter sp., Citrobacter sp. and Aeromonas sp.) isolated from livestock wastewaters were procured from Biological Safety Lab, Chonbuk National University. The log phase culture of the isolates was inoculated in Luria Bertani (LB) broth supplemented with different concentration of AgNPs (1–4 mm) and the flasks were incubated at 25 °C for 12 h. The growth rate was measured in terms of increase in optical density at 600 nm. The culture grown in the absence of AgNPs was used as a control. Results were subjected to two-way analysis of variance using SPSS software version 12 (SPSS, Chicago, IL).
Results and discussion
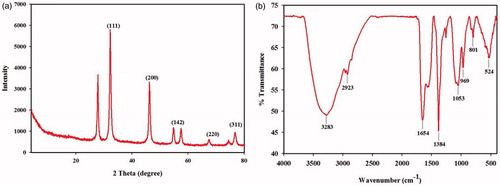
The present study comprises an attempt to synthesize AgNPs using COC extract and evaluate the antibacterial activity of the nanoparticles. After the addition of COC extract, the reaction mixture had turned to brown color within 8 h of incubation at room temperature. The intensity of the color was increased after 12 h of incubation, indicating the reduction of Ag ions. However, the color change was not observed in control flask. The proteins present in the COC extract may reduce the Ag ions present in the solution. This was supported by the results from FTIR studies where stretching vibrations of amines, alkanoids and alkaloids was observed (). The samples were subjected for TEM, XRD and SEM-EDS to confirm the presence of AgNPs. Representative TEM images of the synthesized AgNPs are shown in . The AgNPs were circular in shape and mostly present in aggregates. The size of the particles was varied from 10–70 nm. To validate the presence of Ag, the samples were analyzed in SEM-EDS and the results are shown in . The results showed strong silver signals (3 keV), along with weak oxygen and carbon peaks, which might be originated from the oilcake extract. The results are consistent with previous studies reported the strong peak for AgNPs at 3 keV (Aravinthan et al. Citation2015, Govarthanan et al. Citation2014b).
Figure 1. (a) Transmission electron microscopic image of AgNPs. (b) SEM-EDS spectrum of AgNPs. A strong peak at 3 keV confirming the presence of Ag.
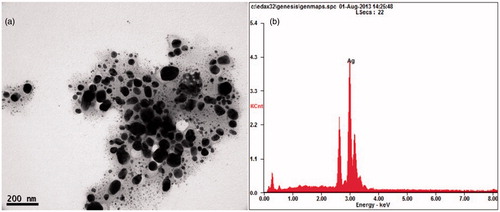
Figure 2. (a) XRD pattern of AgNPs synthesized using oilcake extract. (b) FTIR spectra of AgNPs synthesized using COC extract.
The EDS quantitative analysis showed the presence of silver (100%) without any contaminants. XRD analysis was performed to understand the nature of the AgNPs . The peaks at 2θ values of 46.2, 67.4 and 76.8 correspond to 200, 220 and 311, indicates the cubic nature of AgNPs. The results are in agreement with several studies reported the cubic nature of biologically synthesized AgNPs (Balaji et al. Citation2009, Shaligram et al. Citation2009). FTIR spectrum of AgNPs is shown in . The peak at 3283 cm−1 was assigned to N − H stretching vibration of primary and secondary amines. The strong absorption peaks at 2923, 1654, 1384 and 1053 cm−1 could be ascribed to O − H, N − H and C − O stretching of alcohols, phenols and carboxylic anions (Vivek et al. Citation2012). The plane bends at 969 and 801 cm−1 indicates the heterocyclic compounds such as alkaloids and alkanoids (Praveen Kumar et al. Citation2011). The medium intense band at 524 cm−1 indicates the availability of aliphatic bromides. The results confirmed that proteins present in COC extract could be responsible for the reduction.
The antibacterial activity of AgNPs against multi-ARB is shown in . In general, the growth rate of multi-ARB was decreased according to the increasing concentration of AgNPs. Also, the results indicated that AgNPs exhibited bacteriostatic activity against multi-ARB. Several reasons could be explained for the bacteriostatic activity of the AgNPs toward ARB. The size and shape of the AgNPs, and the concentration of AgNPs used in the growth kinetic studies may limit the antibacterial activity (Pal et al. Citation2007). The interaction of silver cations with bacteria cause the denaturation of protein, rupture of the plasma membrane, depletion of intracellular ATP and finally cell death (Lok et al. Citation2006). The formation of free radical by the AgNPs may consider as another mechanism by which bacterial cells die (Kim et al. Citation2007). However, this phenomenon needs more study. The results are consistent with previous studies reported the limited antibacterial activity of AgNPs toward Gram-negative bacteria (Kumar et al. Citation2014).
Conclusion
This study reports the simple, cost-effective method for the synthesis of AgNPs. The synthesized particles were circular in shape and the elemental composition confirmed the presence of silver without any contamination. The AgNPs reduced the growth rate of multi-ARB such as Citrobacter sp., Aeromonas sp. and Acinetobacter sp. Further work will address the reaction conditions to reduce the size and alter the shape of AgNPs for enhanced antibacterial activity.
Funding information
The research work was supported by the National Research Foundation of Korea (NRF) grant funded by the government (MEST; No. 2011-0020202). This research was also supported by Korea Ministry of Environment as “Eco- Innovation project (Project No. 2014000140003)”.
Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R. 2003. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B Biointerfaces. 28:313–318.
- Ananda Babu S, Gurumallesh Prabu H. 2011. Synthesis of AgNPs using the extract of Calotropis procera flower at room temperature. Mater Lett. 65:1675–1677.
- Aravinthan A, Govarthanan M, Selvam K, Praburaman L, Selvankumar T, Balamurugan R, Kim JH. 2015. Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int J Nanomedicine. 10:1977–1983.
- Balaji DS, Basavaraja S, Deshpande R, Mahesh DB, Prabhakar BK, Venkataraman A. 2009. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B Biointerfaces. 68:88–92.
- Benjamin S, Pandey A. 1996. Lipase production by C. rugosa on copra waste extract. Indian J Microbiol. 45:452–456.
- Elango G, Roopan SM. 2015. Green synthesis, spectroscopic investigation and photocatalytic activity of lead nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 139:367–373.
- Gohl BI. 1970. Animal feed from local products and by-products in the British Caribbean. Rome: FAO. AGA/Misc/70/25.
- Govarthanan M, Lee GW, Park JH, Kim JS, Lim SS, Seo SK, et al. 2014a. Bioleaching characteristics, influencing factors of Cu solubilization and survival of Herbaspirillum sp. GW103 in Cu contaminated mine soil. Chemosphere. 109:42–48.
- Govarthanan M, Selvankumar T, Manoharan K, Rathika R, Shanthi K, Lee KJ, et al. 2014b. Biosynthesis and characterization of silver nanoparticles using panchakavya, an Indian traditional farming formulating agent. Int J Nanomedicine. 9:1593–1599.
- Kim JS, Kuk E, Yu KN, Kim J, Park SJ, Lee HJ, et al. 2007. Antimicrobial effects of silver nanoparticles. Nanomedicine. 3:95–101.
- Kumar DA, Palanichamy V, Roopan SM. 2014. Green synthesis of silver nanoparticles using Alternanthera dentate leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc. 127:168–171.
- Kummerer K. 2009. Antibiotics in the aquatic environment – a review – part II. Chemosphere. 75:435–441.
- Lee KJ, Park SH, Govarthanan M, Wang PH, Seo YS, Cho M, et al. 2013. Synthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogens. Mater Lett. 105:128–131.
- Lok C, Ho C, Chen R, He Q, Yu W, Sun H, et al. 2006. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 5:916–924.
- Otari SV, Patil RM, Nadaf NH, Ghosh SJ, Pawar SH. 2012. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococus sp. Mater Lett. 72:92–94.
- Pal S, Kyung Y, Myong Song J. 2007. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 73:1712–1720.
- Panacek A, Kvítek L, Prucek R, Kolar M, Vecerova R, Pizurova N, et al. 2006. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 110:16248–16253.
- Praveen Kumar K, Paul W, Sharma CP. 2011. Green synthesis of gold nanoparticles with Zingiber officinale extract: characterization and blood compatibility. Process Biochem. 46:2007–2013.
- Ramachandran S, Patel AK, Nampoothiri KM, Francis F, Nagy V, Szakacs G, Pandey A. 2004. Coconut oil cake – a potential raw material for the production of alpha-amylase. Bioresour Technol. 93:169–174.
- Roopan SM, Elango G. 2015. Exploitation of Cocos nucifera a non-food toward the biological and nanobiotechnology field. Ind Crop Prod. 67:130–135.
- Roopan SM, Madhumitha RG, Rahuman AA, Kamaraj C, Bharathi A, Surendra TV. 2013. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crop Prod. 43:631–635.
- Sabu A, Sarita S, Pandey A, Bagar B, Szakacs G, Soccol CR. 2002. Solid-state fermentation for production of phytase by Rhizopus oligosporus. Appl Biochem Biotechnol. 102:251–260.
- Sastry M, Ahmad A, Islam KM, Kumar R. 2003. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 85:162–170.
- Selvakumar P, Ashakumary L, Pandey A. 1998. Biosynthesis of glucoamylase from Aspergillus niger by solid-state fermentation using tea waste as the basis of a solid substrate. Bioresour Technol. 65:83–85.
- Sengottaiyan A, Mythili R, Selvankumar T, Aravinthan A, Kamala-Kannan S, Manoharan K, et al. 2015. Green synthesis of silver nanoparticles using Solanum indicum L. and their antibacterial, splenocyte cytotoxic potentials. Res Chem Intermediat. [Epub ahead of print]. doi:10.1007/s11164-015-2199-7.
- Shaligram NS, Bule M, Bhambure R, Singhal RS, Singh SK, Szakacs G, Pandey A. 2009. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 44:939–943.
- Singh SK, Garg AK, Kalla A, Bhatnagar A. 2003. Oilcakes as protein sources in supplementary diets for the growth of Cirrhinus mrigala (Ham.) fingerlings: laboratory and field studies. Bioresour Technol. 86:283–291.
- Sun DH, Li QB, He N, Huang JL, Wang HX. 2011. Synthesis of silver nanoplates without agitation and surfactant. Rare Metal Mat Eng. 40:148–151.
- Velmurugan P, Shim J, Kamala-Kannan S, Lee KJ, Oh BT, Balachandar V. 2011. Crystallization of silver through reduction process using Elaeis guineensis biosolid extract. Biotechnol Prog. 27:273–279.
- Venkatpurwar V, Pokharkar V. 2011. Green synthesis of silver nanoparticles using marine polysaccharide: study of in-vitro antibacterial activity. Mater Lett. 65:999–1002.
- Vivek R, Thangam R, Muthuchelian K, Gunasekaran P, Kaveri K, Kannan S. 2012. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 47:2405–2410.