Abstract
Dendrimers represents a novel class of macromolecules, which are derived from branches upon branches type structural design. Dendrimers are emerging as promising drug-delivery molecule because of their extraordinary properties including membrane interaction, monodispersity, well-defined size, shape and molecular weight, etc. Drugs interact with dendrimers in three ways; (a) physical encapsulation, (b) electrostatic interactions, and (c) covalent conjugations. Due to compact, globular structure and availability of interior cavity spaces and multiple surface functional groups, drug molecules can be encapsulated both in the interior of the dendrimers (physical encapsulation) as well as attached to the surface functional groups (covalent conjugations).
Introduction
About 40% of recently developed formulations are redundant to pharmaceutical industry and may never help a patient because of poor bioavailability due to low water solubility or cell membrane permeability. Use of conventional formulations is limited by poor release pattern of drugs, poor solubility, and toxicity. New drug-delivery technologies based on nanomaterials may be a ray of hope to overcome these challenges (Bagre et al. Citation2013, Jain et al. Citation2010). Nanotechnology is based on the materials having size range in the dimensions of 1–100 nm. One of the most advanced nanomaterials, i.e., dendrimer, is being explored widely in biomedical applications due to well-defined size and shape (Daraee et al., Citation2014, Dhankhar et al. Citation2010, Drbohlavova et al. Citation2013, Svenson Citation2009). Dendrimers are highly branched spherical molecules having well-defined chemical structure. They are defined as mono-dispersed, 3D, and highly branched polymers (Jain et al. Citation2014a, Jain et al. Citation2014b, Kesharwani et al. Citation2014). In 1978, Fritz Vogtle and coworkers introduced dendrimer chemistry and in 1985 Tomalia synthesized first family of dendrimer. Newkomes group reported the synthesis of dendrimer and named them arborols which is derived from a Latin word Arbor meaning “tree”. Dendrimer is highly branched synthetic polymer and consists of monomer unit having high degree of branching and hence known as polymer of twenty-first century (Kesharwani et al. Citation2014, Tomalia Citation2005).
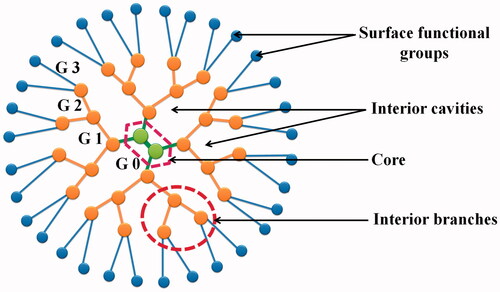
Dendrimers are promising candidates for improving the solubility and to diminish the toxicity of drugs with poor solubility and narrow therapeutic index (Gillies and Fréchet Citation2005). Dendrimers are considered as miracle carriers being investigated for the severe diseases, for example cancer, AIDS, tuberculosis (Gupta et al. Citation2010). Dendrimers have been investigated for encapsulation and controlled delivery of various anticancer drugs attributed to their high drug loading capacity, easy synthesis, stability, transdermal ability, oral drug-delivery potentials. Architectural components of dendrimers include interior core, interior layers (generations) composed of repeating units radially attached to the interior core, and exterior (terminal functionality) attached to outermost interior generation (Jain et al. Citation2015a, Citationb) (). Dendrimers have proved their fineness as carrier for drugs because of their 3D architecture, low polydispersity, and high functionality. Dendrimers may prolong the residence time of drug, increase the stability of bioactive, and protect it from biological environment (Jain and Jain Citation2014b). In addition to this, dendrimers have also been used in gene therapy for intracellular delivery of genetic material into target cells (Jain et al. Citation2013a, Jain et al. Citation2013b).
Dendrimers have been successfully explored for the delivery of anticancer drug as well as for theranostic applications in cancer therapy. Dendrimeric conjugates of anticancer drug have shown ability to bypass efflux transporter, to deliver the drug intracellularly, and to improve bioavailability of loaded molecular cargo. Complexes of cisplatin with dendrimers have showed reduced cytotoxicity with significant anti-proliferative activity. Apart from targeted delivery of chemotherapeutic agents, dendrimers are also being investigated for the delivery of diagnostic agents for targeted imaging of tumor (Li et al., Citation2015a, Li et al. Citation2015b, Nguyen et al. Citation2015, Orocio-Rodríguez et al. Citation2015, Uram et al. Citation2015). In this article, we have discussed in brief about the dendrimers and reviewed the mechanisms of interaction of dendrimers with drug followed by mechanism of release of loaded molecular cargo from dendrimers.
Terms and nomenclature in dendrimer chemistry
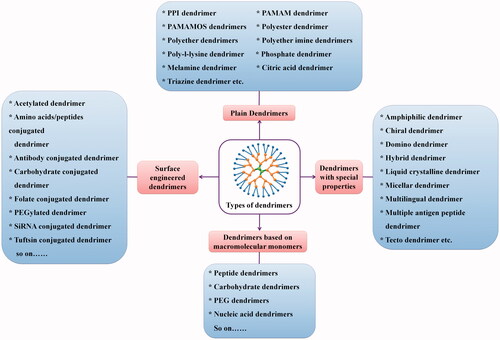
Traditional synthetic polymers have been classified into three major macromolecular structural designs include; (i) linear (class I), (ii) cross-linked (bridged; class II), and (iii) branched types (class III). Structures or architectures of these classes are produced by largely statistical polymerization processes, rather than exact distribution processes. Dendrimers represent the fourth class of polymers which are designed by step-by-step controlled synthesis based on chemical reactions. Dendrimers represent branched macromolecular globular structure with monodispersity, mostly synthesized by convergent or divergent synthesis method (Caminade and Turrin, Citation2014, Kesharwani et al. Citation2014).
Dendrimer chemistry as extra specialized research fields has its own terms as described below.
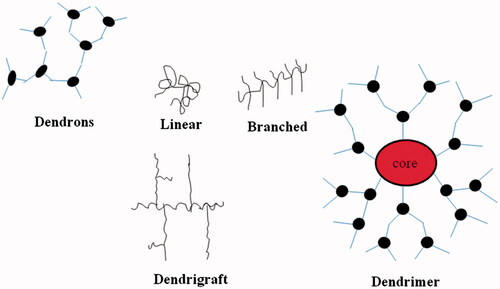
Dendrigrafts
Dendrigraft polymers () are closest to dendrimers structures. Functionalization and grafting reaction are used to synthesize the dendrigrafts. Grafting linear chains onto a linear polymer substrate suitably functionalized with coupling sites yields a comb-branched polymer structure called dendrigrafts (Cadena and Gauthier Citation2010).
Dendrons
Dendrons is the term used about a dendritic wedge without a core, the dendrimer can be prepared from assembling two or more dendrons. These dendrons () have been used in the creation of huge numbers of dendrimers. Properties and characteristics of dendrimers depend upon the dendrons (Woo et al. Citation2007).
Generation
Dendrimers are synthesized by repetition of chemical reaction and grown out of focal point, i.e. core. With each repetition one layer is formed around the core which is known as ‘generation’ number of dendrimers as shown in . The number of focal points when going from the core toward the dendrimer surface is known as generation number. Generation () is common for all dendrimer designs and the hyperbranching when going from the centre of the dendrimer toward the periphery, resulting in homostructural layers between the focal points (branching points). Thus, a dendrimer having five concentric layers when going from the centre to the periphery is denoted as the 5.0 G generation dendrimer (Jain et al. Citation2010, Kesharwani et al. Citation2011, Kesharwani et al. Citation2014).
Shell
The dendrimer shell is the homostructural spatial segment between the focal points, the ‘generation space’. The ‘outer shell’ is the space between the last outer branching point and the surface. The ‘inner shells’ are generally referred to as the dendrimer interior (Meier Citation2000).
End-group
End groups are also generally referred as the ‘terminal groups’ or the ‘surface groups’ of dendrimers. Surface group is slightly more accurate word, in the sense that the dendrimers branches can sometimes fold into the interior of the dendrimers. Dendrimers having amine end-groups are termed ‘amino-terminated dendrimers’ like polypropylene imine (PPI), polyamido amine (PAMAM), and poly-l-lysine (PLL) dendrimers (Jain and Jain Citation2014a, Mehra et al. Citation2014, Citation2015, Shi et al. Citation2015).
Molecular structure and properties of dendrimers
Molecular structure
Dendrimers possess asymmetric shape in 1, 2, and 3 generations as compared with higher generations. During the synthesis of dendrimers, when it reaches to its fourth generation or higher generations, it attains highly branched and globular structures (Klajnert and Bryszewska Citation2000).
Properties of dendrimers
Dendrimers have compact and Globular structure.
Dendrimers are synthesized carefully and in stepwise manner.
Dendrimers have high structural control.
Dendrimers have well-defined architecture.
Dendrimers are monodisperse and highly branched macromolecules with precise molecular weight, shape, and size.
Dendrimers have surface functional groups for drug conjugation and inner cavities for entrapment of drug.
Dendrimer has lower glass temperatures (Kaneshiro and Lu Citation2009, Klajnert and Bryszewska Citation2000, Mishra Citation2011).
Methods for synthesis of dendrimers
Divergent growth method, convergent growth method, hypercore and branched method, double exponential, and mixed growth methods are generally used for synthesis of dendrimers (Kesharwani et al. Citation2014, Klajnert and Bryszewska Citation2000).
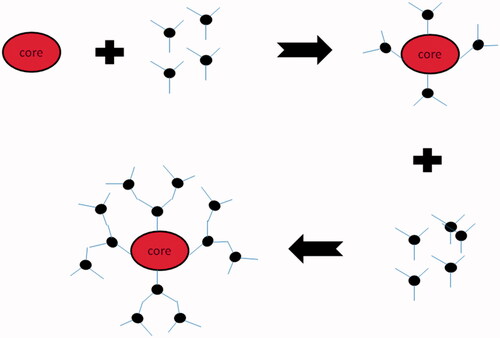
Divergent growth method
In this method, growth of dendrimers starts from core and grows in outward direction (). This method was firstly introduced by Tomalia and Newkome and the name divergent came from the synthesis strategy in which dendrimers grow around the core. Divergent growth methods result in sequential addition of generations to core resulting into 3D architecture of dendrimers with simultaneous increase in number of surface functional groups, molecular weight, and generations (Jain et al. Citation2010, Kesharwani et al. Citation2014).
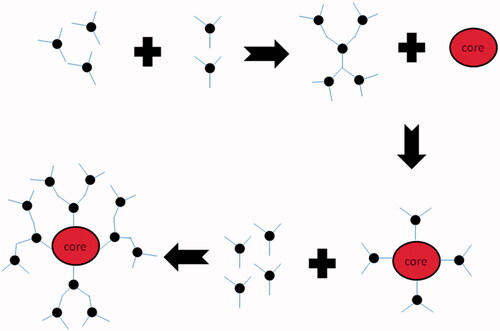
Convergent growth method
Mainly this method begins from core and progresses toward inward (). The branching units are grown and linked to one and other groups. When these branches are large enough, they are attached to core molecule (Jain et al. Citation2010, Kesharwani et al. Citation2014).
Hypercore and branched method
Herein, pre-assembly of oligomers (a polymers whose molecules consist of relatively few repeating units) join together to produce the desired structure of dendrimer in few steps. This is the appropriate method over convergent method because the fewer steps are required for synthesis of higher generations of dendrimer (Jain et al. Citation2010, Kesharwani et al. Citation2014).
Double exponential and mixed growth
In this method, dendrimers are synthesized by both divergent as well as convergent growth methods (Jain et al. Citation2010, Kawaguchi et al. Citation1995, Kesharwani et al. Citation2014). It is the most advanced method of dendrimer synthesis where both divergent and convergent methods are used to form a triangle called ‘Dendrimer’. The triangle may be used to repeat the growth process (Kawaguchi et al. Citation1995).
Dendrimers in anticancer drug delivery
Dendrimer is a nanocarrier having size 1–100 nm. Dendrimers are promising candidates for improving the solubility and diminishing the toxicity of anticancer drugs as well as for targeted delivery to cancer cells and theranostic applications (Jain et al. Citation2012, Jain et al. Citation2013a, Jain et al. Citation2013b) ().
Mechanism of dendrimer–drug interactions
Drug molecules may be covalently conjugated to the end groups of a dendrimer or entrapped inside the core via hydrogen bonding, hydrophobic linkage, or electro–static interactions (Hughes Citation2005). The number of generations influences the drug loading capacity: a relatively high generation number provides more space for guest drugs and has a larger number of functional groups on the surface for drug conjugation. PPI, PAMAM, PLL, polypeptide, polyesters, polyether dendrimers, and dendrimers based on PEG, or carbohydrates have been mainly investigated for delivery anticancer drugs (Shi et al. Citation2015, Turnbull and Stoddart Citation2002). These dendrimers have been investigated frequently as drug carrier, due to availability of high density of functional groups (amine groups) on the surface. In addition, cationic charges of PPI, PAMAM, and PLL dendrimers enable the delivery of nucleic acids. Drug release from dendrimers depends on the type of interactions between a drug and a dendrimer (Howell and Fan Citation2009). Anticancer drug interacts with dendrimers via three different mechanisms, i.e. physical encapsulation, electrostatic interaction, and covalent conjugation which are described in detail in the following sections. A brief summary of mechanism of interaction of anticancer drugs with dendrimers is shown in .
Table 1. Mechanisms of interactions of various anticancer drugs with dendrimers.
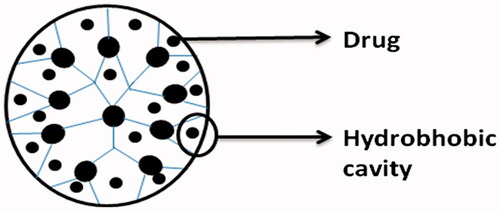
Physical encapsulation
Due to its spherical shape, unfilled internal cavities, and structural design, dendrimers can directly encapsulate guest molecules into the macromolecule interior (). The empty internal cavities of dendrimers are hydrophobic in nature and interact with poorly soluble drugs through hydrophobic interactions (Liu and Frechet Citation1999). Nitrogen or oxygen atoms present in the internal cavities of dendrimers can interact with the drug molecules by hydrogen bond formation (Kesharwani et al. Citation2011). The relationship between the internal cavities of dendrimers and drug molecules may involve some interactions like physical encapsulation, hydrophobic interaction, or hydrogen bonding.
Due to their inherent architecture, drug molecules can be loaded into dendrimers using their well-defined internal crevices by hydrophobic interactions or hydrogen bonding or by electrostatic interactions between drug ionic groups and oppositely charged dendrimer surfaces. The ability of dendrimers to solubilize a variety of drugs including anticancer, anti-HIV, and anti-inflammatory agents has recently been reviewed (Medina and El-Sayed Citation2009). Compared with chemical conjugation, physical incorporation of drugs has the advantage of straightforward, rapid preparation without adversely affecting drug pharmacological activity. Its disadvantages include low stability in terms of storage and premature drug release, batch-to-batch variation in concentration of solubilized drug and low-drug loading capacity (Caminade and Turrin Citation2014, Jain and Jain, Citation2015).
Electrostatic interactions
The large number of amine groups and carboxyl groups on the surface of dendrimers has potential applications in enhancing the solubility of hydrophobic drugs by electrostatic interaction (Milhem et al. Citation2000). Some drugs with carboxyl groups like ibuprofen, ketoprofen, diflunisal, naproxen, and indomethacin have been complexed with dendrimers by electrostatic interactions. Through electrostatic interactions, various ionizable drugs form complexes with the multifunctional surface of dendrimers having large number of ionizable terminal surface groups. In PAMAM dendrimers, both primary amine and tertiary amine groups present on the surface and within the core, respectively, are titrable and has pKa values of 10.7 and 6.5, respectively. Hence, they possess the ionizable groups terminally as well as within their core and thus offer potential sites for drug interactions. Many of the drugs such as ibuprofen, piroxicam, indomethacin, and benzoic acid have shown to form stable complexes through electrostatic interactions (Madaan et al. Citation2014).
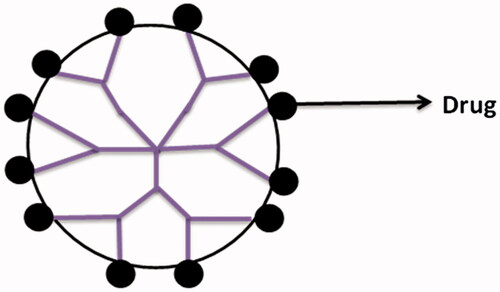
Covalent conjugation
Presence of large numbers of functional groups on the surface of dendrimers makes them suitable for the covalent conjugation of numerous drugs with relevant functional groups () (Patri et al. Citation2005). In the case when drug is covalently bound to dendrimers, its release occurs through chemical or enzymatic cleavage of hydrolytically labile bonds (Patri et al. Citation2005). Moreover, the drugs can be covalently conjugated to dendrimers through some spacers that may include PEG, p-amino benzoic acid, p-amino hippuric acid, lauryl chains, etc., or biodegradable linkages such as amide or ester bonds. This prodrug approach has been found to increase the stability of drugs and has affected their release kinetics significantly. Several researchers have successfully conjugated penicillin V, venlafaxine, 5-aminosalicylic acid, naproxen, propranolol with PAMAM dendrimers. The results have shown enhanced solubility and controlled release of drugs from these complexes in comparison to the plain drug. Apart from these, many anticancer drugs viz. cisplatin, doxorubicin, epirubicin, methotrexate, and paclitaxel have also been conjugated with dendrimers and have shown potential drug targeting. For example, epirubicin prodrug was developed by conjugating it with PEG dendrimers containing aminoadipic acid as branching molecules. These conjugates exhibited increased blood residence time and showed improved therapeutic action. The study revealed that PEG dendrimers increased the stability of bound drug toward chemical degradation and hence can be used potentially in the development of prodrugs of large molecules (Madaan et al. Citation2014).
Some of the drawbacks of physical drug incorporation can be reduced by covalent linking of a drug to the dendrimer using a chemical approach which will also bring about selective drug release in vivo. Such an approach can generate structures with a pre-measured drug content and enhanced stability. Drug release can be obtained by change in the biological microenvironment such as variation in pH, temperature, or concentration of a specific enzyme (Shukla et al. Citation2008).
Mechanism of drug delivery through dendrimers
Due to compact and globular structure and large number of surface functional groups, drug molecules can be encapsulated both in the interior of the dendrimers as well as attached or bound to the surface groups. Dendrimers can be used as drug carriers either by loading drugs within the dendritic structure or by interacting with drugs at their terminal functional groups through electrostatic or covalent bonds (Nanjwade et al. Citation2009). The covalent bond is formed between the drug and exterior surface of dendrimers. The exterior surfaces of dendrimers have been reported as potential sites of interaction with drugs (Wolinsky and Grinstaff Citation2008). The number of drug molecules incorporated into a dendrimers may depend on the structural design of a dendrimers. The loading capacity is increased by the formation of a complex with the huge number of groups present on the surface of dendrimers. With increasing generation of dendrimers the number of groups present on the surface of dendrimers also increases (Medina and El-Sayed Citation2009).
There are two mechanisms for drug delivery
In vivo degradation of covalent bond between drugs dendrimer by suitable enzymes.
Releasing of the drug from dendrimer due to physical changes or stimulus like pH, temperature, etc.
In vitro release of physically incorporated drugs from dendrimers is usually rapid, and depends on several factors such as the drug partition coefficient between hydrophobic and aqueous environments, strength of drug/dendrimer interactions, dendrimer generation, and surface groups (Nanjwade et al. Citation2009). He et al. (Citation2015) Developed dendrimer/drug complexes which showed target specificity toward αvβ3 integrin over-expressing cancer cells. In this study, cyclic arginine–glycine–aspartic acid (RGD) peptide-conjugated 5.0 G PAMAM dendrimers were developed for anticancer drug encapsulation and targeted therapy of cancer cells over-expressing αvβ3 integrins. The anticancer drug doxorubicin (DOX) was physically encapsulated in RGD conjugated multifunctional dendrimers. From in vitro drug release kinetic studies in PBS (pH 7.4) or acetate buffer (pH 5.0) at 37 °C, it was found that 80% DOX was released within just 2 h, whereas in case of dendrimeric complexes ∼10 and 21% of the drug was released within 1 and 24 h, respectively; in acetate buffer (pH 5.0). In comparison, the drug release rate in PBS (pH 7.4) was found to be faster as 35 and 57% of DOX was released from the complex in 1 and 24 h, respectively. The higher release rate of DOX from the complexes at pH 7.4 than at pH 5 could be due to the fact that under acidic pH conditions, the protonated DOX molecules are able to form strong hydrogen bonding with the dendrimer terminal functional groups (deprotonated amines and terminal polar C–O–C bonds of the PEG moieties), leading to as lower release of DOX. In contrast, under slightly basic conditions at pH 7.4, the DOX molecules are able to be partially deprotonated and to form strong hydrogen bonding with the dendrimer terminal functional groups, resulting in a faster release rate of the drug from the dendrimer interior (He et al. Citation2015).
The future of drug release from dendrimeric conjugate depends on the type of linking group used to covalently attach the drug on the surface of dendrimers. Different types of linking bonds or spacers have been used to link drugs to dendrimers including, enzymatically or hydrolytically cleavable esters, amide groups, and reducible disulfides which can be reduced by glutathione in cytosol. The cleavage of an ester linkage is generally more rapid than that of an amide bond. A detailed study on ester and amide-linked naproxen conjugates of PAMAM dendrimers to determine their stability and release was reported by Najlah and D’Emanuele (Citation2006). Dendrimers have shown the intracellular drug delivery potential with ability of bypassing efflux transporters (Najlah and D’Emanuele Citation2006). Finally, it could be concluded that dendrimers-based drug-delivery system could emerge as promising drug-delivery vehicles in future attributed to their potential to efficiently transport the drug molecules across the biological barrier. Hu et al. (Citation2014) compared drug release pattern from pH sensitive hydrazone-linked and pH insensitive carbamate-linked dendrimer–drug conjugates. DOX was covalently attached to an asymmetric biodegradable polyester dendrimer through pH sensitive acyl hydrazone linkage or through a carbamate linkage. Dendrimer–DOX conjugates with hydrazone linkage were more cytotoxic toward colon carcinoma cells (IC50 = 1.4 mg of Dox per mL) as compared with dendrimer–DOX conjugates with carbamate linkage (IC50 = 2.0 mg of Dox per mL) after incubation for 72 h (Hu et al. Citation2014). Kaminskas et al. (Citation2014) developed PEGylated 5.0 G polylysine dendrimers conjugated to methotrexate (MTX) [Dendrimer − MTX(OH)] and α-carboxyl O-tert-butylated [Dendrimer − MTX(OtBu)] and to methotrexate (MTX) and observed differential patterns of deposition and activity in tumor-burdened lymph nodes after intravenous and subcutaneous administration in rats. Dendrimer − MTX(OtBu) resulted in prolonged plasma exposure after subcutaneous (s.c.) and intravenous (i.v.) dosing, lymphatic exposure, and lymph node retention after i.v. (3–5-fold) and s.c. (10–20-fold) administration when compared with MTX. In contrast, Dendrimer − MTX(OH) resulted in limited plasma exposure after i.v. administration and very low plasma exposure after s.c. administration. Absolute lymphatic recovery after s.c. administration was higher than that of MTX, but ∼4-fold lower than that of Dendrimer– MTX(OtBu). After i.v. administration lymph node recovery was similar to that of Dendrimer–MTX(OtBu) and therefore moderately (∼5-fold) higher than that after iv administration of MTX. After sc administration, lymph node retention for Dendrimer–MTX(OH) was very high (∼100-fold higher than for MTX) (Kaminskas et al. Citation2014).
Different mechanisms of loading of drug into dendrimers provide opportunity to tune drug release from the dendrimers according to the need of therapeutic action. Further the type of linking used to conjugate the drug to the surface of dendrimers can play pivotal role in the controlled delivery of bioactives from dendrimeric scaffold.
Future prospects
The main goal of nanomedicine is to produce nanometer scale multifunctional entity, by engineering and designing the appropriate targeting agent which can diagnose, deliver the therapeutic agent, and can monitor the treatment. The dendrimers-based nanomedicines will depend on rational designing of delivery systems and thorough understanding of biological processes. A summary of dendrimer-based marketed formulations is given in .
Table 2. Dendrimer-based-marketed formulations (Menjoge et al. Citation2010, Mishra Citation2011).
Conclusion
Dendrimer provides a unique platform for drug attachment that has the ability to bind and release drugs through several mechanisms. Dendrimer is a delivery vehicle for anticancer agents that are poorly soluble in water and has ability to promote the transport of drugs across biological membranes. Due to compact and globular structure and large number of surface functional groups, drug molecules can be encapsulated both in the interior of the dendrimers as well as attached or bound to the surface groups. Drugs can link with dendrimer through a strong covalent- and cleavable bond. Some problems occur in the case of drugs covalently linked to dendrimers due to the formation of a stable bond. The dendrimer can protect drugs from degradation and inhibit renal clearance. Dendrimers have been used actively or passively to target a payload to particular destinations. Like any other nanomaterials, the possible biomedical or therapeutic applications of dendrimers would demand for an unequivocal point of their safety which is a prerequisite for their regulatory approval. The known cidal activity of some dendrimers could be further exploited in the area of contraception.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bagre AP, Jain K, Jain NK. 2013. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: in vitro and in vivo assessment. Int J Pharm. 456:31–40.
- Cadena LES, Gauthier M. 2010. Phase-segregated dendrigraft copolymer architectures. Polymers. 2:596–622.
- Caminade AM, Turrin CO. 2014. Dendrimers for drug delivery. J Mater Chem B. 2:4055–4066.
- Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A. 2014. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 17:1–13.
- Dhankhar R, Vyas SP, Jain AK, Arora S, Rath G, Goyal AK. 2010. Advances in novel drug delivery strategies for breast cancer therapy. Artif Cells Blood Substit Immobil Biotechnol. 38:230–249.
- Drbohlavova J, Chomoucka J, Adam V, Ryvolova M, Eckschlager T, Hubalek J, Kizek R. 2013. Nanocarriers for anticancer drugs-new trends in nanomedicine. Curr Drug Metab. 14:547–564.
- Fuertes MA, Alonso C, Pérez JM. 2003. Biochemical modulation of Cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev. 103:645–662.
- Gillies ER, Fréchet JM. 2005. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 10:35–43.
- Gupta U, Dwivedi SK, Bid HK, Konwar R, Jain NK. 2010. Ligand anchored dendrimers based nanoconstructs for effective targeting to cancer cells. Int J Pharm. 393:185–196.
- He X, Alves CS, Oliveira N, Rodrigues J, Zhu J, Bányai I, Tomás H, Shi X. 2015. RGD peptide-modified multifunctional dendrimer platform for drug encapsulation and targeted inhibition of cancer cells. Colloids Surf B Biointerfaces. 125:82–89.
- Howell BA, Fan D. 2009. Poly(amidoamine) dendrimer-supported organoplatinum antitumour agents. Proceedings of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, 2009.
- Hu W, Cheng L, Cheng L, Zheng M, Lei Q, Hu Z, et al. 2014. Redox and pH-responsive poly(amidoamine) dendrimer–poly(ethylene glycol) conjugates with disulfide linkages for efficient intracellular drug release. Colloids Surf B Biointerfaces. 123:254–263.
- Hughes GA. 2005. Nanostructure-mediated drug delivery. Nanomed Nanotechnol Biol Med. 1:22–30.
- Jain K, Jain NK. 2014a. Dendrimer: A 21st century nanobiopolymer. Pharmabiz; [cited 2015 Oct 7]. Available from: http://www.pharmabiz.com/ArticleDetails.aspx?aid=81282&sid=21
- Jain K, Jain NK. 2014b. Surface engineered dendrimers as antiangiogenic agent and carrier for anticancer drug: dual attack on cancer. J Nanosci Nanotechnol. 14:5075–5087.
- Jain K, Jain NK. 2015. Dendrimers as Nanobiopolymers in Cancer ChemoTherapy: Nanobiomedicine. New Delhi: Studium Press, pp. 65–90.
- Jain A, Jain K, Kesharwani P, Jain NK. 2013a. Low density lipoproteins mediated nanoplatforms for cancer targeting. J Nanopart Res. 15:1888.
- Jain A, Jain K, Mehra NK, Jain NK. 2013b. Lipoproteins tethered dendrimeric nanoconstructs for effective targeting to cancer cells. J Nanopart Res. 15:2003
- Jain K, Gupta U, Jain NK. 2014a. Dendronized nanoconjugates of lysine and folate for treatment of cancer. Eur J Pharm Biopharm. 3:500–509.
- Jain K, Kesharwani P, Gupta U, Jain NK. 2012. A review of glycosylated carriers for drug delivery. Biomaterials. 33:4166–4186.
- Jain K, Kesharwani P, Gupta U, Jain NK. 2010. Dendrimer toxicity: let's meet the challenge. Int J Pharm. 394:122–142.
- Jain K, Mehra NK, Jain NK. 2014b. Potentials and emerging trends in nanopharmacology. Curr Opin Pharmacol. 15:97–106.
- Jain K, Verma AK, Mishra PR, Jain NK. 2015a. Characterization and evaluation of amphotericin B loaded MDP conjugated poly (propylene imine) dendrimers. Nanomedicine. 11:705–713.
- Jain K, Verma AK, Mishra PR, Jain NK. 2015b. Surface engineered dendrimeric nanoconjugates for macrophage targeted delivery of amphotericin B: formulation development, in vitro and in vivo evaluation. Antimicrob Agents Chemother. 59:2479–2487.
- Kaminskas LM, Mcleod VM, Ascher DB, Ryan GM, Jones S, Haynes JM, et al. 2014. Methotrexate-conjugated PEGylated dendrimers show differential patterns of deposition and activity in tumour-burdened lymph nodes after intravenous and subcutaneous administration in rats. Mol Pharm. 31:343–349.
- Kaneshiro TL, Lu ZR. 2009. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials. 30:5660–5666.
- Kawaguchi T, Walker KL, Wilkins CL, Moore JS. 1995. Double exponential dendrimer growth. J Am Chem Soc. 117:2159–2165.
- Kesharwani P, Jain K, Jain NK. 2014. Dendrimer as nanocarrier for drug delivery. Prog Polym Sci. 39:268–307.
- Kesharwani P, Tekade RK, Gajbhiye V, Jain K, Jain NK. 2011. Cancer targeting potential of some ligand-anchored poly(propylene imine) dendrimers: a comparison. Nanomedicine. 7:295–304.
- Klajnert B, Bryszewska M. 2000. Dendrimers: properties and applications. Acta Biochimica Polonica. 48:199–208.
- Kozub P, Simaljakova M. 2011. Systemic therapy of psoriasis: methotrexate. Bratisl Lek Listy. 112:390–394.
- Lai PS, Lou PJ, Peng CL, Pai CL, Yen WN, Huang MY, Young TH, Shieh MJ. 2007. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Control Release. 122:39–46.
- Li J, Yu F, Chen Y, Oupický D. 2015. Polymeric drugs: advances in the development of pharmacologically active polymers. J Control Release. 219:369–382.
- Li T, Smet M, Dehaen W, Xu H. 2015. Selenium-platinum coordination dendrimers with controlled anti-cancer activity. ACS Appl Mater Interfaces. DOI: https://doi.org/10.1021/acsami.5b07877.
- Liu M., Frechet JM. 1999. Designing dendrimers for drug delivery. Pharm Sci Technol Today. 2:393–401.
- Longley DB, Harkin DP, Johnston PG. 2003. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 3:330–338.
- Madaan K, Kumar S, Poonia N, Lather V, Pandita D. 2014. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 6:139.
- Medina SH, El-Sayed ME. 2009. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem Rev. 109:3141–3157.
- Mehra NK, Jain K, Jain NK. 2014. Novel Triazine Dendrimer. Encyclopedia of Biomedical Polymers and Polymeric Biomaterials. Boca Raton: Taylor & Francis.
- Mehra NK, Jain K, Jain NK. 2015. Pharmaceutical and biomedical applications of surface engineered carbon nanotubes. Drug Discov Today. 20:750–759.
- Meier W. 2000. Polymer nanocapsules. Chem Soc Rev. 29:295–303.
- Menjoge AR, Kannan RM, Tomalia DA. 2010. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 15:171–185.
- Milhem O, Myles C, Mckeown N, Attwood D, Demanuele A. 2000. Polyamidoamine starburst dendrimers as solubility enhancers. Int J Pharm. 197:239–241.
- Mishra I. 2011. Dendrimer: a novel drug delivery system. J Drug Deliv Therapeutics. 1:70–74.
- Najlah M, D’Emanuele A. 2006. Crossing cellular barriers using dendrimer nanotechnologies. Curr Opin Pharmacol. 6:522–527.
- Nanjwade BK, Bechra HM, Derkar GK, Manvi F, Nanjwade VK. 2009. Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci. 38:185–196.
- Nguyen H, Nguyen NH, Tran NQ, Nguyen CK. 2015. Improved method for preparing cisplatin-dendrimer nanocomplex and its behavior against NCI-H460 lung cancer cell. J Nanosci Nanotechnol. 15:4106–4110.
- Ooya T, Lee J, Park K. 2003. Effects of ethylene glycol-based graft, star-shaped, and dendritic polymers on solubilization and controlled release of paclitaxel. J Control Release. 93:121–127.
- Orocio-Rodríguez E, Ferro-Flores G, Santos-Cuevas CL, Ramírez Fde M, Ocampo-García BE, Azorín-Vega E, Sánchez-García FM. 2015. Two novel nanosized radiolabeled analogues of somatostatin for neuroendocrine tumor imaging. J Nanosci Nanotechnol. 15:4159–4169.
- Patri AK, Kukowskalatallo JF, Baker JR. 2005. Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev. 57:2203–2214.
- Shi C, He Y, Feng X, Fu D. 2015. ɛ-Polylysine and next-generation dendrigraft poly-l-lysine: chemistry, activity, and applications in biopharmaceuticals. J Biomater Sci Polym Ed. 26:1343–1356.
- Shukla R, Thomas TP, Desai AM, Kotlyar A, Park SJ, Baker JR. 2008. HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb. Nanotechnology. 19:295102.
- Svenson S. 2009. Dendrimers as versatile platform in drug delivery applications. Eur J Pharm Biopharm. 71:445–462.
- Tomalia DA. 2005. Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog Polym Sci. 30:294–324.
- Turnbull WB, Stoddart JF. 2002. Design and synthesis of glycodendrimers. J. Biotechnol. 90:231–255.
- Uram Ł, Szuster M, Filipowicz A, Gargasz K, Wołowiec S, Wałajtys-Rode E. 2015. Different patterns of nuclear and mitochondrial penetration by the G3 PAMAM dendrimer and its biotin-pyridoxal bioconjugate BC-PAMAM in normal and cancer cells in vitro. Int J Nanomed. 10:5647–5661.
- Wolinsky JB, Grinstaff MW. 2008. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv Drug Deliv Rev. 60:1037–1055.
- Woo S, Lee Y, Sunkara V, Cheedarala RK, Shin HS, Choi HC, Park JW. 2007. “Fingertip”-guided noncovalent functionalization of carbon nanotubes by dendrons. Langmuir. 23:11373–11376.