Abstract
Context Immunotherapy is among the most promising modalities for treatment of cancer. Recently, interleukin 12 (IL-12) has been used as an immunotherapeutic agent in cancer gene therapy. IL-12 can activate dendritic cells (DCs) and boost anti-tumor immune responses. Objective In the current study, we have investigated if IL-12 gene therapy can lead to the regression of tumor mass in a mouse model of fibrosarcoma. Material and methods To investigate the therapeutic efficacy of IL-12, WEHI-164 tumor cells were transfected with murine-IL12 plasmids using Lipofectamine. Enzyme linked immunosorbent assay (ELISA) was used to confirm IL-12 expression in transfected cells. The fibrosarcoma mouse model was established by subcutaneous injection of transfected cells to Balb/C mice. Mice were sacrificed and the tumors were extracted. Tumor sizes were measured by caliper. The expression of IL-12 and IFN-γ was studied with real-time PCR and western blotting. The expression of Ki-67(a tumor proliferation marker) in tumor mass was studied by immunohistochemistry staining. Results and discussion The group treated with IL-12 showed a significant decrease in tumor mass volume (P: 0.000). The results of real-time PCR and western blotting showed that IL-12 and IFN-γ expression increased in the group treated with IL-12 (relative expression of IL-12: 1.9 and relative expression of IFN-γ: 1.766). Immunohistochemistry staining showed that Ki-67 expression was reduced in the group treated with IL-12. Conclusion IL-12 gene therapy successfully led to regress of tumor mass in the fibrosarcoma mouse model. This may serve as a candidate therapeutic approach for treatment of cancer.
Keywords:
Introduction
Sarcomas are relatively rare malignant tumors of mesenchymal origin, but often incurable at the late metastatic stage (Bramante et al. Citation2014). Current cancer treatment strategies largely rely on chemotherapy and/or radiotherapy which exhibit several side effects in patients. Immunotherapeutic approaches for treatment of cancer have the advantage that they act more specifically against tumors and do not damage normal cells (Helmy et al. Citation2013, Yoshimoto et al. Citation2009). Furthermore, targeting neoplastic cells especially in gene transfer can reduce the risks associated with germ line transduction (Wang and Liu Citation2003). Cancer immunotherapy includes approaches that either have specific targets such as the adaptive immune system (e.g., antibodies or T cells) or those that exploit the strong cytotoxic functions of acquired and innate immunity. Immunotherapy strategies include monoclonal antibodies for stimulation of immune system, inhibition of immune inhibitory pathways or direct targeting of tumors, and induction of antibody-dependent cell-mediated cytotoxicity (ADCC) as well as the administration of ex vivo activated T and natural killer (NK) cells, but may not be limited to cancer vaccines. Immunotherapy has proved to be as efficient as conventional cancer therapy methods. In addition, due to the generation of immune memory, immunotherapy can rebalance the equivalence between tumor and host cells. Thus, the overall aim of cancer immunotherapy would be to enhance the defective or weak host immune response to developing tumors. One such strategy is to use cytokines such as IL-12 (Gajewski Citation2012, Kowalczyk et al. Citation2003, Zarour and Ferrone Citation2011).
Gene therapy has provided an opportunity for therapeutic intervention for a large number of genetic and non-genetic disorders. In parallel with the progression in dissecting the biology and genetics of human disease, a huge effort has been focused on devising viral and non-viral vectors that can transfer genetic materials to target cells. In comparison with viral vectors, plasmid mediated gene delivery systems possess several desirable features such as low toxicity, low immunogenicity as well as nuclease resistance. In addition, their efficiency is independent of the size of the genetic cargo (Bathula and Huang Citation2010, Wold and Toth Citation2013).
Cytokines are immune-modulator molecules secreted by immune cells and some other specific cells in human body. The immunemodulatory action can help to control inflammatory reactions (Kresina Citation2001). Cancer gene immunotherapy comprises the delivery of cytokine genes to tumor cells for induction of anti-tumor immune responses (Hallaj-Nezhadi et al. Citation2013). Although the administration of therapeutic proteins can be used for directly affecting the defective biological pathways, gene therapy circumvents the necessity for production and purification of large quantities of recombinant proteins. Furthermore, gene therapy is a more permanent solution (Lotfipour et al. Citation2011).
Interleukin-12 (IL-12) is a 74 kDa heterodimeric cytokine, consisting of a 35 kDa (p35) and a 40 kDa (p40) subunit (Gutiérrez-Ortega et al. Citation2005, Toda et al. Citation2001, Xu et al. Citation2004). In initial studies by Kobayashi et al. in Citation1989 and Gately et al. in Citation1992, IL-12 was shown to be a natural killer-stimulating factor (NKSF) and cytotoxic lymphocyte maturation factor (CLMF) secreted by macrophages, monocytes, and DCs (Toda et al. Citation2001, Xu et al. Citation2004). In comparison with other cytokines, IL-12 has shown superior antitumor effect. IL-12 has also been shown to be effective in the prevention of primary tumor growth (Brunda et al. Citation1993). IL-12 as a multifunctional cytokine, can regulate the production of IFN-γ, induce cytokine production, induce the cytotoxic activity of T cells and NK cells, and development of CD4+ Th1 cells and promote the activity and generation of cytotoxic T lymphocytes (CTLs). The induction of IL-12 by IFN-γ can lead to the up regulation of MHC class I and II molecules, adhesion molecules, and transcription factors such as T-box expressed in T cells (T-bet) (Hara et al. Citation2000, Suzuki et al. Citation2010). Therefore, the antitumor effects of IL-12 in most cases depend on the endogenous production of IFN-γ. Moreover, IL-12 possesses potent anti-angiogenic activity (Voest et al. Citation1995). Taken together, IL-12 is a potent cytokines for cancer immunotherapy (Hara et al. Citation2000, Suzuki et al. Citation2010). The aim of this study was to investigate the effects of plasmid mediated gene therapy with IL-12 in the regression of tumor mass in a fibrosarcoma mouse model.
Materials and methods
Plasmid amplification and isolation
A murine IL-12 expression vector, pUMVC3-mIL-12, was purchased from Aldevron Company (Aldeveron, Fargo, ND). The plasmid DNA (6247 bp) is in size and contains a CMV IE promoter.
The pUMVC3-IL-12 was amplified in an Escherichia coli DH5α strain obtained from Drug Applied Research Center (Tabriz, Iran) and then extracted according to TENS miniprep protocol. The purified plasmid was detected by agarose gel electrophoresis. DNA concentration was determined by measuring absorption at 260 nm using a NanoDrop 1000 Spectrophotometer (Wilmington, DE).
Cell culture
Balb/C mouse fibrosarcoma cells (WEHI-164) were purchased from the Pasteur Institute (Tehran, Iran). The cells were cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (Sigma, Germany), in the presence of penicillin (100 U/mL), streptomycin (100 μg/mL, Sigma-Aldrich, St. Louis, MO), and incubated in a humidified incubator with 5% CO2 at 37 °C.
In vitro transfection studies
WEHI-164 cells were seeded into six-well plate at a density of 4 × 105 cells/well. After 48 h, cells were washed with phosphate-buffer solution (PBS) and 2 ml RPMI-1640 medium (containing no FBS and antibiotics) was added to wells. Then 6 μg of pUMVC3-mIL-12 plasmid was diluted in 250 λ OptiMem in a microtube and 10 λ Lipofectamine 2000 was diluted in 250 λ OptiMem in a seprate microtube. After 5 min, both microtubes gently mixed together and incubated at room temperature for 20 min. The cells were incubated at 37 °C in 5% CO2. After 6 h, the complexes were aspirated and replaced with culture medium. After 48 h, for quantitative analysis of pUMVC3-mIL-12 expression, culture supernatants were harvested and collected supernatants were analyzed by mouse IL-12 enzyme-linked immunosorbent assay kit was used according to the manufacturer’s instructions (Koma Biotech Company, Seoul, Korea). The amount of the protein was determined as picogram per mL.
Animal experiments
Female Balb/c mice (6–8 weeks old) were purchased from the Pasteur Institute (Tehran, Iran). One million of WEHI-164 cells were inoculated subcutaneously into the right flank of the Balb/c mice to establish a tumor in the control group and 1 million of WEHI-164 cells transfected with pUMVC3-mIL-12 were injected subcutaneously into the right flank of the Balb/c mice to establish a tumor in the group treated with IL-12 (case group). The viability of the cells used for inoculation was over 90% as determined by dye exclusion with 0.4% trypan blue. Tumor growth was monitored three times a week with external calipers after tumor challenge. Tumor volume (mm3) was calculated by the following formula: 0.5 × (length × width2).
RNA extraction and real time PCR
Following tumor mass extraction, total RNA was extracted with AccuZol reagent (Bioneer, Daedeok-Gu, Daejeon, Korea) as described by the manufacturer’s instructions. Complementary DNA (cDNA) was generated from 1 μg of total RNA by use of oligo-dT18 primer and MMLV reverse transcriptase (Promega, Madison, WI). Real-time PCR amplification was performed with SYBR Premix Ex Taq (Takara Bio, Otsu, and Shiga, Japan) in the Rotor-GeneTM 6000 instrument (Corbett Life Science, Mortlake, NSW, Australia). cDNAs were diluted 1:4 in nuclease-free water and 5 μL of diluted cDNA was added to 20 μL of PCR mixture containing SYBR Premix Ex Taq (Takara Bio, Otsu, Shiga, Japan) and 0.2 μmol/L of each primer. Primer sequences were for mouse IL-12p40 forward, 5′-GAGCACTCCCCATTCCTACT-3′ and reverse, 5′-GCATTGGACTTCGGTAGATG, for mouse 5′-IFN-γ forward, 5′-TCAGCAACAGCAAGGCGAAAAAG-3′, and reverse, 5′-ACCCCGAATCAGCAGCGACTC-3′ and for GAPDH forward, 5′-CCTCGTCCCGTAGACAAAA-3′ and reverse, 5′-AATCTCCACT-TTGCCACTG-3′. The initial denaturation step at 95 °C for 10 min was followed by 45 cycles at 95 °C for 20 s and 60 °C for 1 min. Relative gene expression was calculated with the 2−(ΔΔCT) 17, using GAPDH as the endogenous expression standard.
Immunohistochemical analysis
Ki-67 expression level as a proliferation index in the tumor mass was determined by immunohistochemistry. Briefly, Four-micrometer frozen sections were cut, air-dried, fixed in acetone, and rehydrated in PBS containing 0.05% Tween-20. Non-specific binding sites were blocked by blocking solution for 30 min at room temperature. Slides were incubated with primary antibody (Purified anti-mouse Ki-67), (Biolegend, UK, London) for 60 min. Subsequently, slides were washed in PBS/Tween-20 and then incubated with HRP labeled secondary antibody [Rabbit Polyclonal secondary antibody to Rat IgG (HRP-conjugated)] (Abcam, Cambridge, MA) for 30 min. H2O2 was added to a DAB solution (Substrate solution) and then slides incubated with DAB/H2O2 for 5 min and washed with PBS/Tween-20. Sections were viewed with an invert microscope. For studying of Ki-67 expression, image processing software was used to count both the total number of cell nuclei in each image, and the number of positively stained nuclei. Data were then expressed as percent of positively stained nuclei, derived from these counts. t-Test was used for statistical analysis.
Western blot analysis
Fifty micrograms of total protein from each samples was heated for 5 min at 95 °C before loading and separated on 12% SDS polyacrylamide gels using a mini-gel apparatus (Bio-Rad Laboratories, Hercules, CA). Proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). Following on, membranes blocked with 5% non-fat dry milk in TBS/Tween-20 (0.05%, v/v) for 1 h at room temperature and then incubated overnight at 4 °C with the appropriate primary antibodies [Anti-mouse IL-12 and Anti-mouse IFN-γ (Biolegend, UK, London) and Anti-mouse β-Actin (Abcam, Cambridge, MA)] in TBS/T buffer (in a 1:1000 dilution) followed by incubation with the appropriate HRP-conjugated secondary antibody (1:1000 dilution; Abcam) for 2 h at room temperature. After washing, protein bands visualized using enhanced chemiluminescence detection Kit (GE Healthcare, Piscataway, NJ) and autoradiography films (Fuji Photo Film Co., Ltd., Tokyo, Japan) according to the manufacturer’s instruction.
Statistical analysis
t-Test was used to determine the significant differences between two groups. All statistical analyses were performed by SPSS software Version 16 (SPSS Inc., Chicago, IL). P values < 0.05 was considered to be statistically significant.
Results
Confirmation of murine IL-12 expression by enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay test was carried out for confirmation of IL-12 expression by tumor transfected cells. IL-12 concentration in the supernatant of the tumor transfected cell culture was assessed by spectrophotometer in 540 nm (λ). The IL-12 concentration in the supernatant of tumor transfected cell culture was found to be 1000 pg/mL.
Antitumor effects of IL-12 in vivo
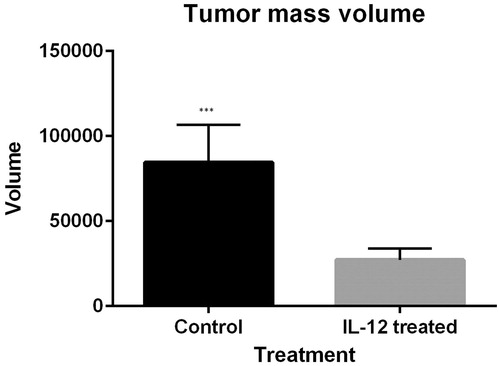
Twenty-one days after tumor challenge, the volume of tumor masses of the mice injected with fibrosarcoma/IL-12 cells was reduced significantly incomparable in the mice injected with fibrosarcoma as a control group (mean volume of tumor masses in the control group is 84604.42857 and mean volume of tumor masses in the IL-12 treated group is 27230.27285 (P = 0.000). Hence, gene therapy with IL-12 has an effect in the regression of tumor masses ().
Expression of murine IL-12 and murine IFN-γ mRNA and protein in tumor tissue
To investigate whether fibrosarcoma/IL-12 cells can express IL-12 in vivo, tumor mass were harvested from mice treated with IL-12 and the control group 21 days after tumor inoculation respectively. Total RNA was extracted from tumor masses and cell lyset was prepared to confirm IL-12 and IFN-γ expression by immunoblotting. The results of real-time PCR indicated that the expression of IL-12 and IFN-γ was significantly (P = 0.000) enhanced in the group treated with IL-12 in comparison to the control group ( and ). Furthermore, the results of immunoblotting showed that the expression of IL-12 and IFN-γ was enhanced in the group treated with GM-CSF in comparison to control group (.
Figure 2. (A) Relative expression of IL-12. IL-12 gene expression in the group treated with IL-12 is UP-regulated (compared to the control group) by a mean factor of 1.9 (P = 0.000; standard error range is 1.90–1.90). (B) Relative expression of IFN-γ. IFN-γ gene expression in the group treated with IL-12 is up-regulated (compared to the control group) by a mean factor of 1.76 (P = 0.00; SE range is 1.76–1.76).
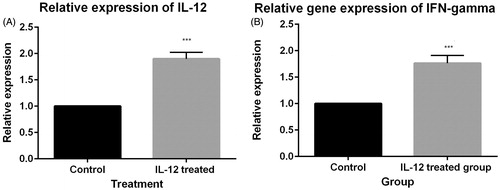
Figure 3. The cytokine expression results of western blot: IL-12 and IFN-γ expression has been proved by western blotting analysis. One sample of each group has been showed in picture. (A) expression of β-actin. (A) Proteins were equalized by use of β-actin expression. (B) expression of IL-12. (B) Western blotting results showed that IL-12 expression was enhanced in group treated with IL-12 incomparable to control group. (C) expression of IFN-γ. (C): Western blotting results showed that IFN-γ expression was enhanced in group treated with IFN-γ incomparable to control group.
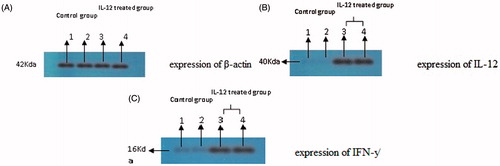
Table 1. Expression of IL-12 and IFN-γ in tumors.
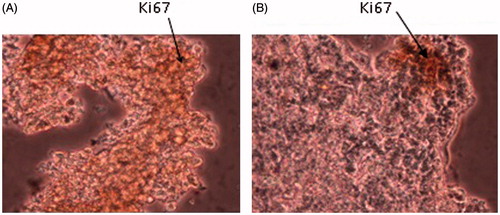
Expression of Ki-67 in tumor sections
The expression of Ki-67 in tumor mass was studied with immunohistochemical staining. The results showed that the expression of Ki-67 was significantly reduced in the group treated with IL-12, incomparable to control group (P < 0.05; .
Discussion
Efficient transduction of a cytokine gene into the tumor cells and modulating immune responses is a viable strategy for treatment of cancer (Wang and Liu Citation2003). Cancer gene therapy can be based on two main modalities; one approach is to cause a direct effect on cancer cells by transferring suicide siRNAs (for ontogenesis) (Shi et al. Citation2008) or proteins related to the cell cycle (Nogawa et al. Citation2005), and apoptosis (Beh et al. Citation2008, Folini et al. Citation2009). In this method, transfer the therapeutic gene into the cancer cells is necessary to induce cytotoxicity. The second approach works by indirect activation of antitumor immunity by introducing a cytokine gene such as IL-12 into tumor cells (Bhatia et al. Citation2013). In such cytokine gene therapy, the therapeutic gene does not need to be delivered into all cancer cells because the cytokine is secreted from the cells. Therefore, even a local supply of IL-12 in tumor is an effective immunotherapeutic approach with decreases the systemic adverse effects. IL-12 gene delivery has already shown promising antitumor effects (Conwell and Huang Citation2005, Lehrman Citation1999, Newman and Bettinger Citation2007, Shen et al. Citation2008, Taniyama et al. Citation2002, Walser et al. Citation2007). IL-12 induces an effective cellular immune response against syngeneic tumors, which is concomitant with a local enhancement in inflammatory Th1 cytokines in the tumor microenvironment. These altered immune responses are followed by a systemic protective immunity and thus, IL-12 has proven to be a promising immunotherapeutic approach for cancer therapy (Pertl et al. Citation2001). For example, neuroblastoma cells producing IL-12 have been showed to induce a CD8+ T cell-dependent local antitumor effect, NK- or T cell-independent anti-angiogenic effect induced by IP-10 and an antimetastatic effect (Lode et al. Citation1998). Adenoviral-mediated IL-12 gene therapy has also shown an antimetastatic effect in prostate cancer with partial dependency on NK cells (Nasu et al. Citation1999). This is while in bladder carcinoma and colon cancer, adenoviral-mediated IL-12 gene therapy mainly depends on the local antitumor effects of T cells (Mazzolini et al. Citation1999).
Although the transfection efficiency of the non-viral gene delivery vectors is lower than viral systems, they are safe (Newman and Bettinger Citation2007). The low transduction efficiency is not an actual hindrance to cytokine gene therapy in cancer, since the transfer the cytokine gene into all cancer cells is not required (Pertl et al. Citation2001, Taniyama et al. Citation2002, Walser et al. Citation2007). In this study, we investigated the antitumor effect of IL-12 gene therapy using plasmid DNA on fibrosarcoma in mice. IL-12 gene delivery with Lipofectamine 2000 suppressed tumor growth. The results of our study showed that IL-12 secretion with tumor cells is an effective method for achieving a high local concentration of IL-12 with low risk of systemic toxicity. The antitumor effect of IL-12 gene therapy was dependent largely on NK cells and partially on T cells. Another appealing observation was that the effect of IL-12 on anti neo-vascularization of the tumor was also dependent on the two cellular components. In some recent reports, NK cells and T cells have been shown to inhibit tumor angiogenesis by production of IFN-γ (Cohen Citation1995, Leonard et al. Citation1997, Li et al. Citation2004, Qin et al. Citation2003, Tare et al. Citation1995). IFN-γ is a potent activator of NK cells and boosts their cytolytic activity. Our study showed that the tumor volume in the group treated with IL-12 was significantly reduced in comparable to the control group (P = 0.000). The results of real time PCR and western blotting showed that the expression of IL-12 and IFN-γ was enhanced in the IL-12 treated group. Furthermore, gene therapy with IL-12 could decrease Ki-67 expression in the fibrosarcoma tumor masses and this finding showed that IL-12 gene therapy could decrease cancerous cell proliferation. Ki-67 is a protein associated with cell proliferation and is present in all other cell cycle phases except G0 (Konsti et al. Citation2011). In addition, a significant level of intra-tumoral IFN-γ was also observed as an inducer of two important antitumor chemokines, IP-10, and MIG (Tare et al. Citation1995). MIG expression in the tumor microenvironment induces T cells and NK cells to exert their antitumor cytotoxic activities (Shen et al. Citation2008). These findings are in agreement with previously reported results.
In conclusion, our results demonstrated that gene therapy with IL-12 could regresses tumor in inoculated tumor models.
Declaration of interest
Author states no conflict of interest. This work was supported by a grant from the Drug Applied Research Center, Tabriz University of Medical Sciences.
References
- Bathula SR, Huang L. 2010. Gene Therapy with Plasmid DNA. Burger's Medicinal Chemistry and Drug Discovery. New York: Wiley.
- Beh CW, Seow WY, Wang Y, Zhang Y, Ong ZY, Ee PLR, Yang YY. 2008. Efficient delivery of Bcl-2-targeted siRNA using cationic polymer nanoparticles: downregulating mRNA expression level and sensitizing cancer cells to anticancer drug. Biomacromolecules. 10:41–48.
- Bhatia S, Menezes ME, Das SK, Emdad L, Dasgupta S, Wang XY, Sarkar D, Fisher PB. 2013. Innovative approaches for enhancing cancer gene therapy. Discov Med. 15:309–317.
- Bramante S, Koski A, Kipar A, Diaconu I, Liikanen I, Hemminki O, et al. 2014. Serotype chimeric oncolytic adenovirus coding for GM‐CSF for treatment of sarcoma in rodents and humans. Int J Cancer. 135:720–730.
- Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately M. 1993. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 178:1223–1230.
- Cohen J. 1995. IL-12 deaths: explanation and a puzzle. Science. 270:908.
- Conwell CC, Huang L. 2005. Recent advances in non‐viral gene delivery. Adv Genetics. 53:1–18.
- Folini M, Pennati M, Zaffaroni N. 2009. RNA interference-mediated validation of genes involved in telomere maintenance and evasion of apoptosis as cancer therapeutic targets. siRNA and miRNA Gene Silencing. Berlin: Springer.
- Gajewski TF. 2012. Cancer immunotherapy. Mol Oncol. 6:242–250.
- Gately MK, Wolitzky AG, Quinn PM, Chizzonite R. 1992. Regulation of human cytolytic lymphocyte responses by interleukin-12. Cell Immunol. 143:127–142.
- Gutiérrez-Ortega A, Sandoval-Montes C, De Olivera-Flores TJ, Santos-Argumedo L, Gómez-Lim MÁ. 2005. Expression of functional interleukin-12 from mouse in transgenic tomato plants. Transgenic Res. 14:877–885.
- Hallaj-Nezhadi S, Valizadeh H, Baradaran B, Dobakhti F, Lotfipour F. 2013. Preparation and characterization of gelatin nanoparticles containing pDNA encoding IL-12 and their expression in CT-26 carcinoma cells. Future Oncol. 9:1195–1206.
- Hara I, Nagai H, Miyake H, Yamanaka K, Hara S, Micallef MJ, et al. 2000. Effectiveness of cancer vaccine therapy using cells transduced with the interleukin-12 gene combined with systemic interleukin-18 administration. Cancer Gene Ther. 7:83–90.
- Helmy KY, Patel SA, Nahas GR, Rameshwar P. 2013. Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv. 4:1307–1320.
- Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 170:827–845.
- Konsti J, Lundin M, Joensuu H, Lehtimäki T, Sihto H, Holli K, et al. 2011. Development and evaluation of a virtual microscopy application for automated assessment of Ki-67 expression in breast cancer. BMC Clin Pathol. 11:3.
- Kowalczyk DW, Wysocki PJ, Mackiewicz A. 2003. Cancer immunotherapy using cells modified with cytokine genes. Acta Biochim Pol. 50:613–624.
- Kresina TF. 2001. An Introduction to Molecular Medicine and Gene Therapy. New York: Wiley Online Library.
- Lehrman S. 1999. Virus treatment questioned after gene therapy death. Nature. 401:517–518.
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, et al. 1997. Effects of single-dose interleukin-12 exposure on interleukin-12–associated toxicity and interferon-γ production. Blood. 90:2541–2548.
- Li S, Xia X, Mellieon FM, Liu J, Steele S. 2004. Candidate genes associated with tumor regression mediated by intratumoral IL-12 electroporation gene therapy. Mol Ther. 9:347–354.
- Lode HN, Dreier T, Xiang R, Varki NM, Kang AS, Reisfeld RA. 1998. Gene therapy with a single chain interleukin 12 fusion protein induces T cell-dependent protective immunity in a syngeneic model of murine neuroblastoma. Proc Natl Acad Sci USA. 95:2475–2480.
- Lotfipour F, Hallaj-Nezhadi S, Valizadeh H, Dastmalchi S, Baradaran B, Jalali MB, Dobakhti F. 2011. Preparation of chitosan-plasmid DNA nanoparticles encoding interleukin-12 and their expression in CT-26 colon carcinoma cells. J Pharm Pharm Sci. 14:181–195.
- Mazzolini G, Qian C, Xie X, Sun Y, Lasarte JJ, Drozdzik M, Prieto J. 1999. Regression of colon cancer and induction of antitumor immunity by intratumoral injection of adenovirus expressing interleukin-12. Cancer Gene Ther. 6:514–522.
- Nasu Y, Bangma C, Hull G, Lee H, Hu J, Wang J, et al. 1999. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther. 6:338–349.
- Newman C, Bettinger T. 2007. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 14:465–475.
- Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K, et al. 2005. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Investig. 115:978–985.
- Pertl U, Luster AD, Varki NM, Homann D, Gaedicke G, Reisfeld RA, Lode HN. 2001. IFN-γ-inducible protein-10 is essential for the generation of a protective tumor-specific CD8 T cell response induced by single-chain IL-12 gene therapy. J Immunol. 166:6944–6951.
- Qin Z, Schwartzkopff J, Pradera F, Kammertœns T, Seliger B, Pircher H, Blankenstein T. 2003. A critical requirement of interferon γ-mediated angiostasis for tumor rejection by CD8 + T cells. Cancer Res. 63:4095–4100.
- Shen Z, Brayman A, Chen L, Miao C. 2008. Ultrasound with microbubbles enhances gene expression of plasmid DNA in the liver via intraportal delivery. Gene Ther. 15:1147–1155.
- Shi X, Liang Z, Ren X, Liu T. 2008. Combined silencing of K-ras and Akt2 oncogenes achieves synergistic effects in inhibiting pancreatic cancer cell growth in vitro and in vivo. Cancer Gene Ther. 16:227–236.
- Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, et al. 2010. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release. 142:245–250.
- Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, et al. 2002. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 105:1233–1239.
- Tare NS, Bowen S, Warrier RR, Carvajal DM, Benjamin WR, Riley JH, Anderson TD, Gately MK. 1995. Administration of recombinant interleukin-12 to mice suppresses hematopoiesis in the bone marrow but enhances hematopoiesis in the spleen. J Interferon Cytokine Res. 15:377–383.
- Toda M, Martuza R, Rabkin S. 2001. Combination suicide/cytokine gene therapy as adjuvants to a defective herpes simplex virus-based cancer vaccine. Gene Ther. 8:332–339.
- Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. 1995. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 87:581–586.
- Walser TC, Ma X, Kundu N, Dorsey R, Goloubeva O, Fulton AM. 2007. Immune-mediated modulation of breast cancer growth and metastasis by the chemokine Mig (CXCL9) in a murine model. J Immunother. 30:490–498.
- Wang JH, Liu XY. 2003. Targeting strategies in cancer gene therapy. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao. 35:311–316.
- Wold SM, Toth K. 2013. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 13:421–433.
- Xu D, Gu P, Pan PY, Li Q, Sato AI, Chen SH. 2004. NK and CD8 + T cell‐mediated eradication of poorly immunogenic B16‐F10 melanoma by the combined action of IL‐12 gene therapy and 4‐1BB costimulation. Int J Cancer. 109:499–506.
- Yoshimoto T, Morishima N, Okumura M, Chiba Y, Xu M, Mizuguchi J. 2009. Interleukins and cancer immunotherapy. Immunotherapy. 1:825–844.
- Zarour HM, Ferrone S. 2011. Cancer immunotherapy: progress and challenges in the clinical setting. Eur J Immunol. 41:1510.