?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Soft tissue adhesives made from natural hydrogel are attractive in clinical applications due to their excellent properties, such as high water content, good biocompatibility, low immune, good biodegradability. Hydrogels derived from natural polysaccharides and proteins are ideal components for soft tissue adhesive since they resemble the extracellular matrices of the tissue composed of various sugar and amino acids-based macromolecules. In this paper, a series of novel tissue adhesives mixed by aldehyde sodium alginate (ASA) with amino gelatin (AG) were developed and characterized. The effect of aldehyde content in ASA and amino group content in AG on the properties of ASA/AG cross-linked hydrogel was measured. The results showed the gelling time, swelling behavior and the bonding strength of the hydrogel can be tuned by varying the content of aldehyde groups in ASA and the content of amino groups in AG. The gelation time could be controlled within 4–18 min. When the aldehyde content of ASA is 75.24% and the amino content of AG is 0.61 mmol/g, the hydrogel almost has the adhesive strength equal to the commercially available adhesive fibrin glue. So, this tunable ASA/AG hydrogels in this study could be a promising candidate as soft tissue adhesive and have a wide range of biomedical applications.
Introduction
Tremendous wounds need to be repaired after traumatic or surgical injury each year in the world. Among them, merely traumatic wounds are more than 12 million (Stussman Citation1996). Injuries are treated by sutures traditionally, while sutures are not ideal because the suture material does not actively participate in healing and the procedure is inherently invasive (Shahinian and Brown Citation1977). Besides, the suture line could injure the surrounding tissue and is not suitable for preventing the liquid outflow after complex surgery. Compared with the traditional sutures, soft tissue adhesive has an effective hemostasis, requires less equipment, operates more quickly, and can avoid the secondary injury without removing. Therefore, more researchers are paying attention to the application of soft tissue adhesives.
In the past few years, much research (Cai et al. Citation2010, Mehdizadeh and Yang Citation2013, Taskin et al. Citation2013) has been carried out on polymeric used for soft tissue adhesives. Among them, the commercialized soft tissue adhesives mainly include α-cyanoacrylate, gelatin, and fibrin glue. Although they have great advantage over traditional suture, none of them completely satisfied the requirement of ideal soft tissue adhesive. Generally speaking, an ideal soft tissue adhesive should be satisfy the following requirements: high water content, safe, ease of application, degradable, and proper adherence (Balakrishnan Citation2005, Mizrahi et al. Citation2011, Smith et al. Citation2010). The α-cyanoacrylate adhesive could produce toxicity causing inflammation and its roughness could injure the surrounding soft tissues (Mizrahi et al. Citation2011). Fibrin is extracted from the plasma (Jackson Citation2001), making it very expensive and which easily spreads disease. The mechanical strength of cross-linked gelatin adhesives is not sufficient (Bae et al. Citation2002, Kim et al. Citation2011). All these urged us to study a new type of adhesive by choosing or developing new materials. Hydrogels derived from naturally occurring polymers have received considerable attention in the past 50 years. Many researches have demonstrated that hydrogels have the potential to direct the migration, growth, and organization of cells during regeneration, repair the cartilage tissue (Kim et al. Citation2011), wound healing (Mizrahi et al. Citation2011, Mo et al. Citation2010), transplanted cells and drug delivery (Ludwig Citation2005). Hydrogel has a high water content to maintain a physiologically moist microenvironment, good biological properties that could minimize bacterial infection at the wound site and mimic many features of extracellular matrix made natural hydrogel available for wound healing (Ahmed Citation2015).
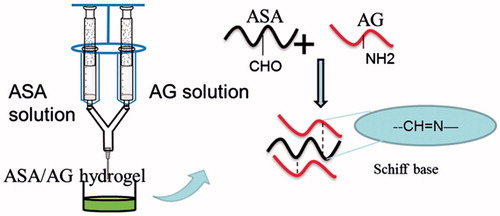
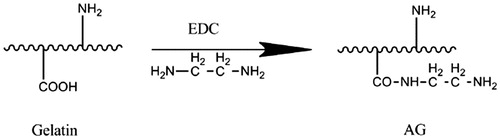
Here, we make an effort to develop a new soft tissue adhesive made of natural materials that are nontoxic (Ding et al. Citation2009, Giri et al. Citation2012), low immune rejection, and low price. We used polysaccharide-sodium alginate and proteins-gelatin for soft tissue adhesive, which is similar to extracellular matrices of the tissue comprised of various sugars and amino-based macromolecules (Ahmed Citation2015). Alginate is a natural polysaccharide extracted from marine algae, and the structure is similar to the glycosaminoglycan of human body that has been widely used in wound dressings. But, the problem of sodium alginate is its non-biodegradablility. Recently it has been reported that its di-aldehyde derivative is biodegradable (Yang et al. Citation2011). So, in this paper we oxidized sodium alginate with sodium periodate to get aldehyde sodium alginate (ASA) to make it biodegradable and get the functional groups for chemical reaction. Gelatin is a water-soluble natural polymer derived from collagen, it has many amino and carboxyl groups which provide modified site. We modified gelatin with ethylenediamine (ED) in the presence of water-soluble 1-ethyl-3 (3-dimethylaminopropyl) carbodiimide (EDC) to introduce additional amino groups to get amino gelatin (AG) that increased the fluidity at room temperature. Upon mixing the ASA and AG solutions, a gelation occurs by Schiff’s base reaction between aldehyde groups of ASA and amino groups of AG to form hydrogels. ASA with different aldehyde contents were generated by controlling the oxidization of alginate with sodium periodates, and AG with different amount of amino groups was developed by converting carboxyl groups into amino groups via ED conjugation. The sol–gel transition temperature was measured and tuned using different alginate and gelatin solutions. Furthermore, porcine skin was used as a soft tissue model to evaluate their adhesive strength. The introduction of amino groups changed the fluidity of AG at room temperature. The modified of sodium alginate and gelatin allowing the ASA/AG hydrogels are suitable for soft tissue adhesive.
Experimental
Materials
The sodium alginate (medium viscosity grade) was obtained from Mingyue Alginate Corporation Limited (Shandong Province, China). Gelatin (from pigskin) was obtained from Luosailuo Gelatin Corporation Limited (Jilin Province, China). Diethyl ether, sodium periodate, ethanediamine, phosphate-buffered saline (PBS, pH = 7.4) were purchased from Sigma-Aldrich (Shanghai, China), and used as received. EDC hydrochloride was supplied by GL Biochem (Shanghai, China). Dialysis tube (MW = 3500 or 14,000 Da) was obtained from Jingkehongda Biotechnology Co. (Shanghai, China). All other chemicals, unless specified otherwise, were of reagent grade and purchased from Sigma-Aldrich.
Methods
Preparation of aldehyde sodium alginate (ASA)
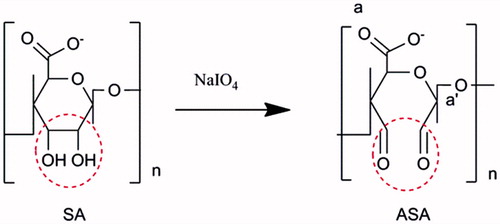
The ASA macromolecules were prepared as previously described (Yuan et al. Citation2014), as shown in . Briefly, sodium alginate (5 g) was dissolved in ultrapure deionized water (DI H2O, 500 mL) at room temperature (25 °C). Then NaIO4 dissolved in water was added drop wise to alginate solutions, followed with oxidization reaction as predetermined periods of time (0.5 h, 2 h, 4 h, 6 h, 16 h, 24 h) under stirring in the dark at room temperature (25 °C), and terminate the reaction by adding ethylene glycol (molar ratio of ethylene glycol: sodium periodate = 1:1). The product was purified by dialysis (Mw = 14,000) against DI H2O for 3 d, and lyophilized to obtain the final product of ASA, the yield was 88%.
Preparation of amino gelatin (AG)
AG was prepared according to our previously described method (Mo Citation2000, Yuan et al. Citation2014). Briefly, 5 g of gelatin was dissolved in 100 mL (0.1 mol/L) sodium dihydrogen phosphate (NaH2PO4) solution (pH = 5). Then, a specific amount of ED and EDC were added, and the pH was adjusted back to 5 by hydrochloric acid, the reaction was allowed to proceed at room temperature overnight. After that, the reaction mixture was dialyzed (Mw = 3500) against ultrapure water for 3 d to remove the excess ED and EDC, and lyophilized to obtain the AG, the yield was 90%.
Determination of the oxidized degree of ASA
Oxidized degree (OD) is defined as the mole ratio of oxidized uronic acid unit (dialdehyde contents) to the total sodium alginate uronic acid unit, so the OD can present the dialdehyde content. We measured the OD of ASA using the method reported previously with iodometric titration and hydroxylamine hydrochloride (Balakrishnan Citation2005, Balakrishnan et al. Citation2014). Briefly, 15 g hydroxylamine hydrochloride was dissolved in 30 mL DI H2O, and further mixed with 40 mL anhydrous ethanol, 100 mL 4.2% sodium hydroxide (NaOH) in ethanol, and 5 mL 0.45% bromophenol blue solution in DI H2O. Then, the mixed solution was placed overnight to get the supernatant for following quantitative analysis.
ASA (0.1000 g) was placed in a flask, followed by adding 5 mL of the above-mentioned hydroxylamine hydrochloride solution and 5 mL of anhydrous ethanol, the mixture was heated to reflux for 2 h and then cooled to room temperature. The hydrochloric acid was calibrated by 0.1 mol/L NaOH titrate solution showed yellow–green colour. Blank experiment without ASA was carried out in the similar procedure. The OD of the ASA was calculated according to EquationEq. (1)(1)
(1) :
(1)
(1)
where V1 is the volume of hydrochloric acid added to the experimental group (L). V2 is the volume of hydrochloric acid added to the blank group (L). N is the concentration of hydrochloric acid used for the titration (mol/L). W is the weight of the ASA (g). 198 is the molecular weight of a unit of alginate (g/mol).
Determination of the amino group in AG
The amount of amino groups in the gelatin or modified gelatin was determined using the trinitro-benzene-sulfonic acid (TNBS) method (Yuan et al. Citation2014). Briefly, 1.0 mL of 4 wt% sodium bicarbonate (NaHCO3) aqueous solution and 1.0 mL of 0.1 wt% 2,4,6-TNBS sodium aqueous solution were added to 1 mL 0.50 mg/mL gelatin or modified gelatin in the PBS solution (PH = 7.4). Reaction was set in the dark for 2 h at 40 °C, and the TNBS and gelatin reaction equations is shown in .
The optical density was then determined at 415 nm (Hitachi UN-2500 spectrometer, Japan). β-Alanine with known concentrations was measured in the same manner as described above. Then, plot a relationship between β-alanine concentration and an absorbance as the standard curve was drawn.
Products generated by sulfonic group of TNBS and amino group of gelatin had a characteristic absorption peak at 415 nm, using the absorbance the amino content of AG was converted to standard curve, then the unite mmol/L was changed to mmol/g. That is, 1 g of gelatin or AG contains amino moles.
The sol–gel transition temperature of gelatin and AG solutions
The vial-inverting approach (Geng et al. Citation2012) was used to determine the sol–gel transition temperature of gelatin and AG solutions. Each sample with a given concentration was stored in a vial at 4 °C , and the vials with polymer gels were immersed in a water bath which has a temperature increment of 1 °C/min, and the samples were regarded as a “gel” in the case of no visual flow within 30 s by inverting the vial.
Preparation of ASA and AG cross-linked hydrogels
Hydrogels were prepared using a double mix syringe glue applicator fitted with a common needle (). One syringe was filled with ASA aqueous solution and the other with an equal volume of AG aqueous solution. The two solutions were mixed in the needle by pushing the applicator and cross-linked by Schiff’s base reaction in a few seconds to form the AG–ASA hydrogels.
Swelling behavior of ASA and AG cross-linked hydrogels
The swelling test of the hydrogels (diameter = 5 mm, thickness = 4 mm) was performed according to the reported method (Geng et al. Citation2012). Briefly, the cylindrical hydrogel disks were prepared as described in the section “Preparation of ASA and AG cross-linked hydrogels” and immersed in PBS at 37 °C for overnight to reach a swelling equilibrium. After wiping off the surface water of the swollen hydrogels, the weight of the hydrogels was measured and recorded as (Ws). The samples were further frozen and lyophilized to obtain the dry weight (Wd). The swelling ratio (Qo) was calculated by EquationEq. (2):(2)
(2)
(2)
(2)
Adhesive strength test of ASA and AG cross-linked hydrogels for soft tissue adhesive
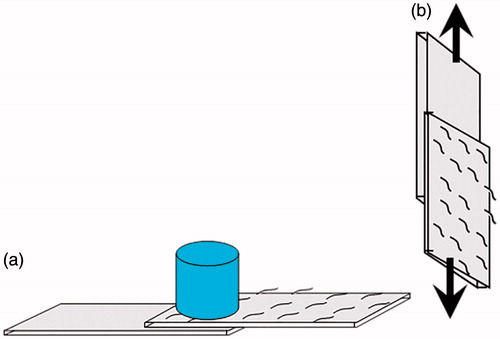
Porcine skin was used as a soft tissue model for investigating the adherence properties of hydrogels. The fatty layer of porcine skin was removed using a scalpel, and the fatty layer-free porcine skin was cut into pieces (1 cm × 2 cm). Then the ASA solutions and AG solutions were mixed in double mix syringes to form hydrogels, and the hydrogels were injected on the the dermal side of skin slice. After that, two skin sheets were immediately overlapped with a bonding area 1 × 1 cm2 and loaded 50 g, as shown in . After 1 h (to make sure the gelation of ASA/AG finished), the bonding strength was measured in the tension mode at room temperature using a Dejie DXLL-20000 materials testing instrument (Shanghai, China), as shown in . The bonding strength was defined as the maximum strength in the stress–strain curve (Jeon et al. Citation2012).
Gelling time determination
ASA solutions (1 mL) were mixed with 1 mL AG solutions in glass vials (diameter 20 mm) by magnetic stirring at 37 °C, the gelling time was recorded as the time required for the magnetic bar to stop, according to Mo (Citation2000).
Cytotoxicity assay by MTT
Cytotoxicity assay was carried out in the hydrogel extraction solutions with L929 mouse fibroblast cells. L929 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Shanghai, China) supplemented by 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. The complete medium was replaced every other day. The hydrogel extraction solution was prepared by incubating the hydrogel in media (99% DMEM and 1% penicillin/streptomycin) without serum for 24 h, 48 h, and 72 h at 37 °C. After the extraction process, the supernatant was taken by centrifuging the extracted liquid and then 0.22 μ2 microporous filter was used to filter the supernatant to remove any impurities and germs. Before using the extraction if culture cells, suitable amount of serum was added to make the serum concentration to10%. To evaluate the potential cytotoxicity of OSA/AG, hydrogels were used for the (3–(4,5-dimethylthiazol-2yl) 2,5-diphenyl tetrazoliumbromide) (MTT) assay (Zorlutuna et al. Citation2011). This assay is used specifically for mitochondrial function and the hydrogel toxicity from the cell viability was evaluated. Cells were cultured in 48-well culture plates at a density of 10,000 cells/well and incubated by the hydrogel extraction solutions. The cells were incubated at different time periods with 0.5 mg/mL MTT at 37 °C and 5% CO2 for 4 h. The solutions were then removed followed by the addition of dimethyl sulfoxide for 0.5 h to ensure the diffusion of purple formazan salts, and finally the absorbance of the resulting solution was measured at 492 nm using a multidetection microplate reader (MK3, Thermo, MA). Three parallels were averaged for each specimen. Reported values are mean of the three replicates.
Statistical analysis
All results are represented as mean ± standard deviation. The data were analyzed by one-way ANOVA, followed by Tukey’s test for the evaluation of specific differences with Origin Pro 8.0 (Origin Lab, Guangzhou, China). p < 0.05 was considered to represent a significant difference.
Results and discussion
Characterization of sodium alginate and aldehyde sodium alginate
The properties of sodium alginate solutions
Before modifying the sodium alginate, we need to know about its properties to obtain a good application. We measured the dissolved time and viscosity of 1% (W/V) sodium alginate at different temperatures (4 °C, 25 °C, 40 °0), the viscosity was measured by LVDV-1 rotational viscometer ().
Table 1. The relationship of dissolved temperature, dissolved time, and viscosity.
The oxidized reaction of sodium alginate
Polysaccharides with adjacent hydroxyl structure, such as cellulose, dextran, and sodium alginate, can be oxidized by strong oxidizer to form an aldehyde group structure, which has been widely applied for hydrogel preparation. Tan et al. (Citation2009) prepared oxidation hyaluronic acid using NaIO4, then prepared the injectable hydrogel by cross-linking the aldehyde group of oxidized hyaluronic acid and amino groups of chitosan. Weng et al. (Citation2008) used strong oxidizer to open the ring of dextran from aldehyde dextran, then mixed with carboxymethyl chitosan, and obtained in situ cross-linking hydrogels which can be used for rapid prototyping.
Sodium alginate contains vicinal hydroxyl groups that can be oxidized with sodium periodate to convert 1,2-hydroxyl groups into aldehyde groups to get ASA ().
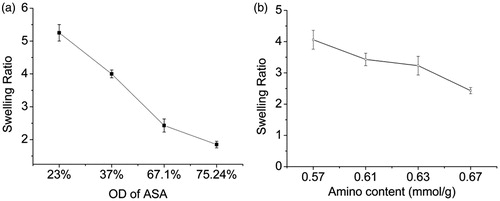
The influence of reaction time on the OD of ASA was measured and calculated by the method described in the section “Determination of the oxidized degree of ASA”. We can see the OD was 61.5% after 0.5 h reaction; extension of the reaction time did not significantly increase the OD (). After reacting for 16 h, it was only 66.3%, so the reaction basically completed in 2 h. Thus, 2 h oxidation of sodium alginate should be sufficient for the generation of the largest amount of aldehyde groups. The influence of oxidizer (NaIO4) dosage for the OD of ASA was also measured (). The results showed the OD increased with the increase of oxidizer dosage used, from 23% for ASA-1 to 75.24% for ASA-4, but decreased to 53.56% for ASA-5. Here, OD did not always increase with the dosage of increasing oxidizer. When the dosages of sodium periodate exceeded a certain critical value, the aldehyde content reduced. This explains that partial aldehyde group could conjugate the adjacent hydroxyl to form hemiacetal, hindering the residual hydroxyl group’s continued oxidation (Painter and Larsen Citation1970), so the OD cannot reach 100%. Also, the excessive sodium periodate could further oxidize the aldehyde group into carboxyl group, so the aldehyde content was reduced. When the molar ratio of NaIO4 and sodium alginate repeat unit was 1.5, the OD was maximum (75.24%).
Figure 5. The influence of reaction time on the oxidized degree of ASA, with the molar ratio of oxidizer (NaIO4)/SA repeat unit is 1.
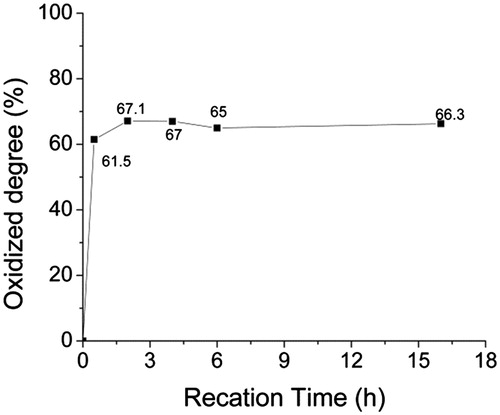
Table 2. The OD of ASA with different molar ratios of oxidizer (NaIO4) to SA unit.
Characterization of amino gelatin
Gelatin is a kind of protein denaturation, it have many amino groups (–NH2) and carboxyl groups (–COOH) which provide modified site. Gelatin was modified with ED in the presence of water-soluble EDC to introduce additional amino groups to form AG, as described in the section “Preparation of amino gelatin (AG)”. The synthesis route of AG is shown in .
shows the amount of amino group in AG measured by TNBS assay, by controlling the molar ratios of ED/COOH for the transition of carboxyl to amino; the amino group content in AG was increased from 0.51 mmol/g of primary gelatin as AG-0 to 0.67 mmol/g of modified gelatin as AG-4.
Table 3. The amount of amino group in AG measured by TNBS assay, after the change of carboxyl groups in primary AG into amino groups with different molar ratios of ED/COOH.
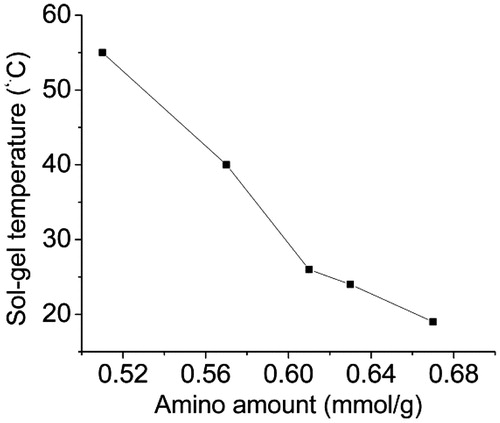
We further measured the sol–gel temperature of AG solutions with different amount of amino groups. As shown in , the sol–gel temperature decreased obviously with amino group content increasing, from 55 °C for AG-0 to 19 °C for AG-4. Thus, the fluidity of AG solutions becomes better than natural gelatin because gelatin is a typical amphiphilic polyelectrolytes. The polymer chains have carboxyl (–COOH) groups with negative charge and amino (–NH2) group with positive charge. Through ED-grafted gelatin, the carboxyl group is obtained and hence more amino groups are also obtained due to the electrostatic attraction between the amino group and carboxyl group. So AG has a lower sol–gel temperature and performs a better fluidity than natural gelatin at room temperature.
Characterization of ASA/AG cross-linked hydrogels
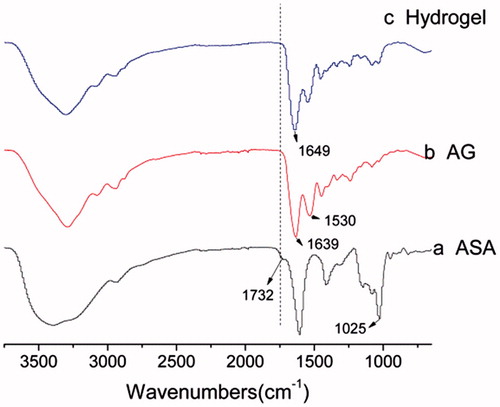
ASA/AG hydrogels were formed by the Schiff-based reaction between the aldehyde groups of ASA and amino groups of AG when mixing ASA solutions and AG solutions together. ATR-FTIR confirmed the mechanism of this reaction (). ASA (a) showed a specific absorption peak at 1732 cm−1 attributing to the aldehyde group, and peak at 1025 cm−1 attributing to the C–O–C structure. AG (b) showed two different peaks at 1639 cm−1 and 1530 cm−1 belonging to the amino group and amide group, respectively. As shown in , hydrogel (c) showing the absorption peak of aldehyde group at 1732 cm−1 decreased dramatically after cross-linking, which means the aldehyde content indeed reduced in the process of forming hydrogel. At the same time, a strong absorption peak at 1649 cm−1 was observed for C = N. The aldehyde group decreasing and C = N group arising indicated the Schiff’s base reaction occurred in the ASA/AG hydrogel.
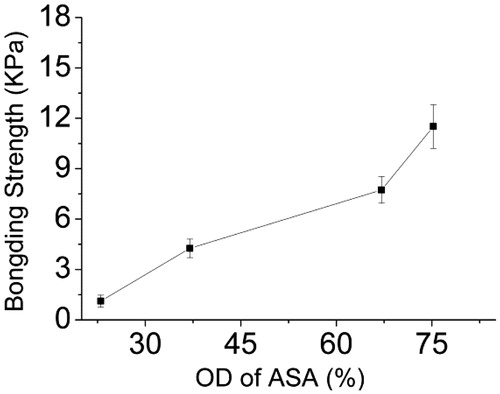
The effect of the amino content of AG and the OD of ASA on the bonding strength was evaluated by measuring porcine skin adhesive ability: Forty wt% ASA-1 (OD is 23%) solutions were mixed with 40% (w/v) AG with different amount of amino groups. shows increase in the amino contents of AG and the bonding strength of the adhesive. When the amino content increased to 0.67 (mmol/g), the bonding strength of the adhesive attains the maximum value of 10.28 ± 1.69 KPa. These results show that more amino content of AG with the aldehyde content of ASA could generate a denser cross-linked network, resulting in greater cohesion strength of the adhesive. shows the effect of the OD of ASA on the bonding strength of porcine skin adhered to 40 wt% AG-2 solution (amino content is 0.57 mmol/g) mixed with 40% (w/v) ASA with different aldehyde contents. As shown in , the bonding strength increased with the increasing OD of ASA, the highest bonding strength is 11.51 ± 1.30 KPa for ASA-4/AG-2. This is because ASA with more aldehyde groups could also form a denser network, then further enhancing the adhesion strength in porcine skin.
Figure 9. The bonding strength of porcine skin adhered by mixing solution of 40 wt% ASA-1 (OD is 23%) and 40% (w/v) AG with different amount of the amino groups.
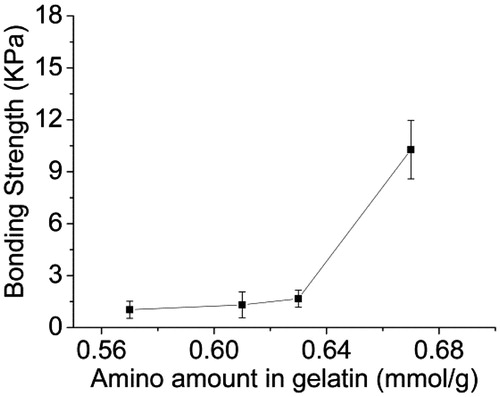
Figure 10. The bonding strength of porcine skin adhered by mixing solution of 40% (w/v) ASA with different OD and 40% (w/v) AG-2.
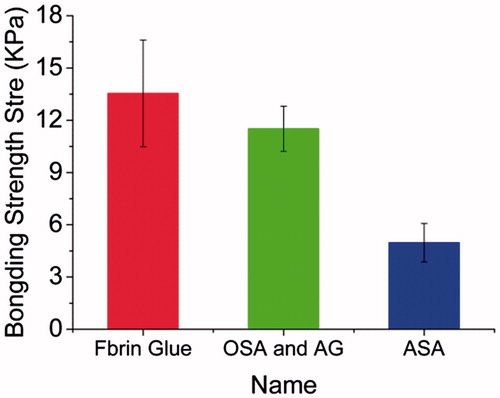
Furthermore, the adhesive effect of ASA-based hydrogels were compared with the commercially available fibrin glue. As shown in , when only one component of ASA-4 (OD is 75.24%) is used to adhesive the porcine skin, the bonding strength was about 4.97 ± 1.10 KPa, which was significantly lower than 11.51 ± 1.30 KPa for ASA-4/AG-2. This is because the cross-linking density of one component of ASA is less than two components of ASA/AG hydrogels, while the adhesive bonding strength of ASA/AG hydrogels were closer to the commercial fibrin glue which has the bonding strength of 13.54 ± 3.07 KPa.
Figure 11. The bonding strength of porcine skin adhered by commercial fibrin glue, 40% (w/v) ASA-4 and mixing solution of 40% (w/v) ASA-4 with 40% (w/v) AG-2.
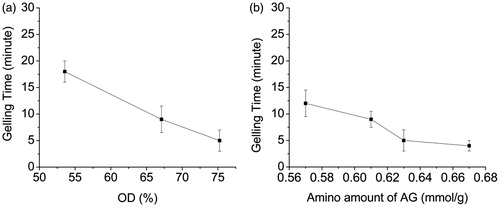
We measure the gelation time dependence on different OD of ASA and different amino amount of AG. shows that the gelling time decrease with the increase in OD when the amine group content is 0.67 mmol/g for AG-4 (). Furthermore, the gelling time also decreased with increase in the amino amount of AG when the OD of ASA is 75.24% for ASA-4, as shown in . The gelation time could be controlled within 4∼18 min. This phenomenon demonstrates that the more the aldehyde groups or amino groups, the more collisions will occur between aldehyde groups and amino groups, so the faster the hydrogels are formed. The gelation time of ASA and AG is controllable through change in the aldehyde group content in ASA or amino group content in AG.
Infiuence of the OD (dialdehyde content) of ASA and amino content of AG on hydrogel swelling
Hydrogels are formed by physical or chemical bonds between polymer chains. Cross-linking structure of the polymer cannot be dissolved by the water solvent, only absorbing a certain amount of solvent and swelling to form hydrogel. Studying the swelling characteristics of hydrogel networks is important in various applications. The influence of the OD of ASA and amino content of AG on hydrogel swelling was measured as mentioned above. As shown in , the swelling ratio of ASA/AG hydrogel decreased with the increase of OD of ASA () and the amino content of AG (). This could be explained by the hydrogel swelling ratio which is generally influenced by the cross-linking density. The cross-linking density increased with the increase of OD in ASA and amino content in AG. As a result, the network structure of hydrogels tend to be more tightly preventing water penetration into the scaffold, causing a lower swelling ratio.
Cytotoxicity test
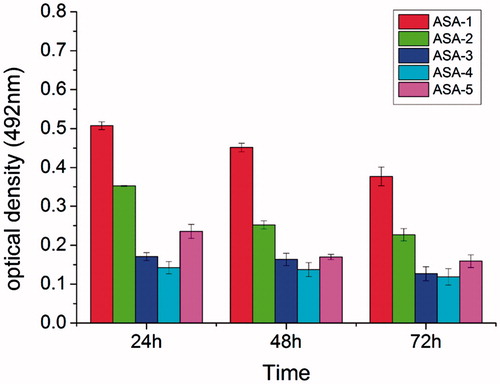
To evaluate the cytotoxicity nature of the ASA/AG hydrogels, a preliminary test using L929 mouse fibroblast cells was carried out. The MTT assay was implemented to quantitatively investigate the metabolic activity of L929 cells. The more cells present, the higher the optical density that can be detected. As shown in , the results of MTT assay for different aldehyde contents of ASA cross-linked with AG-2 to form hydrogels, and the hydrogels extraction solution was used to culture cells after 24 h, 48 h, and 72 h. The obtained statistical analysis of optical density showed that the hydrogels extraction solution could induce cells growth, which demonstrated that the ASA/AG hydrogels can provide an excellent environment for cell growth. The optical density of hydrogels decreased slightly with the increase in culture time because, with the time increasing, the cross-linked hydrogels began to degrade. Besides, the optical density reduced when the aldehyde content of ASA increased at the same time point. This is due to the excessive aldehyde groups in hydrogel. The existence of the aldehyde groups is unfavorable for the cell culture.
Conclusions
Alginate-based hydrogels are potential biomaterials for many applications, particularly in the areas of soft tissue adhesive. The results demonstrate that the OD was increased with the increase in oxidizer dosage. The most suitable reaction time was about 2–6 h. The introduced amino group content increased with the increasing ED concentration. With the increase in amino content of AG, the sol–gel temperature decreased from 55 °C to 19 °C. The bonding strengths of hydrogel were dependent on the OD of ASA and amino group content of AG. The bonding strength had no significant difference between ASA-4/AG-2 and the commercially available fibrin glue. The gelation time could be controlled within 4–18 min. The swelling ratio test confirmed denser network when the aldehyde groups or amino groups increased. The cytotoxicity test by MTT confirmed that the ASA/AG hydrogels are nontoxic for cells. The entire test suggesteded that the ASA/AG hydrogels could be a promising candidate as soft tissue adhesive.
Funding information
This research was supported by National Nature Science Foundation of China (31470941, 31271035), the Innovation Fund Designated for Graduate Students of Donghua University (Item No. CUSF-DH-D-2015032), Science and Technology Commission of Shanghai Municipality (15JC1490100, 15441905100), Ph.D. Programs Foundation of Ministry of Education of China (20130075110005), and light of textile project (J201404).
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the research group project no. RGP-201.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ahmed EM. 2015. Hydrogel: preparation, characterization, and applications. A review. J Adv Res. 6:105–121.
- Bae SK, Sung TH, Kim JD. 2002. A soft-tissue gelatin bioadhesive reinforce with a proteinoid. J Adhes Sci Technol. 16:361–372.
- Balakrishnan B, Jayakrishnan A. 2005. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 26:3941–3951.
- Balakrishnan B, Joshi N, Jayakrishnan A, Banerjee R. 2014. Self cross-linked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 10:3650–3663.
- Cai ZX, Mo XM, Zhang KH, Fan LP, Yin AL, He CL, Wang HS. 2010. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int J Mol Sci. 11:3529–3539.
- Ding Y, Zhao Y, Tao X, Zheng YZ, Chen JF. 2009. Assembled alginate/chitosan micro-shells for removal of organic pollutants. Polymer. 50:2841–2846.
- Geng X, Mo X, Fan L, Yin A, Fang J. 2012. Hierarchically designed injectable hydrogel from oxidized dextran, amino gelatin and 4-arm poly(ethylene glycol)-acrylate for tissue engineering application. J Mater Chem. 22:25130–25139.
- Giri TK, Thakur D, Alexander A, Ajazuddin, Badwaik H, Tripathi DK. 2012. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: present status and applications. Curr Drug Deliv. 9:539–555.
- Jackson MR. 2001. Fibrin sealants in surgical practice: an overview. Am J Surg. 182:1S–7S.
- Jeon O, Alt DS, Ahmed SM, Alsberg E. 2012. The effect of oxidation on the degradation of photocrosslinkable alginate hydrogels. Biomaterials. 33:3503–3514.
- Kim IL, Mauck RL, Burdick JA. 2011. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 32:8771–8782.
- Lee KY, Mooney DJ. 2012. Alginate: properties and biomedical applications. Prog Polym Sci. 37:106–126.
- Ludwig A. 2005. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev. 57:1595–1639.
- Mehdizadeh M, Yang J. 2013. Design strategies and applications of tissue bioadhesives. Macromol Biosc. 13:271–288.
- Mizrahi B, Weldon C, Kohane DS. 2011. Tissue adhesives as active implants. Stud Mechanobiol Tissue Eng. Biomaterials. 8:39–56.
- Mo X. 2000. Soft tissue adhesive composed of modified gelatin and polysaccharides. J Biomater Sci Polym Ed. 11:341–351.
- Mo X, Iwata H, Ikada Y. 2010. A tissue adhesives evaluated in vitro and in vivo analysis. J Biomed Mater Res. 94:326–332.
- Painter T, Larsen B. 1970. Formation of hemiacetals between neighbouring hexuronic acid residues during the periodate oxidation of alginate. Acta Chem Scand. 24:813–833.
- Shahinian L Jr., Brown SI. 1977. Postoperative complications with protruding monofilament nylon sutures. Am J Ophthalmol. 83:546–548.
- Smith TJ, Kennedy JE, Higginbotham CL. 2010. Rheological and thermal characteristics of a two phase hydrogel system for potential wound healing application. J Mater Sci Technol. 45:2884–2891.
- Stussman BJ. 1996. National Hospital Ambulatory Medical Care Survey: 1994 Emergency Department Summary. Adv Data. 275:1–20.
- Tan H, Chu CR, Payne KA, Marra KG. 2009. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 30:2499–2506.
- Taskin AK, Yasar M, Ozaydin I, Kaya B, Bat O, Ankarali S, Yildirom U, Aydin M. 2013. The hemostatic effect of calcium alginate in experimental splenic injury model. Ulus Travma Acil Cerrahi Derg. 19:195–199.
- Weng L, Romanov A, Rooney J, Chen W. 2008. Non-cytotoxic, in situ gelable hydrogels composed of N-carboxyethyl chitosan and oxidized dextran. Biomaterials. 29:3905–3913.
- Yang JS, Xie YJ, He W. 2011. Research progress on chemical modification of alginate: a review. Carbohydr Polym. 84:33–39.
- Yuan L, Xiaohua G, Xiumei M. 2014. Aldehyde-sodium alginate and amino-gelatin preparation as soft tissue adhesive. J Donghua Univ. 31:503–506.
- Zorlutuna P, Jeong JH, Kong H, Bashir R. 2011. Stereolithography-based hydrogel microenvironments to examine cellular interactions. Adv Funct Mater. 21:3642–3651.