?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Hydrophobic modification of Bletilla striata polysaccharide (BSP) was performed by grafting fatty acids to BSP backbone and then characterized on their physicochemical properties. All neutral derivatives were able to self-assemble into spherical particles within the size range of 250–400 nm, their size and critical micelle concentration decreased with increasing hydrophobicity and substitution degree of the fatty acids. Also, the BSP-stearic acid conjugates showed a preferable performance on hemolysis test and cytotoxicity analysis on HepG2 cells, which suggested their potential application as a drug delivery vector.
Introduction
Amphiphilic copolymers or hydrophobically modified polymers have attracted increasing attention as potential drug delivery nanocarriers. These polymers, containing hydrophilic and hydrophobic segments in their structure, can spontaneously self-assemble into a variety of supramolecular organizations like micelles and vesicles with hydrophobic core inside and hydrophilic shell outside in aqueous media (Bonferoni et al. Citation2014, Bui et al. Citation2012, Chiang et al. Citation2012, Hassani et al. Citation2012). Thus, the resulted nanoassembly can be applied to deliver hydrophobic drugs for improved solubility (Cao et al. Citation2010) and therapeutic efficacy (Boudou et al. Citation2012) of drugs. In recent years, natural polysaccharides have been extensively studied for hydrophobic modification and formation of self-assembled nanoparticles, due to their biodegradability (Chen et al. Citation2011), biocompatibility (Guo et al. Citation2012) and solubilization property (Chen et al. Citation2015). Some polysaccharides, including chitosan (Hu et al. Citation2008, Shan et al. Citation2014), hyaluronic acid, pullulan (Sallustio et al. Citation2004), heparin, and starch (Namazia et al. Citation2011), have been reported on their chemical modifications by grafting hydrophobic groups.
Bletilla striata is a perennial herb mainly distributed in East Asia, whose tuber has been used as a traditional medicine in China for 1000 years (Xiang et al. Citation2014). Bletilla striata polysaccharide (BSP), which is extracted from the tubers of Bletilla striata, has been confirmed to exhibit similar therapeutic effects of anti-fibrosis (Wang et al. Citation2014), antimitotic (Morita et al. Citation2005), and antityrosinase activity (Ye et al. Citation2010). BSP is predominantly consisted of glucomannan with a backbone of (1→4)-β-d-mannose and glucose at a molar ratio of 3:1 (Peng et al. Citation2014), which provided a large number of groups for chemical modification. BSP is commonly used as bioadhesive materials in pharmaceutical formulations, but seldom applied as drug carriers.
In the present work, the hydrophobically modified Bletilla striata polysaccharides (hm-BSPs) were prepared by linking different fatty acids (dodecanoic acid, palmitic acid, and stearic acid) to BSP according to a simple esterification method (Sallustio et al. Citation2004). The resulted hm-BSPs were clarified by FTIR and 1HNMR, their self-assembly behaviors and micelle properties were characterized by fluorescence spectroscopy, transmission electronic microscopy (TEM), and dynamic light scattering (DLS) analysis, respectively. Furthermore, the hemolysis and cytotoxicity of hm-BSP conjugated with stearic acid were also investigated to provide reference data for potential application of hm-BSP nanoparticles in drug delivery.
Materials and methods
Materials
The tubers of Bletilla striata were purchased from Mingde Chinese Medicine Crude Slice Co., Ltd. (Yinchuan, China). BSP was extracted and purified from Bletilla striata tubers (Wang et al. Citation2006). Its polysaccharide, moisture, and protein contents were 95.70, 0.50, and 0.14% determined by phenol-sulfuric acid method, thermogravimetric analysis, and Bradford procedure, respectively. Dodecanoic acid and palmitic acid were obtained from Tianjin Chemical Company (Tianjin, China), and stearic acid was supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). N,N'-dicyclohexylcarbodiimide (DCC) was acquired from Bioduly (Nanjing, China) and dimethylaminopyridine (DMAP) was from Kelong Chemical Regent Co., Ltd. (Chengdu, China). Pyrene was supplied by Sigma-Aldrich Co., Ltd. (Germany) and recrystallized before use. Dimethyl sulfoxide (DMSO) was from Tianjin Chemical Company (Tianjin, China) and stored over 3A molecular sieves for drying. Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal calf serum (FCS) were obtained from Thermo Fisher Scientific Inc. (Nunclon, Denmark). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan). All other reagents were of analytical grade and used as received. Purified water was used for all solutions and dilutions.
Synthesis of hm-BSP
Bletilla striata polysaccharide (BSP) was modified with fatty acids (FA) according to a previously reported method (Sallustio et al. Citation2004). Briefly, BSP (100 mg) was dispersed in 20 mL DMSO and stirred at room temperature until completely dissolved. FA (1.0 mmol) in 5 mL DMSO was activated by the addition of DCC (1.0 mmol) and DMAP (1.2 mmol) and stirring for 2 h in an ice water bath. The obtained mixture was then dropped into the above BSP solution and stir-mixed. The mixture was kept stirring for 2 h at 80 °C, and for another 24 h at room temperature. Following this, the reaction mixture was dialyzed (MWCO 3400) against 1000 mL water for 72 h, and the dialysis water was exchanged every 6 h. Finally, the purified solution was freeze-dried for 30 h by a FD-1C freeze-dryer (Beijing, China) to obtain the hm-BSP products.
Structure analysis of hm-BSP
Fourier transform infrared (FTIR) spectra of BSP and hm-BSP derivatives were analyzed as KBr pellets using a FTIR spectrometer (TENSOR37, Bruker, Ettlingen, Germany). Prior to analysis, samples were mixed with KBr at weight ratio of 1:15. The pellets were placed in the sample holder and spectral scanning was taken in the wavelength range between 4000 and 400 cm−1 at a resolution of 4 cm−1 with the scan speed of 1 cm−1/s.
Determination of substitution degree of hm-BSP
The degree of substitution (DS) was determined by 1H-NMR (Namazia et al. Citation2011) which was recorded on AV400 NMR instrument (Bruker, Ettlingen, Germany). The samples were dissolved in deuterated DMSO at 10 mg/mL. The spectra were obtained at 25 °C with a coupling constant of 150 Hz, a delay time of 1 s and an acquisition time of 80 ms. All chemical shifts were given in parts per million (ppm). Three protons of the methyl terminal of acyl chain were observed as a triplet at 0.85 ppm. And, the peaks between 3.50 and 5.50 ppm corresponded to the signals from four protons of the anhydroglucose structure. The DS was calculated from the ratio of the area of the proton peak at 0.85 ppm to that of the proton peak between 3.50 and 5.50 ppm.
Measurement of fluorescence spectroscopy
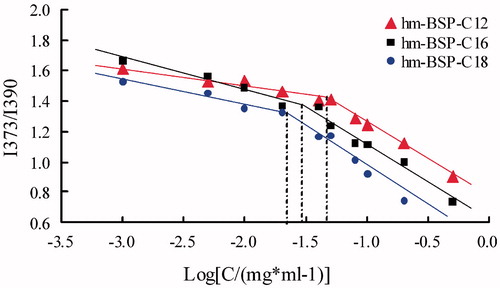
The self-aggregation behavior and the critical micelle concentration (CMC) of hm-BSP derivatives were determined by fluorescence spectroscopy using pyrene as a fluorescent probe. A certain amount of pyrene solution (3.0 × 10−4 mol/L) in acetone was added into a series of test tubes and evaporated under a vacuum to remove the solvent. Then, 10 mL of hm-BSP suspension at various concentrations were added to each test tube, and the final concentration of pyrene was fixed at 6.0 × 10−7 mol/L. The mixtures were shaken overnight at 25 °C in water bath, and then used to measure the steady-state fluorescence spectra by a fluorescence spectrophotometer (970CRT, Shanghai, China). The excitation wavelength was set at 332 nm and the emission spectra were obtained in the range of 320–450 nm at an integration time of 2.0 s. The slit widths for excitation and emission were 5.0 and 2.5 nm, respectively. The ratio of the intensity at 373 nm (I373) to that at 390 nm (I390) was calculated and plotted against the common logarithm of hm-BSP concentration. The CMC of the sample was determined as the corresponding concentration of hm-BSP at the turning point in the plot.
Preparation and characterization of self-assembled hm-BSP nanoparticles
Self-assembled nanoparticles of different hm-BSP derivatives were prepared by an ultrasonic method (Ye et al. Citation2008). Briefly, 25 mg hm-BSP powders were dispersed into 10 mL water and stirred at 37 °C for 4 h until completely dissolved. The solution was treated by a JY99-II ultrasonic processor (Scientz, Ningbo, China) at a power input of 200 W for 10 min in an ice water bath. The period of ultrasound burst was set at 2 s with a pause of 3 s between two bursts. The resulting suspension was then filtered through a 0.45 μm syringe filter, and used for immediate analysis or freeze-drying.
The mean particle size and size distribution of hm-BSP nanoparticles were determined by a NICOMP™380 submicron particle analyzer (PSS Nicomp, Santa Barbara, CA). The morphology of the nanoparticles was observed by an H-7650 transmission electron microscopy (TEM, Hitachi, Japan). One droplet of samples was placed on carbon-coated copper grid, and the excess solution was removed with a filter paper. After air-dried, the samples were viewed and photographed.
Hemolysis test
First, 4 mL of rabbit blood sample was added to 10 mL of normal saline, and then red blood cells (RBCs) were isolated from serum by centrifugation at 2500 rpm for 10 min. The RBCs were further washed five times with 10 mL saline, and then diluted to a concentration of 2.0% (v/v) (Lian et al. Citation2011). Then, the hm-BSP solutions at selected concentrations were added to the tubes containing 2.5 mL of 2.0% RBCs suspensions and further diluted to 5 mL by saline. RBCs suspensions diluted with pure saline or distilled water were used as the negative or positive control, respectively. All samples were incubated at 37 °C and observed at predetermined time intervals. Finally, the samples were remixed by gently inverting the tubes eight times to observe the agglutination of RBCs (Zu et al. Citation2013).
Cytotoxicity test
The tetrazolium-8-[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium] monosodium salt assay was used to assess the cytotoxicity of hm-BSP-C18. Human hepatoblastoma HepG2 cell lines were cultured in DMEM equilibrated with 90% humidified atmosphere of 5% CO2 in air at 37 °C. The medium was supplemented with 10% FCS. HepG2 cells were seeded in 96-well plates at 3 × 104 cells per well and incubated for 24 h. The medium was then replaced with 100 μL of medium containing various concentrations of hm-BSP-C18. The untreated cells were used as the control. The plates were incubated for another 24 h (or 48 h, 72 h), and the cytotoxicity was analyzed using CCK-8 kits according to the manufacturer’s protocol. The absorbance was measured at a test wavelength of 450 nm using a Model 550 microplate reader (Bio-Rad, Segrate, Italy). The percentage of cell viability (CV%) was calculated as follows.
where Atreat and Acontrol were the absorbance of the treated cells and the control, respectively. Experiments were carried out in six wells and tested for three times. The statistical analysis was performed by Student’s t-test using SPSS statistics software version 16.0 (SPSS Inc., Chicago, IL).
Results and discussion
Synthesis of hm-BSP
As presented in , the amphiphilic BSP derivatives were synthesized through the reaction between the carboxylic groups of FA and the hydroxyl groups of BSP. The hm-BSPs with different aliphatic side chain length were defined as follows: BSP dodecanoate (hm-BSP-C12), BSP palmitate (hm-BSP-C16), and BSP stearate (hm-BSP-C18).
Figure 1. Synthesis of hydrophobically modified Bletilla striata polysaccharides with different fatty acids (m: the amount of carbon).
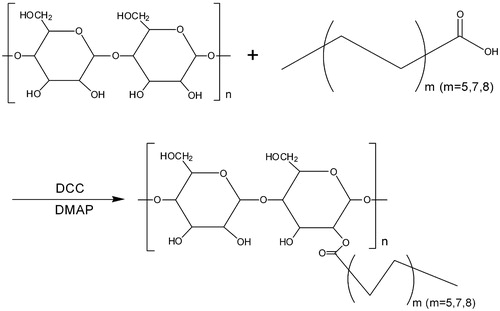
The carboxyl group of FA was chemically coupled with the primary hydroxyl group of BSP via esterification using DCC/DMAP. DCC is called “zero length” cross-linker because the ester linkage was formed without leaving a spacer molecule (Barar and Omidi Citation2014). The remaining DCC and by-products can be easily removed by dialysis against water. Lyophilization resulted in solid, light yellow-colored powder of BSP-FA conjugates.
Structure analysis of hm-BSP
The formation of hm-BSP was verified by FTIR and 1H NMR spectroscopy. shows the FTIR spectra of BSP and hm-BSPs. In the spectrum of BSP, the broad band around 3429 cm−1 was attributed to the strong O–H stretching, the peaks at 2920, 1370, and 1062 cm−1 were due to stretching vibration of –CH2– groups, the absorption at 1742 cm−1 was assigned to stretching vibration of the C–O in carbonyl groups. The characteristic absorption at 812 cm−1 showed that BSP contained α-mannose, while a peak of 875 cm−1 revealed the existence of β-glucose residues (Kong et al. Citation2015). The presence of pyranose was revealed by the absorption at 1028 cm−1. Compared with BSP, obvious changes at 2920 cm−1 (indicating the existence of the methyl groups and methylene groups and 1730 cm−1 (indicating the C = O group in ester bond) can be observed in the spectra of hm-BSPs, demonstrating that FA was successfully introduced into BSP backbone during the reaction.
Figure 2. FT-IR spectra of BSP and its derivatives (a: BSP, b: hm-BSP-C12, c: hm-BSP-C16, d: hm-BSP-C18).
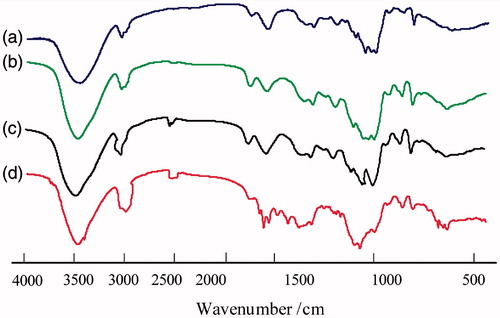
Figure 3. The 1H NMR spectra of BSP and its derivatives (a: BSP, b: hm-BSP-C12, c: hm-BSP-C16, d: hm-BSP-C18) in deuterated DMSO.
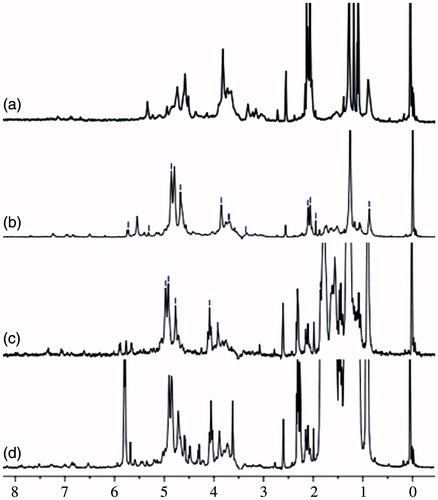
The chemical structure of BSP and hm-BSPs was also analyzed by 1H NMR spectroscopy. The 1H NMR spectrum of BSP showed two obvious peaks in the anomeric region, where the dominant peaks were centered on 4.64 and 4.40 ppm. We assigned 1H-chemical shifts of the protons at 4.70 ppm to H-1, 4.06 ppm to H-2, 3.72 ppm to H-3, 3.75 ppm to H-4, 3.50 ppm to H-5, and 3.89/3.69 ppm to H-6 of 1,4-linked β-mannose, respectively. The signal of 4.46 ppm was attributed to H-1 of 1,4-linked β-glucose. The high field signal, at 2.10 ppm, might be assigned to the methyl of an acetyl group (Zhang et al. Citation2014). The peak at 2.10–2.25 ppm was due to the methylene group beside the carbonyl group, and the one at 1.45 ppm was the methylene group directly before the carbonyl group. All other methylene groups showed a peak at 1.22 ppm (Namazia et al. Citation2011). The broadening peaks for the methylene groups (at 2.10 ppm) close to the ester bond (at 1.45 ppm) indicated successful esterification by comparing BSP to its derivatives. Thus, DS could be determined from the ratio of the normalized, integrated intensities of the signals of methyl group from the acyl chain to that of the protons from anhydroglucose units, according to the following equation and the results were listed in .
where 3 is the number of protons from the signal of the methyl proton, and IAGU is the integral for the 4 protons of the AGU from 3.50 to 5.50 ppm. It clearly showed that DS value increased from 3.70 to 10.56 with increasing alkyl chain length of FA.
Table 1. Properties of hm-BSPs.
Measurement of fluorescence spectroscopy
The aggregation behavior of hm-BSPs in aqueous medium was investigated by fluorescence spectrometry using pyrene as a fluorescence probe (Frizzo et al. Citation2015). Due to its poor solubility and self-quenching property, the pyrene prefers to stay in the hydrophobic microdomains formed in aqueous solution and shows strong emission, and the emission turns to be weak when quenched in polar media. The intensity of I373 will be increased in polar solvent, and no such effect is observed on that of I390 (Huh et al. Citation2000). Thus, the CMC of hm-BSPs can be determined from the change in intensity ratio (I373/I390) of pyrene.
showed the intensity ratio (I373/I390) as a function of hm-BSP concentration. Two straight lines were obtained by extrapolating the intensity ratio (I373/I390) at low and high concentration regions, and their cross-point was estimated as the CMC value for hm-BSP (Yang et al. Citation2014). The CMC value was 0.058, 0.040, and 0.028 mg/mL for hm-BSP-C12, hm-BSP-C16, and hm-BSP-C18, respectively. The CMC values decreased with the increasing chain length of FA, which may be resulted from the enhanced hydrophobicity of inner core as the DS increasing. These results were observed in similar works that authors mentioned in their earlier reports (Gao et al. Citation2008). In addition, the CMC of hm-BSPs were much lower than that of low molecular weight surfactants, such as 2.3 mg/mL for sodium dodecyl sulfate and 1.0 mg/mL for deoxycholic acid (Coello et al. Citation1996). These results showed that the hm-BSP conjugates were a series of novel amphiphilic polymers and could self-aggregate into nanoparticles at low concentration in aqueous medium.
Preparation and characterization of hm-BSP self-assembled nanoparticles
The self-assembled nanoparticles were successfully prepared from hm-BSPs using a diafiltration method. As shown in , the original BSP solution was totally transparent since no assembly took place in aqueous medium. On the contrary, all hm-BSP solutions were turbid, which indicated that hm-BSP molecules self-assembled into nanoparticles. It was also found that the turbidity of hm-BSP solution decreased with the increasing chain length of FA, which indicates reduced particle size. The fact was confirmed by the determination of the mean diameter of hm-BSP nanoparticles by DLS analysis.
Figure 5. Appearance for BSP and hm-BSP solutions (a: hm-BSP-C12, b: hm-BSP-C16, c: hm-BSP-C18, d: BSP).

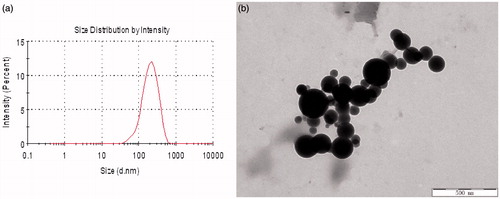
As shown in , the average diameters of hm-BSP nanoparticles with aliphatic side chain length of C12, C16, and C18 were 400, 340, and 250 nm, respectively. A longer hydrophobic chain of FA will form more compact aggregates of micelles, so that the particle size of hm-BSP nanoparticles was decreased with increasing length of side chain. In particular, the nanoparticles of hm-BSP-C18 showed a minimal diameter with a relatively narrow size distribution (PI < 0.20).
Zeta potentials were also determined by DLS. All three samples were nearly neutral, which might be mainly assigned to the fact that BSP itself was a non-ionic polysaccharide and no electric charge was existed on the surface of the nanoparticles.
shows the morphology and size distribution of hm-BSP polymers considering hm-BSP-C18 as an example. Nanoparticles with an average diameter of less than 400 nm were observed, which was similar with the data reported by other authors (Jiang et al. Citation2006). However, the size of hm-BSP-C18 under TEM was relatively smaller than that by DLS analysis, which may due to the fact that DLS analysis were performed in aqueous solution while TEM observation was under air-dried condition. Besides, some particles with diameter of around 100–150 nm were also observed under TEM, which may be caused by different DS of FA chain grafted to BSP.
In vitro toxicity test of hm-BSP-C18
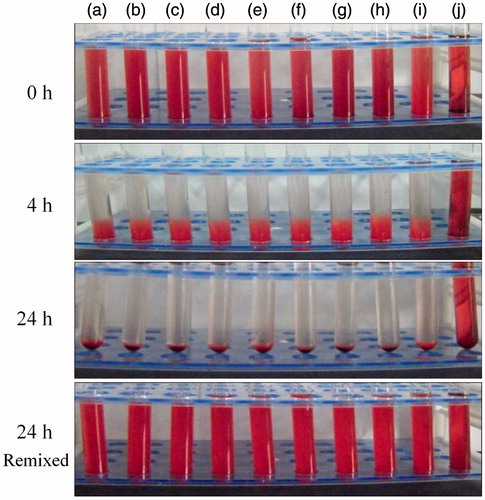
Hemolysis of the blood is an important initial assessment of toxic effect of biomaterials. The membrane breakage of RBCs will cause the release of the hemoglobin and other internal components into the surrounding fluid. In this study, the hemocompatibility of hm-BSP conjugate was evaluated by measuring hemolytic activity. shows the appearance of tested samples at different time intervals. For all vials containing hm-BSP-C18 at various concentrations () or normal saline (), red blood cells were slowly settled down to the bottom of the vials and left a colorless and transparent supernatant, which suggested that no hemolysis was happened to these samples. In addition, the red blood cells were easily re-dispersed by gently re-mixing at the end of the experiment, and no apparent erythrocyte agglutination was observed. However, the vial of positive control () showed no sedimentation throughout the experiment, and its color was darker than the re-dispersed samples, indicating the occurrence of significant hemolysis. These results demonstrate that hm-BSP-C18 is highly blood compatible and is less likely to cause hemolysis after intravenous administration.
Figure 7. Visual observation of hemolysis caused by hm-BSP-C18 at preset time intervals with different concentrations (mg/mL): (a) 0.2, (b) 0.4, (c) 0.6, (d) 0.8, (e) 1.0, (f) 1.5, (g) 2.0, (h) 4.0, (i) normal saline (negative control), and (j) distilled water (positive control).
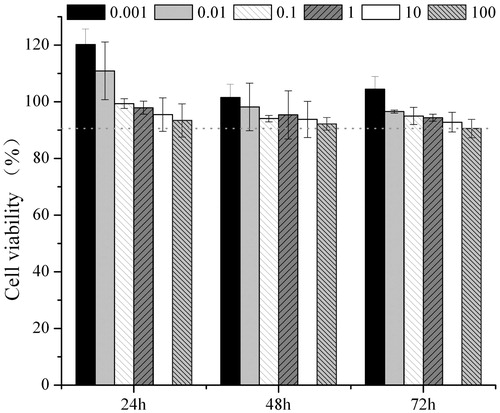
shows the viability of HepG2 cells measured at varied concentrations of hm-BSP-C18 solutions after incubated for different time intervals, respectively. In general, the cell viabilities decreased with the increasing of hm-BSP concentration and incubation time, and the differences were narrowed after incubated for 48 or 72 h. The cell viability remained over 90% even though the concentration went up to 100 μg/mL, suggesting a preferable cytocompatibility of hm-BSP-C18.
Conclusion
A series of novel amphiphilic BSP derivatives modified by FA were synthesized by a simple esterification reaction. The physicochemical properties of hm-BSPs with different side-chain lengths were investigated and compared. The in vitro hemolysis and cytotoxicity of the bioconjugates were also tested. TEM observation showed that hm-BSP nanoparticles were spherical, with their average particle sizes of 250–400 nm and CMC values of 0.028–0.058 mg/mL. The hm-BSP nanoparticles were nearly neutral on zeta potentials. The hm-BSP-C18 showed no hemolysis effect and high cell viability on HepG2 cell lines. In conclusion, the above results suggested that the self-aggregated nanoparticles of hm-BSPs would be applied as promising nanocarriers in pharmaceutical and biomedical fields, especially in the delivery of bioactive agents with poor aqueous solubility due to their excellent hemocompatibility as well as low cytotoxicity.
Declaration of interest
The authors declare no conflicts of interest. This work was supported by the National Natural Science Foundation of China (81160521).
References
- Barar J, Omidi Y. 2014. Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. Bioimpacts. 4:3–14.
- Bonferoni MC, Sandri G, Dellera E, Rossi S, Ferrari F, Mori M, Caramella C. 2014. Ionic polymeric micelles based on chitosan and fatty acids and intended for wound healing. Comparison of linoleic and oleic acid. Eur J Pharm Biopharm. 87:101–106.
- Boudou T, Kharkar P, Jing J, Guillot R, Pignot-Paintrand I, Auzely-Velty R, Picart C. 2012. Polye-lectrolyte multilayer nanoshells with hydrophobic nanodomains for delivery of Paclitaxel. J Control Release. 159:403–412.
- Bui L, Abbou S, Ibarboure E, Guidolin N, Staedel C, Toulmé JJ, Lecommandoux S, Schatz C. 2012. Encapsidation of RNA-polyelectrolyte complexes with amphiphilic block copolymers: toward a new self-assembly route. J Am Chem Soc. 134:20189–20196.
- Cao Y, Gu Y, Ma H, Bai J, Liu L, Zhao P, He H. 2010. Self-assembled nanoparticle drug delivery systems from galactosylated polysaccharide-doxorubicin conjugate loaded doxorubicin. Int J Biol Macromol. 46:245–249.
- Chen MM, Liu Y, Yang WZ, Li XM, Liu LR, Zhou ZM, et al. 2011. Preparation and characterization of self-assembled nanoparticles of 6-O-cholesterol-modified chitosan for drug delivery. Carbohydr Polym. 85:1244–1251.
- Chen Q, Sun Y, Wang J, Yan G, Cui Z, Yin H, Wei H. 2015. Preparation and characterization of glycyrrhetinic acid-modified stearic acid-grafted chitosan micelles. Artif Cells Nanomed Biotechnol. 43:217–223.
- Chiang WH, Lan YJ, Huang YC, Chen YW, Huang YF, Lin SC, Chern CS, Chiu HC. 2012. Multi-scaled polymersomes from self-assembly of octadecanol-modified dextrans. Polymer. 53:2233–2244.
- Coello A, Meijide F, Nuneu ER, Tato JV. 1996. Aggregation behavior of bile salts in aqueous solution. J Pharm Sci. 85:9–15.
- Frizzo CP, Gindri IM, Bender CR, Tier AZ, Villetti MA, Rodrigues DC, Machado G, Martins MAP. 2015. Effect on aggregation behavior of long-chain spacers of dicationic imidazolium based ionic liquids in aqueous solution. Colloids Surf a Physicochem Eng Asp. 468:258–294.
- Gao FP, Zhang HZ, Liu LR, Wang YS, Jiang QY, Xin D, Zhang QQ. 2008. Preparation and physicochemical characteristics of self-assembled nanoparticles of deoxycholic acid modified-carboxymethyl curdlan conjugates. Carbohydr Polym. 71:606–613.
- Guo YZ, Wang XH, Li D, Du H, Wang XY, Sun RC. 2012. Synthesis and characterization of hydrophobic long-chain fatty acylated cellulose and its self-assembled nanoparticles. Polym Bull (Berl). 69:389–403.
- Hassani LN, Hendra F, Bouchemal K. 2012. Auto-associative amphiphilic polysaccharides as drug delivery systems. Drug Discov Today. 17:608–614.
- Hu FQ, Wu XL, Du YZ, You J, Yuan H. 2008. Cellular uptake and cytotoxicity of shell crosslinked stearic acid-grafted chitosan oligosaccharide micelles encapsulating doxorubicin. Eur J Pharm Biopharm. 69:117–125.
- Huh KM, Kwon IC, Kim YH, Kim C, Jeong SY, Lee KY. 2000. Synthesis of triarmed poly(ethylene oxide)–deoxycholic acid conjugate and its micellar characteristics. Langmuir. 16:10566–10568.
- Jiang GB, Quan DP, Liao K, Wang HH. 2006. Preparation of polymeric micelles based on chitosan bearing a small amount of highly hydrophobic groups. Carbohydr Polym. 66:514–520.
- Kong L, Yu L, Feng T, Yin X, Liu T, Dong L. 2015. Physicochemical characterization of the polysaccharide from Bletilla striata: effect of drying method . Carbohydr Polym. 125:1–8.
- Lian H, Sun J, Yu YP, Liu YH, Cao W, Wang YJ, et al. 2011. Supramolecular micellar nanoaggregates based on a novel chitosan/vitamin E succinate copolymer for paclitaxel selective delivery. Int J Nanomedicine. 6:3323–3334.
- Morita H, Koyama K, Sugimoto Y, Kobayashi J. 2005. Antimitotic activity and reversal of breast cancer resistance protein-mediated drug resistance by stilbenoids from Bletilla striata. Bioorg Med Chem Lett. 15:1051–1054.
- Namazia H, Fathi F, Dadkhah A. 2011. Hydrophobically modified starch using long-chain fatty acids for preparation of nanosized starch particles. Sci Iran C. 18:439–445.
- Peng Q, Li M, Xue F, Liu H. 2014. Structure and immunobiological activity of a new polysaccharide from Bletilla striata. Carbohydr Polym. 107:119–123.
- Sallustio S, Galantini L, Gente G, Masci G, La Mesa C. 2004. Hydrophobically modified pullulans: characterization and physicochemical properties. J Phys Chem B. 108:18876–18883.
- Shan P, Shen JW, Xu DH, Shi LY, Gao J, Lan YW, Wang Q, Wei XH. 2014. Molecular dynamics study on the interaction between doxorubicin and hydrophobically modified chitosan oligosaccharide. RSC Adv. 4:23730–23739.
- Wang C, Sun J, Luo Y, Xue W, Diao H, Dong L, Chen J, Zhang J. 2006. A polysaccharide isolated from the medicinal herb Bletilla striata induces endothelial cells proliferation and vascular endothelial growth factor expression in vitro. Biotechnol Lett. 28:539–543.
- Wang Y, Liu D, Chen SJ, Wang Y, Jiang HX, Yin HP. 2014. A new glucomannan from Bletilla striata: Structural and anti-fibrosis effects. Fitoterapia. 92:72–78.
- Xiang YL, Ye Q, Li WB, Xu WL, Yang HJ. 2014. Preparation of wet-spun polysaccharide fibers from Chinese medicinal Bletilla striata. Mater Lett. 117:208–210.
- Yang J, GAo C, Lü S, Zhang X, Yu C, Liu M. 2014. Physicochemical characterization of amphiphilic nanoparticles based on the novel starch-deoxycholic acid conjugates and self-aggregates. Carbohydr Polym. 102:838–845.
- Ye Y, Chou GX, Mu DD, Wang H, Chu JH, Leung AK, Fong WF, Yu ZL. 2010. Screening of Chinese herbal medicines for antityrosinase activity in a cell free system and B16 cells. J Ethnopharmacol. 129:387–390.
- Ye YQ, Yang FL, Hu FQ, Du YZ, Yuan H, Yu HY. 2008. Core-modified chitosan-based polymeric micelles for controlled release of doxorubicin. Int J Pharm. 352:294–301.
- Zhang MS, Sun L, Zhao WC, Peng XX, Liu FQ, Wang YP, et al. 2014. Cholesteryl-modification of a glucomannan from Bletilla striata and its hydrogel properties. Molecules. 19:9089–9100.
- Zu Y, Meng L, Zhao X, Ge Y, Yu X, Zhang Y, Deng Y. 2013. Preparation of 10-hydroxycamptothecin loaded glycyrrhizic acid-conjugated bovine serum albumin nanoparticles for hepatocellular carcinoma-targeted drug delivery. Int J Nanomedicine. 8:1207–1022.