Abstract
Zedoary turmeric oil, a plant extract currently in clinical use, may provide potent pharmacological actions such as liver injury. In the previous study, to improve the in vivo absorption of ZTO and produce a high oral bioavailability, chitosan was employed to prepare sustained-release microspheres containing ZTO. In this study, a portability liver cancer model of rats was established successfully to compare the pharmacodynamic of ZTO microspheres and injection. In vitro results showed that microspheres had almost uniformly spherical shapes and were well dispersed by a relatively dynamic stable system. In vivo, compared with the control group, all ZTO microspheres groups resulted in growing inhibition of walker-256 cells transplanted solid tumor and the obvious controlled tumor size. At the same dose, ZTO microspheres suggested a better effect. The data showed that three doses of ZTO microspheres could prolong the average survival time considerably. None of the severe signs such as the pimelosis, fibrotic changes and fibrous septum were detected in the histopathology study.
Introduction
Transcatheter hepatic arterial chemoembolization (TACE) is a non-surgical tumor treatment method developed in the 1980s. It exerts good effect on liver cancer and has even been recommended as the best solution in non-surgerical treatment. Some articles reported that (Erhardt et al. Citation2014, Wiggermann et al. Citation2011, Wu et al. Citation2015) applying the treatment method of hepatic arterial embolization could increase the effectiveness of treatment and extend the life time of non-disease progress. Both international and domestic reports recorded some individual patients who had survived more than 10 years after TACE treatment. However, clinically, patients who had serious liver decompensation and portal vein main trunk cancer patients with obturating embolism should not use this method.
Zedoary turmeric oil (ZTO) is extracted from the dry rhizome of Curcuma zedoaria and mainly contains hepatoprotective sesquiterpenes (Hikino et al. Citation1968, Liju et al. Citation2011), which include furanodiene, Germacrone, curdione, neocurdione, curcumenol, isocurcumenol, aerugidiol and zedoarondiol (Matsuda et al. Citation1998). A series of studies of ZTO indicate that it has potent pharmacological actions, including protective effect on dgalactosamine/lipopolysaccharide-induced liver injury, tumor suppression, anti-bacterial effects, increasing white blood cells, anti-thrombotic effect, and gastrointestinal motility enhancing effect (Li et al. Citation2002, Wei et al. Citation2003). However, the feasibility of developing new formulations was limited because of its irritant properties, instability, and volatility. In addition, poor water solubility results in low oral bioavailability of ZTO formulations.
In the previous study, to improve the vivo absorption of ZTO and produce a high oral bioavailability, chitosan was employed to prepare sustained-release microspheres containing ZTO. Chitosan is a polymer with good biocompatibility and ability to open the intracellular tight junction (Benhabiles et al. Citation2012, Wei et al. Citation2003). It has been suggested as a suitable polymeric material for controlling drug release in the form of fibers, membranes, microspheres, and capsules (Raafat et al. Citation2008). Chitosan has the most desirable properties with its biodegradability and good biocompatibility, which makes chitosan and its derivatives widely used in biomedical fields, such as wound healing, drug delivery and tissue engineering, especially for developing nano/microspheres as a carrier system (Bernkop-Schnurch and Dunnhaupt Citation2012, Kim et al. Citation2012).
Thus in this research, the author firstly built a mode of rat's portability liver cancer, and then evaluated the drug effect on inhibiting tumor under three doses of high, medium and low ZTO-loaded chitosan microsphere. ZTO injection and blank microsphere were used as control. It was hoped that through the research of pharmacodynamics, we could provide more clinical data for drug development and clinical test in the future.
Materials and methods
Materials
ZTO (purity 97.4%) was supplied by Meilian BioPharma Co., Ltd. (Shanghai, China); ZTO chitosan microspheres of three doses were prepared by our lab. ZTO injection was donated by Changzhen Xinkai Pharma Co., Ltd. (Suzhou, China). Walker-256 cell line was purchased from Shanghai Xinyu BioPharma Co., Ltd. (Shanghai, China). All other chemicals and reagents were of at least analytical grade. Purified water prepared from a Milli-Q deionization unit (Millipore, Bedford, MA) was used throughout the study.
Animals
The experiments were performed on Sprague-Dawley rats weighing between 150 and 180 g and mice 60–80 g. The animals purchased from Shanghai Experimental Animal Center were kept in cages in a room at a temperature of 25 ± 2°C and with a 12:12 light–-dark cycle. Food and water were freely available. All experiments were performed in strict accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the China National Institutes of Health.
Walker-256 cell recovery and preparation of mice with tumor
Briefly, the researchers thawed the frozen Walker-256 cell strain, and then added PBS to prepare a cell suspension with a density of around 1 × 108/ml. Three milliliters of it was injected to each of the five male mice's abdominal cavity. Seroperitoneum was found after 7 days, indicating that the recovery was successful. Then, the seroperitoneum of mice was collected, and put under 1000 rpm centrifugation for 3 min. Then, the supernatant was abandoned, the rest was washed three times with PBS to prepare a 2 × 107 ml−1 cell suspension. 0.5 ml of the suspension was injected under the mice's skin. After about 1 week, the growing mice was killed, the subcutaneous tumor tissue was collected, sliced into 60 pieces of 1 mm× 1 mm × 1 mm size and placed in PBS. After homogenate by using refiner and centrifuged at 1000 rpm for 3 min, the supernatant was abandonedand the left was washed three times with PBS as backup.
Animals’ model preparation
Model group (80 rats): Each rat was anesthetized by intraperitoneal injection of 0.4 ml of 2% napental. After regular disinfection, under ensisternum median line, abdominal wall was cut by 2 cm to expose the left liver; a 1-ml injector was used to puncture into the liver surface by 1 cm at an angle of 30–45° to inoculate cytoplasm and then a latin sponge was used for hemostasis by compression for 3 min. Then, the belly was stitched up ().
Control group (12 rats): Same treatment measures with the model group were employed. The only difference was to inject same amount of physiological saline.
Treatment method
Eighty rats in the model group were divided into five groups at random. On the seventh day after the liver was inoculated with tumor, rats were fixed after being anaesthetized on the abdominal cavity and then cut again (around 2–3 cm) to expose the liver. The biggest diameter (a) and smallest diameter (b) of the tumor surface were measured. The arteriae gastroduodenalis, hepatic artery and arteriae hepatica propria were separated. The remote end of arteriae gastroduodenalis was ligatured. Silver clamp was used to temporarily block common hepatic artery. The researchers then cut a small opening on arteriae gastroduodenalis under surgical operation microscope, then inserted a special tube with an outside diameter of around 0.3 mm into the arteriae hepatica propria. When the blood was back, following the grouping and dose criteria (), researchers separately inserted relevant preparation into each group of rats. After the insertion, the tube was drew and the proximal end of arteriae gastroduodenalis was ligatured. The silver clamp on the arteriae hepatica propria was released, the opening was stitched up at every layer and cleaned, and the chlorotetracycline ointment was applied exteriorly. The rats were fed in different cages at a constant temperature, water and food were provided automatically.
Table I. Grouping and dosage (n = 16).
Evaluation criterion
Eight rats in each group were sacrificed on the seventh day after treatment. The maximum diameter (a) and the minimal diameter (b) of the tumor were measured again. The tumor volume was calculated by formula: V = ab2/2. Growth rate (GR) of the tumor was calculated based on the tumor volume before and after treatment: GR = tumor volume after the treatment/tumor volume before the treatment.
The rest of the rats in each group (n = 8) were fed in the same condition and observed for a few days. The life extension rate of the rats in the treatment group was calculated: (average survival days in treatment group/average survival days in control group) × 100%.
Histopathology
After the treatment, the livers of sacrificed rats were pressed between filter pads, weighed and then fixed in 10% neutral formalin using standard techniques and stained with hematoxylin and eosin (H&E) for histopathological examination. All tissue samples were observed using a light microscope (Axiovert 200MAT, Carl Zeiss, Oberkochen, Germany). The normal saline group was used as a control.
Statistical analysis
Results were expressed as the mean ± SD. Analysis of variance was used to test the statistical significance of differences among groups. Statistical significance was evaluated by using Student’s t-test for the single or multiple comparisons of experimental groups, respectively. A p < 0.05 was considered statistically significant.
Results
Characterization of ZTO microspheres
According to the previous experience, we have prepared ZTO-loaded chitosan microspheres by solvent evaporation method. Briefly, moderate ZTO and chitosan were co-dissolved in the organic solvent consisting of dichloromethane. The mixture solution was then added dropwise into the aqueous solution containing 1% (w/v) of Tween-80 with stirring at 200 rpm. The coarse emulsion was subjected to 300 W of ultrasonic treatment for 2 min using a high-intensity probe ultrasonicator with water bath (0 °C). In the state of reduced pressure, organic solvent was removed from the dispersion system by rotary evaporator. The prepared microspheres were collected by the method of ultracentrifugation, and washed with distilled water at least three times to remove the surfactant. The final product was stored in a vacuum desiccator at room temperature.
The morphology of microspheres determined by SEM is shown in . The particles had almost uniformly spherical shapes and were well dispersed. Microspheres had a particle size of 62–135 μm with a low PDI. In this study, the average zeta potential of preparation microspheres was about −25.6 ± 0.7 mV, indicating that the microspheres obtained was a relatively dynamically stable system. An important issue with respect to the use of microspheres as drug carriers is their capacity for drug loading and drug entrapment efficiency. In this study, the entrapment efficiency and drug-loading capacity of microspheres were 88.6 ± 5.4% and 15.1 ± 1.8%, respectively.
In vivo tumor growth inhibition study
To test the antitumor activity of ZTO-loaded chitosan microspheres, rats bearing hepatocellular carcinoma cells line walker-256 tumors were administered with three doses of ZTO microspheres and ZTO injection in 7 days. As is shown in , no obvious difference (p > 0.05) was found between each group’s tumor volume before the treatment. It indicated that the preparation of tumor model was successful. Compared with the control group, all ZTO groups resulted in inhibition growth of walker-256 cells transplanted solid tumor and a notably controlled tumor size. The tumors’ mean volumes and GR of each group are provided in . In the high dose group of ZTO microspheres, there was only a slight increase of tumor volume. The GR of tumor was only 4.75 ± 0.67 (p < 0.05, ). In addition, although the dose of ZTO injection and ZTO microspheres median was the same, the increase of tumor volume was different. The GR of two groups were 9.53 ± 2.13 and 6.93 ± 1.01 (p < 0.05, ) respectively, suggesting that a better effect might be expected from the formulation of microspheres. In the low dose group, ZTO microspheres also showed the ability to inhibit the tumor with a GR of 8.17 ± 1.43. It was significantly different from the normal saline group, but not different from the ZTO injection group.
Figure 3. Changes of tumor volume in rats transplanted with hepatocellular carcinoma cells line walker-256 on seventh day (n = 8) *p < 0.05 the tumor volume before and after treatment.
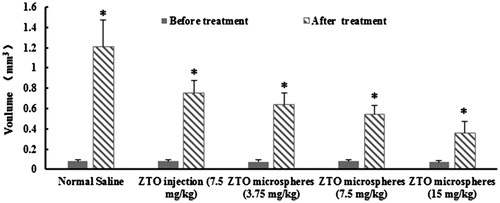
Table II. The effect of ZTO-loaded chitosan microspheres and ZTO injection on rats of walker-256 cells (n = 8).
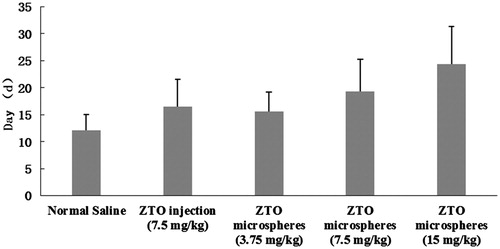
shows the survival time of the rats transplanted with hepatocellular carcinoma cells line walker-256 on the seventh day after the arterial infusion. The data analysis showed that the average survival time of rats infused with three doses of ZTO microspheres was all longer than the control group (p < 0.05). Meanwhile, the ZTO injection group could also prolong the survival time of rats, but with no obvious difference compared to the same dose of ZTO microspheres (7.5 mg/kg).
Histopathology
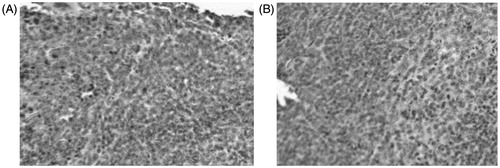
Results of histological study, shown in (images at 200× magnification), provided good support for the conclusions drawn from the therapeutic study. Normal rat’s liver section slide stained by H&E method () was used as a control. In this group, liver cells were regularly arranged and the lobular architecture was intact, only a small number of fibers were observed in blood vessel walls and biliary duct walls. However, in the group of ZTO microspheres (, 7.5 mg/kg), none of the severe signs including the pimelosis, fibrotic changes and fibrous septum were detected.
Discussion
The in vitro experiment of ZTO (Lim et al. Citation2010, Liu et al. Citation2009, Zhou et al. Citation2015) showed that it can inhibit and break some kinds of cancer cells including the human liver cancer cell cultured in vitro; it can also influence the nucleic acid metabolism of the cancer cell, regulate and promote immune response, and strengthen the active immune protective effect. ZTO’s mechanism (Jiang et al. Citation2005, Xia et al. Citation2012) of action could have the following aspects: (1) Direct cell toxic action: ZTO and its extractive β-elemene had direct cell toxic action on L615 leukemia cell, both could lead to qualitative tumor cell changes and death with certain deactivation. (2) Inducement of tumor cell death: elemene could block the tumor cell’s entry into the G2M period from S period, and inhibit its multiplication and quickly induce its apoptosis. (3) Inducement of tumor cell's abnormal multiplication: ZTO can reduce the DNA absorbance value of mouse's HePA liver cancer cell, its seed dimension and DNA index. It can increase the percentage of diploid cells in tumor cells, and decrease the percentage of pentaploid. (4) Influence on cancer cell's nucleic acid metabolism: β-elemene could considerably decrease the nucleic acid quantity of the Ehrlich's ascite cell with its obvious inhibiting effect on RNA polymerase and combinability with DNA. (5) Enhancing immunity: the zedoary turmeric could boost cellular immunity and humoral immunity and had direct or indirect effect on nonspecific immunity. (6) Active immunity of tumor vaccine: oil of zedoary turmeric and β-elemene's L615 tumor vaccine active immunity could induce an immunity protection effect, while the latter had tumor specificity and the immunity protection effect β-elemene tumor vaccine could be strengthened through chemical drugs or virus combined treatment. (7) Influence on cancer cell membrane conductivity: rcumenol had effect on the receptor protein on the cancer cell membrane, and could change the permeability of channel protein to change membrane conductivity, and then influence the cellular metabolism and kill the cancer cell in the end.
Thus, clinical infusion of ZTO in liver artery increased the drug concentration in the cancerous place more than systemic administration, with a certain effect. However, direct infusion of ZTO could hardly maintain the high concentration of drugs inside tumor tissues with shorter tumor treatment effect (Singh et al. Citation2013, Zhao et al. Citation2014).
The key point of hepatic artery embolism was that the embolism substance entered the peripheral vessel and effectively stopped the blood, causing local tissue ischemia, hypoxia, increased vascular permeability, release of the drug from microspheres and its entry into tissues. Embolization of microspheres was also related to the carrier material and drug. Hydrophilic polymer type of microspheres, once entering the blood vessel, would become penetrative in water and could easily form thrombus (Acosta et al. Citation2015, Aramwit et al. Citation2015, Ganguly et al. Citation2015). Thus, apart from mechanical embolism, it also had the effect of thrombus to produce long-term embolization. In addition, the porous structure on polymer surface and other characters made it sensitive to blood cells. Blood cells could easily adhere to or enter into microspheres. In such a situation, the particle size of sponge that absorbed stone embolization of platelet and its mechanical strength were both increased in favor of embolism. The re-clone of cells and whether embolism could adhere to blood vessel forever depended on the net drilling of polymer.
However, this type of microspheres usually easily causes proximal embolization, and form collateral circulation, while inert material microspheres did not had such effects. Also, because it increases blood pressure after embolism, the great vessel wall elasticity would led to a fatal weakness – remote embolism. Thus, we chose hydrophilic material chitosan as the stroma. Drugs effective during the systematic administration were not applicable to chemotherapy embolism because it led to ischemia in target organ, lower PH value, less oxygen pressure and lower energy. This required the drug to have activity under those circumstances. In addition, drug should have certain affinity with carrier to effectively stop sudden release and be compatible with the internal environment. Thus, under the circumstance of embolism, it may cause changes in the microscopic physiological environment and drug absorption of metabolism.
Declaration of interest
The authors report no conflicts of interest in this study.
References
- Acosta N, Sánchez E, Calderón L, Cordoba-Diaz M, Cordoba-Diaz D, Dom S, et al. 2015. Physical Stability studies of semi-solid formulations from natural compounds loaded with chitosan microspheres. Mar Drugs. 13:5901–5919.
- Aramwit P, Ekasit S, Yamdech R. 2015. The development of non-toxic ionic-crosslinked chitosan-based microspheres as carriers for the controlled release of silk sericin. Biomed Microdevices. 17:84. doi: 10.1007/s10544-015-9991-4.
- Benhabiles MS, Salah R, Lounici H, Drouiche N, Goosen MFA, Mazmeri N. 2012. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocolloids. 29:48–56.
- Bernkop-Schnurch A, Dunnhaupt S. 2012. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 81:463–469.
- Erhardt A, Kolligs F, Dollinger M, Schott E, Wege H, Bitzer M, et al. 2014. TACE plus sorafenib for the treatment of hepatocellular carcinoma: results of the multicenter, phase II SOCRATES trial. Cancer Chemother Pharmacol. 74:947–954.
- Ganguly K, Kulkarni AR, Aminabhavi TM. 2015. In vitro cytotoxicity and in vivo efficacy of 5-fluorouracil-loaded enteric-coated PEG-crosslinked chitosan microspheres in colorectal cancer therapy in rats. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2015.1089955.
- Hikino H, Agatsuma K, Takemoto T. 1968. Structure of curzerenone, epicurzerenone, and isofuranogermacrene (curzerene). Tetrahedron Lett. 9:2855–2858.
- Jiang Y, Li ZS, Jiang FS, Deng X, Yao CS, Nie G. 2005. Effects of different ingredients of zedoary on gene expression of HSC-T6 cells. World J Gastroenterol. 11:6780–6786.
- Kim S, Kang Y, Krueger CA, Sen M, Holcomb JB, Chen D, et al. 2012. Sequential delivery of BMP-2 and IGF-1 using a chitosan gel withgelatin microspheres enhances early osteoblastic differentiation. Acta Biomater. 8:1768–1777.
- Li GD, Xu F, Shen AJ. 2002. Progression of studies on zedoary turmeric oil. Chin Pharm J. 37:806–809.
- Liju VB, Jeena K, Kuttan R. 2011. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa. L. Indian J Pharmacol. 43:526–531.
- Lim CB, Ky N, Ng HM, Hamza MS, Zhao Y. 2010. Curcuma wenyujin extract induces apoptosis and inhibits proliferation of human cervical cancer cells in vitro and in vivo. Integr Cancer Ther. 9:36–49.
- Liu HY, Pen AB, Liao AJ, Shi W. 2009. [Preliminary research on the mechanism of apoptosis hepatic stellate cells induced by zedoary turmeric oil]. Zhonghua Gan Zang Bing Za Zhi. 17:790–791.
- Matsuda H, Ninomiya K, Yoshikawa M. 1998. Inhibitory effect and action mechanism of sesquiterpenes from Curcuma zedoaria rhizome on d-galactosamine/lipopolysaccharide-induced liver injury. Bioorg Med Chem Lett. 8:339–344.
- Raafat D, von Bargen K, Haas A, Sahl HG. 2008. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol. 74:3764–3773.
- Singh V, Jain M, Misra A, Khanna V, Rana M, Prakash P, et al. 2013. Curcuma oil ameliorates hyperlipidaemia and associated deleterious effects in golden Syrian hamsters. Br J Nutr. 110:437–446.
- Wei LF, Zou BC, Wei MX. 2003. Effect of zedoary on the gastric dynamics in rats. ShangHai J Traditional Chin Med. 37:46–48.
- Wiggermann P, Sieron D, Brosche C, Brauer T, Scheer F, Platzek I, et al. 2011. Transarterial chemoembolization of child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE). Med Sci Monit. 17:CR189–195.
- Wu H, Liu S, Zheng J, Ji G, Han J, Xie Y. 2015. Transcatheter arterial chemoembolization (TACE) for lymph node metastases in patients with hepatocellular carcinoma. J Surg Oncol. 112:372–376.
- Xia Q, Wang X, Xu DJ, Chen XH, Chen FH. 2012. Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential Oil. Thromb Res. 130:409–414.
- Zhao L, Zhang H, Yang Y, Zheng Y, Dong M, Wang Y, et al. 2014. Serum metabonomic analysis of protective effects of Curcuma aromatica oil on renal fibrosis rats. PLoS One. 9:e108678.
- Zhou Y, Shen J, Xia L, Wang Y. 2015. Curcuma zedoaria (Berg.) Rosc. essential oil and paclitaxel synergistically enhance the apoptosis of SKOV3 cells. Mol Med Rep. 12:1253–1257.