Abstract
Herein, we investigate whether curcumin nanoparticles (Cur NPs) are effective for the treatment of monocrotaline (MCT)-induced pulmonary arterial hypertension in Sprague Dawley rat. Echocardiography was performed at the start of the study and 28 days after MCT injection. Compared to MCT only animals, Cur NP administration was associated with reduced right ventricular (RV) wall thickness and a decreased right ventricle weight/body weight ratio. Cur NPs also attenuated MCT induced increase in RV mRNA expression of TNF-α and IL-1β. These changes were also associated with decreased RV expression of nitrotyrosine, fibronectin and myosin heavy chain-β.
Introduction
Pulmonary arterial hypertension (PAH) is a debilitating disease characterized by progressive obstruction of the pulmonary arteries, which can cause a rise in pulmonary vascular resistance (PVR) and right ventricular (RV) hypertrophy (Agarwal and Gomberg-Maitland Citation2011). The median survival rate in PAH patients without treatment is 2.8 years after diagnosis and the estimated survival rates at 1, 3 and 5 years are 68%, 48% and 34%, respectively (D'Alonzo et al. Citation1991). Not unexpectedly, RV failure is the most common cause of death in PAH patients (∼30–50% of deaths) (Humbert et al. Citation2010). Current therapeutic treatments, although effective in improving symptomology, only delay disease progression. As of yet, a cure for PAH has not been found and long-term survival remains poor (Umar et al. Citation2010).
Curcumin (diferuloylmethane) is a polyphenolic compound in turmeric that has been used in Indian Ayurvedic medicine for the treatment of inflammatory diseases (Garodia et al. Citation2007). Recent data have shown that curcumin exhibits anti-inflammatory, anti-oxidant and anti-proliferative activities (Aggarwal and Harikumar Citation2009), most likely through its ability to scavenge free radicals, diminish cytokine expression [e.g., tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 and inhibit the activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB)] (Anand et al. Citation2008, Begum et al. Citation2008). Curcumin has been used to improve left ventricular function in rabbits subjected to pressure-overload (Yao et al. Citation2004) and inhibit cardiac inflammation and fibrosis in rats (Morimoto et al. Citation2008).
Thus far, the biomedical application of curcumin has been hampered due to its poor water solubility and short biological half-life (Pathak and Khandelwal Citation2008). Recent studies, using microparticle-based systems, have shown increased curcumin bioavailability (Shahani et al. Citation2010). Here, we examine whether the systemic administration of curcumin nanoparticles (Cur NPs) can diminish PAH-induced cardiac hypertrophy in the rats. To induce PAH, rats were subjected to a single subcutaneous injection of monocrotaline (MCT; 60 mg/kg). MCT is a macrocylic pyrrolizidine alkaloid that when metabolized by cytochrome P450 (CYP3A4) is converted to dehydromonocrotaline, which selectively injuries the vascular endothelium of lung causing an increase in pulmonary arterial pressure (Gomez-Arroyo et al. Citation2012). Given the pivotal role that inflammation plays in the development of PAH (Archer et al. Citation2010), we hypothesized that Cur NP treatment would diminish the expression of inflammatory mediators and that this reduction in inflammation would be associated with decreased RV hypertrophy.
Methods
PAH model and experimental design
All procedures were approved by the Institutional Animal Care Committee of Marshall University using the criteria outlined by the American Association of Laboratory Animal Care (AALAC). Male Sprague Dawley rats (7 weeks old, 175–200 g) were obtained from Hilltop Laboratories (Scottsdale, PA) and were housed two per cage in an AALAC approved vivarium at Marshall University. The animal housing facility was operated with a 12 h:12 h dark–light cycle and maintained at 22 ± 2 °C. Rats were provided with food and water ad libitum and allowed to acclimate to the housing facilities for at least 2 weeks before experimentation. Animals were randomly assigned to one of three experimental groups: (1) Control (n = 6), (2) MCT only (n = 6) or (3) MCT + Cur NP treatment (n = 6). PAH was induced by a single injection of MCT (60 mg/kg s.c.) (Sigma-Aldrich, St. Louis, MO) as described previously (Kolli et al. Citation2014). Animals injected with MCT were given either Cur NPs (50 mg/kg i.p.) or vehicle [deionized (DI) water] once daily for 7 days following the MCT injection.
Preparation of Cur NPs
Curcumin (100 mg) was solubilized in dichloromethane (10 ml), and 1 ml of this solution was sprayed into DI water (25 ml) dropwise at a flow rate of 0.2 ml/min in 5 min under ultrasonic conditions, with an ultrasonic power of 100 W and a frequency of 30 kHz. After sonication for 10 min, the contents were stirred at 400–800 rpm at room temperature for about 30 min until a clear orange-colored solution was obtained. The solution was concentrated under reduced pressure at 50 °C and then dried at 40 °C to obtain an orange powder.
Scanning electron microscopy
Cur NP size and morphology were characterized using scanning electron microscope (SEM) (JEOL’s JSM 7400 SEM, Akishima, Tokyo). The SEM was operated at 5 kV. The samples were prepared by fixing the Cur NPs to a microscope holder using a conducting carbon strip.
Atomic force microscopy
Cur NPs (1 µg/µl) were deposited on freshly cleaved mica (V1 mica, SPI Inc., West Chester, PA). After a 10 min incubation period, the surface was gently rinsed with DI water to remove any unbound nanoparticles and the sample was dried under nitrogen. Particles were imaged in noncontact mode at a 319 kHz with a scan speed 0.5 Hz using a Nano-R microscope (Pacific Nanotechnology, Santa Clara, CA) equipped with a TM300-A probe (Sensa Probes Inc., Santa Clara, CA).
Echocardiography
Echocardiography was performed using a Philips 5500 ultrasound machine with an S-12 transducer frequency at the beginning of the study and at 4 weeks to assess pulmonary and RV structure and function as outlined previously (Koskenvuo et al. Citation2010). Briefly, rats were anesthetized with an intraperitoneal injection of ketamine and xylazine (4:1, 50 mg/kg). Once anesthetized, the ventral thorax area was shaved and covered with ultrasonic transmission gel. Electrocardiograms were recorded simultaneously to monitor heart rate. Echocardiography images were obtained in the two-dimensional guided M-mode and Doppler M-mode from parasternal long-axis and parasternal short-axis (PSAX) views. M-mode measurements of the RV, LV and wall thickness were performed from PSAX image plane. Pulmonary artery flow was measured at the pulmonary valve level and pulmonary artery diameter was measured at the level of pulmonary out flow tract during systole using the PSAX view. An apical four-chamber view was employed to measure end-diastolic RV area. Measurements from six rats in each group were obtained for the assessment of cardiac structure and function.
Tissue processing and immunoblotting
RV cardiac muscles were pulverized in liquid nitrogen using a mortar and pestle until a fine powder was obtained and homogenized in TPER lysis buffer (Pierce, Rockford, IL) containing protease (P8340, Sigma-Aldrich, Inc., St. Louis, MO) and phosphatase inhibitors (P5726, Sigma-Aldrich, Inc.). After incubation on ice for 30 min, the homogenate was collected by centrifuging at 12,000 g for 5 min at 4 °C. The protein concentration of homogenates was determined in triplicate via 660 nm assay (Thermo Scientific, Rockford, IL). Homogenate samples were boiled in a Laemmli 2 × sample buffer (Sigma-Aldrich, Inc.) for 5 min before loading forty micrograms of total protein from each sample on 10% PAGEr Gold Precast gels (Lonza, Rockland, ME). Proteins were transferred onto nitrocellulose membranes using standard conditions (Manne et al. Citation2013). For immunodetection, membranes were blocked for 1 h at room temperature in blocking buffer [5% non-fat dry milk in TBS-T (20 mM Tris–base, 150 mM NaCl, 0.05% Tween-20), pH 7.6], serially washed in TBS-T at room temperature, then probed with primary antibodies for the detection of β-myosin heavy chain (M 8421, Sigma Aldrich), fibronectin (AB23750, Abcam, Cambridge, MA), HSP-27 (#2442s), HSP-70 (#4872), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (#2118), (from Cell Signaling Technology, Inc., Beverly, MA) and 3-nitrotyrosine (SC-55256) (from Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Membranes were incubated overnight at 4 °C in primary antibody buffer (5% BSA in TBS-T, pH 7.6, primary antibody diluted 1:1000), followed by washing in TBS-T (3 × 5 min), and incubation with HRP-conjugated secondary antibody [anti-rabbit (#7074) or anti-mouse ((#7076)], Cell Signaling Technology, Inc., Danvers, MA) in blocking buffer for 1 h. After removal of the secondary antibody, membranes were washed (3 × 5 min) in TBS-T and protein bands were visualized following reaction with ECL reagent (Amersham ECL Western Blotting reagent RPN 2106, GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Target protein levels were quantified by AlphaView SA image analysis software (Alpha InfoTech, San Leandro, CA) and normalized to GAPDH.
Determination of cardiac TNF-α and IL-1β mRNA levels
RV tissue was homogenized in TRI reagent (Ambion, Austin, TX) at a concentration of 1 ml for every 100 mg of tissue as detailed elsewhere (Thulluri et al. Citation2012). Briefly, after 5 min of incubation with TRI reagent, the homogenates were centrifuged (12,000 g for 15 min at 4 °C). The supernatant was collected and mixed with 200 μl of chloroform (Sigma-Aldrich, Inc.). After a 10 min incubation at room temperature, the aqueous phase of the mixed solution was collected via centrifugation (12,000 g for 15 min at 4 °C) and then mixed with 500 μl of isopropanol (Sigma-Aldrich, Inc.). RNA pellets were precipitated (12,000 x g for 10 min at 4 °C), washed with 75% ethanol, and then dried under vacuum to remove residual ethanol. RNA pellets were dissolved in 50 μl of 10 mM Tris, pH 8.0 and 1 mM EDTA. RNA was quantified at 260 nm using a NanoVue UV–Vis Spectrophotometer (GE Healthcare), and RNA integrity was confirmed using a RNA Nanokit and Agilent 2100 Bio-analyzer (Agilent Technologies, Santa Clara, CA). RNA samples were stored at −80 °C until further use.
Reverse transcription coupled with RT-PCR was used to quantify the relative abundance of TNF-α and IL-1β mRNA levels in control, MCT and MCT + Cur NP animals. Complementary DNA (cDNA) libraries were synthesized using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., Foster City, CA) as detailed elsewhere (Thulluri et al. Citation2012).
RT-PCR was performed using the Power SYBER Green PCR Master Mix (Applied Biosystems Inc.) as outlined previously (Feng et al. Citation2009). PCR reactions were run on an ABI 7000 Real-Time PCR system (Applied Biosystems Inc.) using the following cycle conditions: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min as detailed elsewhere (Wu et al. Citation2010). All experiments were repeated in triplicate. β-actin or GAPDH were used as internal controls. The forward and reverse primers were purchased from Integrated DNA technologies (San Diego, CA). TNF-α Gene bank accession number: NM_012675.3 with forward primer (5′–3′) – CCAGAACTCCAGGCGGTGTC, reverse primer (5′–3′) – GGCTACGGGCTTGTCACTCG, IL-1β Gene bank accession number: NM_031512.2 forward primer (5′-3′) – TGCCCGTGGAGCTTCCAGGA, reverse primer (5′–3′) – TCCAGCTGCAGGGTGGGTGT. The expression of TNF-α and IL-1β mRNA levels was normalized to the geometric means of β-actin and GAPDH mRNA levels, and the 2−Δct method was used to calculate the relative mRNA expression levels.
Data analysis
Results are presented as mean ± SEM. Data were analyzed using SigmaPlot 11.0 software (Systat Software Inc., San Jose, CA). One-way analysis of variance (ANOVA) was used for overall comparisons. The Student–Newman–Keuls post hoc test was used where appropriate to determine significant differences between groups. The level of significance accepted a priori was P ≤ 0.05.
Results
Characterization of Cur NPs
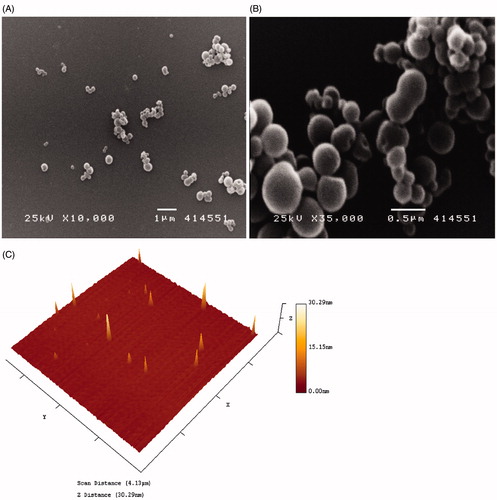
The particle size analysis of the nanoparticles was performed by SEM and atomic force microscope (AFM). The SEM of powdered sample showed the particle size to be in the range of 300 ± 50 nm (, Panels A and B) and AFM of aqueous dispersion showed the particles to be approximately 50–100 nm in size (, Panel C). The change in size of measured particles is believed to be the result of dehydration induced agglomeration events in the powdered samples used in the SEM. The aqueous dispersion is more representative of the biologically relevant particle size.
Curcumin NP treatment decreases PAH induced RV tissue weight
Compared to control animals, body weight was 19% less in the MCT and MCT + Cur NP rats, respectively (P < 0.05) (). Conversely, the ratio of right ventricle (RV) weight to body weight ratio was 27% and 10% higher in the MCT and MCT + Cur NP animals (P < 0.05) ().
Table 1. Body and organ weight for control, MCT only and MCT + Cur NP rats collected 4 weeks after injection of MCT (60 mg/kg).
Curcumin NP treatment decreases MCT-induced changes in pulmonary flow and cardiac remodeling
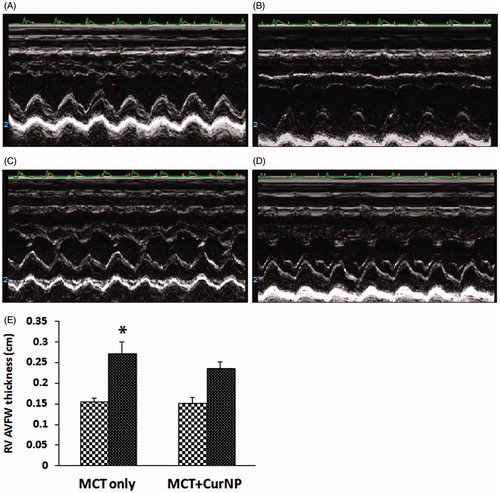
Compared to the MCT + Cur NP animals, echocardiographic analysis of pulmonary arterial flow demonstrated a mid-systolic notch in the MCT animals (, Panels A–D). Similar to our findings at the whole tissue level, echocardiography analysis demonstrated that Cur NP treatment attenuated MCT-induced increases in RV anterior free wall thickness (, Panels A–E).
Figure 2. Cur NP treatment improves MCT-induced decrease in pulmonary artery flow. Representative image from a MCT only animal at day 1 (Panel A) and at day 28 (Panel B). Note mid-systolic notch at day 28. Images from a MCT + Cur NP treated animal at day 1 (Panel C) and at day 28 (Panel D).
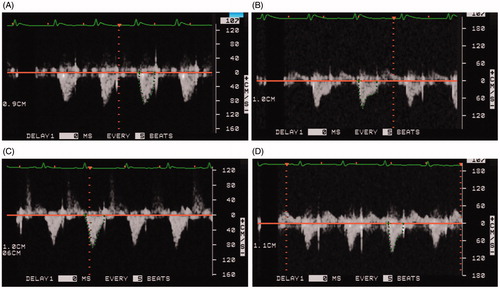
Figure 3. Cur NP treatment attenuates PAH induced increase in RV anterior free wall thickness. M-mode echocardiography images of a MCT only animal at day 1 (Panel A) and at day 28 (Panel B). Images from a MCT + Cur NP treated animal at day 1 (Panel C) and at day 28 (Panel D). Quantification of RV AVFW echocardiography (Panel E). Values are expressed as mean ± SEM; n = 6 for each group. *P < 0.05 versus base line of MCT only group.
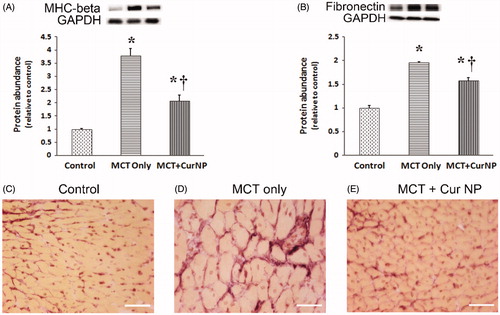
To examine the molecular changes that might accompany PAH-induced changes in RV thickness we next performed immunoblotting to examine myosin heavy chain expression. Compared to the control animals, myosin heavy chain (MHC)-β expression was 278% and 106% higher in the MCT and MCT + Cur NP treated animals, respectively (, Panel A, P < 0.05). We next examined the effect of Cur NP treatment on fibronectin expression with MCT insult. Compared to that observed in the control animals, fibronectin protein levels were 95% and 57% higher in the MCT and Cur NP MCT animals, respectively (, Panel B, P < 0.05).
Figure 4. Cur NP treatment decrease PAH-induced cardiac remodeling. Myosin heavy chain-β protein expression in the RV as determined by immunoblotting and normalized to GAPDH (Panel A). Fibronectin protein expression normalized to GAPDH (Panel B). Graphical data are expressed as mean ± SEM; n = 6 for each group. *P < 0.05 versus control group; †P < 0.05 versus MCT + Cur NP group.
Cur NP treatment decreases TNF-α and Il-1β
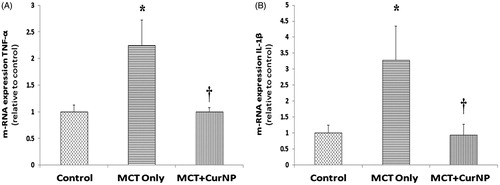
Compared to the control animals, the expression of TNF-α mRNA was 124% and 2% higher in the MCT and MCT + Cur NP animals, respectively (, Panel A, P < 0.05) and IL-1β mRNA level was 227% higher and 7% lower in the MCT and MCT + Cur NP animals, respectively (, Panel B, P < 0.05).
Cur NP treatment decreases MCT-induced increases in oxidative stress signaling
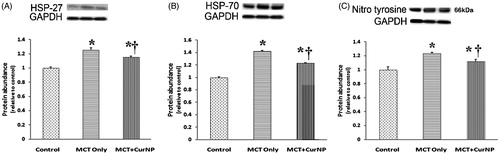
Compared to the control animals, the amount of HSP-27 was 25% and 15% higher in the MCT and MCT + Cur NP animals, respectively (, Panel A, P < 0.05). Like that observed for HSP-27, HSP-70 protein levels were 42% and 22% higher in the MCT and MCT + Cur NP animals, respectively (, Panel B, P < 0.05). Similarly, the levels of 3-nitrotyrosine were 23% higher in the MCT animals and 11% higher in the MCT + Cur NP animals (, Panel C, P < 0.05).
Figure 6. Cur NP treatment attenuates PAH-associated increase in heat shock protein and protein nitrosylation. The expression of Hsp-27 (Panel A), Hsp-70 (Panel B) and nitrotyrosine (Panel C) was determined by immunoblotting and normalized to the expression of GAPDH. Values are expressed as mean ± SEM; n = 6 for each group. *P < 0.05 versus control group; †P < 0.05 versus MCT + Cur NP group.
Discussion
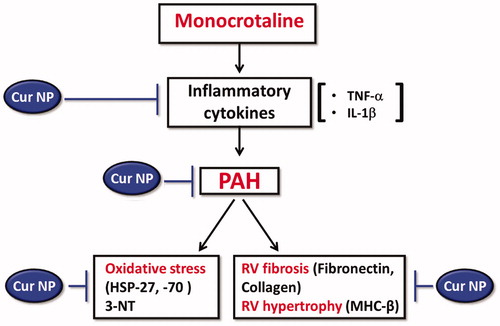
Recent studies have suggested that curcumin may be effective for the treatment of neurodegenerative, pulmonary, autoimmune and neoplastic diseases (Aggarwal and Harikumar Citation2009). In addition, other data have demonstrated that curcumin is able to prevent cardiac hypertrophy and heart failure in a murine model of salt-induced hypertension (Morimoto et al. Citation2008). In an effort to extend these findings, the present study investigated whether treatment with Cur NPs was able to attenuate the development of MCT-induced RV hypertrophy following PAH. Herein, we demonstrate that the administration of Cur NPs (50 mg/kg/i.p.) for 7 days is capable of diminishing the development of RV remodeling in the rat MCT model of PAH ().
Figure 7. Schematic representation of proposed mechanism of action of Cur NP against MCT-induced cardiac remodeling.
The most commonly used animal model of PAH utilizes MCT to selectively injure the pulmonary vasculature, which causes an increase in PVR (Schultze and Roth Citation1998, Wilson et al. Citation1992). Similar to previous reports (Boissiere et al. Citation2005, Koskenvuo et al. Citation2010), our echocardiography data demonstrated that MCT insult resulted in the development of mid-sytolic notch in pulmonary arterial flow and that Cur NP attenuated this response (). These changes are also associated with attenuation in RV mass and free wall thickness (, ).
In an effort to better understand the nature of RV hypertrophy we next examined if MCT insult was associated with changes in contractile protein expression and an increase in non-contractile tissue (). Consistent with previous data (Bogaard et al. Citation2009, Okada et al. Citation2009), we found that MCT-insult was associated with an increase in the amount of slow β-MHC isoform, and fibronectin protein expression in the pressure overloaded right ventricle.
How Cur NP administration may function to diminish cardiac hypertrophy and the development of fibrosis is currently unclear. Previous reports have suggested that curcumin can act as an anti-fibrotic agent and that the chronic administration of curcumin can attenuate the development of pulmonary fibrosis in the rat. It has been hypothesized that this effect may be related to the ability of curcumin to reduce inflammation (Punithavathi et al. Citation2000). Consistent with this possibility, and the work of others using bulk curcumin preparations (Fu et al. Citation2008), we found that Cur NP diminished RV TNF-α and IL-1β mRNA expression (). Whether these changes in TNF-α regulation are directly responsible for the changes we see in cardiac remodeling is unknown and will require further experimentation.
It has been reported that the increases in tissue inflammation/oxidative stress seen during pressure-overload induced RV hypertrophy can cause an up-regulation in heat shock protein expression (Faber et al. Citation2007, Takeuchi et al. Citation2003). Supporting this contention, we observed that PAH induced cardiac hypertrophy was associated with increased expression of HSP-27 and HSP-70 in the right ventricle (). Consistent with our hypertrophy data, we also noted that Cur NP administration was associated with a down-regulation of the expression of HSP-27 and HSP-70 () and decreased levels of oxidative stress. In addition to increases in HSP expression, pressure overload has also been shown to increase tissue oxidative and nitrosative stress (Henderson et al. Citation2007). Similar to our HSP data, we found that MCT-insult caused an elevation in 3-nitrotyrosine levels and that 3-nitrotyrosine levels were diminished with Cur NP administration ().
Conclusion
The data of the present study suggest that Cur NP treatment can attenuate the development of RV hypertrophy in the rat MCT model of PAH. Although the exact mechanism of action is not clear, our data suggest that the attenuated hypertrophy we observe following Cur NP administration may be related to decreases in TNF-α levels, and diminished oxidative stress (). Although prior work studies have reported that curcumin administration can prevent cardiac remodeling it should be noted that the curcumin levels used in the previous study were much higher (150 mg/kg/day for 7 weeks) than those used in the present study (Ghosh et al. Citation2010). Whether Cur NP treatment is effective in preventing other types of cardiac hypertrophy will require additional study.
Declaration of interest
This work was supported in part from DOE grant (DE-PS02-09ER-01 to E.R.B).
References
- Agarwal R, Gomberg-Maitland M. 2011. Current therapeutics and practical management strategies for pulmonary arterial hypertension. Am Heart J. 162:201.
- Aggarwal BB, Harikumar KB. 2009. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 41:40.
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. 2008. Curcumin and cancer: an “old-age” disease with an ”age-old” solution. Cancer Lett. 267:133.
- Archer SL, Weir EK, Wilkins MR. 2010. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 121:2045.
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, et al. 2008. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther. 326:196.
- Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. 2009. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 135:794.
- Boissiere J, Gautier M, Machet MC, Hanton G, Bonnet P, Eder V. 2005. Doppler tissue imaging in assessment of pulmonary hypertension-induced right ventricle dysfunction. Am J Physiol Heart Circ Physiol. 289:H2450.
- D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. 1991. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 115:343.
- Faber MJ, Dalinghaus M, Lankhuizen IM, Bezstarosti K, Verhoeven AJ, Duncker DJ, Helbing WA, Lamers JM. 2007. Time dependent changes in cytoplasmic proteins of the right ventricle during prolonged pressure overload. J Mol Cell Cardiol. 43:197.
- Feng J, Gu Z, Wu M, Gwazdauskas FC, Jiang H. 2009. Growth hormone stimulation of serum insulin concentration in cattle: nutritional dependency and potential mechanisms. Domest Anim Endocrinol. 37:84.
- Fu Y, Zheng S, Lin J, Ryerse J, Chen A. 2008. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 73:399.
- Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. 2007. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 5:25.
- Ghosh SS, Salloum FN, Abbate A, Krieg R, Sica DA, Gehr TW, Kukreja RC. 2010. Curcumin prevents cardiac remodeling secondary to chronic renal failure through deactivation of hypertrophic signaling in rats. Am J Physiol Heart Circ Physiol. 299:H975.
- Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. 2012. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 302:L363.
- Henderson BC, Sen U, Reynolds C, Moshal KS, Ovechkin A, Tyagi N, et al. 2007. Reversal of systemic hypertension-associated cardiac remodeling in chronic pressure overload myocardium by ciglitazone. Int J Biol Sci. 3:385.
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. 2010. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 122:156.
- Kolli MB, Manne ND, Para R, Nalabotu SK, Nandyala G, Shokuhfar T, et al. 2014. Cerium oxide nanoparticles attenuate monocrotaline induced right ventricular hypertrophy following pulmonary arterial hypertension. Biomaterials. 35:9951.
- Koskenvuo JW, Mirsky R, Zhang Y, Angeli FS, Jahn S, Alastalo TP, et al. 2010. A comparison of echocardiography to invasive measurement in the evaluation of pulmonary arterial hypertension in a rat model. Int J Cardiovasc Imaging. 26:509.
- Manne ND, Lima M, Enos RT, Wehner P, Carson JA, Blough E. 2013. Altered cardiac muscle mTOR regulation during the progression of cancer cachexia in the ApcMin/+ mouse. Int J Oncol. 42:2134.
- Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, et al. 2008. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. 118:868.
- Okada M, Harada T, Kikuzuki R, Yamawaki H, Hara Y. 2009. Effects of telmisartan on right ventricular remodeling induced by monocrotaline in rats. J Pharmacol Sci. 111:193.
- Pathak N, Khandelwal S. 2008. Comparative efficacy of piperine, curcumin and picroliv against Cd immunotoxicity in mice. Biometals. 21:649.
- Punithavathi D, Venkatesan N, Babu M. 2000. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br J Pharmacol. 131:169.
- Schultze AE, Roth RA. 1998. Chronic pulmonary hypertension--the monocrotaline model and involvement of the hemostatic system. J Toxicol Environ Health B Crit Rev. 1:271.
- Shahani K, Swaminathan SK, Freeman D, Blum A, Ma L, Panyam J. 2010. Injectable sustained release microparticles of curcumin: a new concept for cancer chemoprevention. Cancer Res. 70:4443.
- Takeuchi M, Takahashi T, Taga N, Iwasaki T, Ohe K, Shimizu H, et al. 2003. Right ventricle of patients undergoing congenital cardiac surgery differentially expresses haem oxygenase-1 and heat shock protein 70 genes. J Int Med Res. 31:413.
- Thulluri S, Wu M, Blough ER, Manne ND, Litchfield AB, Wang B. 2012. Regulation of iron-related molecules in the rat hippocampus: sex- and age-associated differences. Ann Clin Lab Sci. 42:145.
- Umar S, Steendijk P, Ypey DL, Atsma DE, van der Wall EE, Schalij MJ, van der Laarse A. 2010. Novel approaches to treat experimental pulmonary arterial hypertension: a review. J Biomed Biotechnol. 2010:702836.
- Wilson DW, Segall HJ, Pan LC, Lame MW, Estep JE, Morin D. 1992. Mechanisms and pathology of monocrotaline pulmonary toxicity. Crit Rev Toxicol. 22:307.
- Wu M, Hall JB, Akers RM, Jiang H. 2010. Effect of feeding level on serum IGF1 response to GH injection. J Endocrinol. 206:37.
- Yao QH, Wang DQ, Cui CC, Yuan ZY, Chen SB, Yao XW, Wang JK, Lian JF. 2004. Curcumin ameliorates left ventricular function in rabbits with pressure overload: inhibition of the remodeling of the left ventricular collagen network associated with suppression of myocardial tumor necrosis factor-alpha and matrix metalloproteinase-2 expression. Biol Pharm Bull. 27:198.