?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, chromatographic performance of Cu2+-attached pumice particles embedded to monolithic cryogels (Cu2+-APPsEMC) for human serum albumin (HSA) was investigated. Monolithic composite cryogels were prepared by means of polymerization of gel-forming precursors at sub-zero temperatures. The chemical composition of pumice and surface of composite cryogels were determined by X-ray fluorescence spectrometer and scanning electron microscopy, respectively. The highest adsorption capacity (549.5 mg/g pumice) of cryogels was achieved at phosphate buffer of pH 8.0 with initial HSA solution of 3 mg/ml. SDS-PAGE analysis was performed for the samples studied on human serum to determine HSA adsorption/desorption performance of cryogel qualitatively.
Introduction
Human serum albumin (HSA) an important plentiful protein has major roles such as regulation of osmotic pressure, transport, and storing numerous endogenous and exogenous compounds (including bilirubin, calcium, long-chain fatty acids, etc.) in human plasma (Bo and Pawliszyn Citation2006). More importantly, albumin has an important function to manage fluids of patients with acute illness. There are many studies reported earlier in literatures for confirmed therapy of albumin (hypovolemia of shock, burns, hypoalbuminemia, cardiopulmonary bypass, hemodialysis, hyperbilirubinemia, sequestration of protein-rich fluids in acute peritonitis, and extensive cellulitis) in the United States (Wilkes and Navickis Citation2001). Hence, due to its significant potential for the production of blood protein, many researchers have given great importance for the separation of albumin.
Many techniques and media are available for separation and purification of protein-like biological materials with important role for medical treatment. Among them, affinity interactions are the most favored due to the fact that they are highly specific and selective toward target molecule. However, it has restricted adaptation in commercial field due to fragile make-up of affinity ligands, being hard to immobilize in desired orientation, prospective outflow problems of immunogenic substances, and expensive experimental step such as synthesis, separation, etc. (Sharma and Agarwal Citation2001). Therefore, to perform cost-effective and applicable methods is very crucial variables in the science of separation and purification of biological materials.
Porath et al. first formulated and introduced immobilized metal affinity chromatography (IMAC) providing a number of efficacy being easy to prepare and cost-effective, having superior adsorption performance, structural persistence, and very effective reusability score without any considerable loss by comparison with other affinity chromatography methods, especially immunoaffinity chromatography (Arnold Citation1991, Odabaşı et al. Citation2003, Citation2007, Porath et al. Citation1975; Ribeiro et al. Citation2008). In proteins, three side groups of amino acids (i.e., imidazole, indoyl, and thiol groups of histidine, tryptophan, and cysteine, respectively) are mainly responsible for interaction. Aromatic and amino end groups of the peptides were expressed as other less contributed groups, too (Yip and Hutchens Citation1994).
Macroporous composite cryogels are a new class of separating media for improving the binding capacity (Gedikli et al. Citation2014, Yao et al. Citation2006). Their applications in the experiments performed to separate and purify biological macromolecules (DNA, proteins, cell organelles, viruses, and enzymes) were achieved quite effectively in downstream processes (Arvidsson et al. Citation2003, Dainiak et al. Citation2004, Derazshamshir et al. Citation2008, Wang et al. Citation2008, Williams et al. Citation2005, Yavuz et al. Citation2005). Cryogels are prepared via the polymerization of gel-forming precursors at sub-zero temperatures. The requisite solvent crystallization is the main necessary property for the cryotropic gelation technique. The polymerization reaction proceeds in the non-frozen microphase, whereas the solvent crystals act as a pattern for pores connected via micro or macro tunnels at the end of removal of water after dissolution. In adsorption and elution process, very low retention time and pressure drop, and short diffusion distance are provided the supermacroporous media, with pore size up approximately to 100 μm, synthesized with this technique (Derazshamshir et al. Citation2008). Due to low cost and exceptional permeability of networks of supermacropores, cryogels show some promises for the use in large-scale bioseparation systems (Alkan et al. Citation2009, Persson et al. Citation2004).
Recently, we published some natural materials that were used for purification of various biomolecules (Ceylan and Odabaşı Citation2013, Erzengin et al. Citation2011, Ünlü et al. Citation2011). High adsorption capacities of these materials with significant properties are provided with high pore volume and surface area, specific active sites for ligand attachments. They have economical adsorptive properties and quite significant features such as being chemically highly reactive, hydrophilic, and having non-toxicities for biomolecule separation. Especially, among the natural materials, pumice has been used for both removal of pollutants (organic and inorganic materials) and research performed in biotechnology field (Gurbuz and Codd Citation2008).
In this study, a new generation of monolithic composite cryogels was designed with Cu2+-APPs embedding under very low gelation temperature. Adsorption performance of cryogels synthesized was enhanced substantially with the achievement of extended interaction area and thus functional groups via embedding of pumice particles (PPs). Scanning electron microscopy (SEM) was used to determine the surface morphology and pore structure of cryogels.
Materials and methods
Materials
2-Hydroxyethyl methacrylate (HEMA), N,N'-methylene-bis-acrylamide (MBAAm), N,N,N′,N′-Tetramethylethylenediamine (TEMED), HSA, and ammonium persulfate (APS) were supplied by Sigma-Aldrich (St. Louis, MO). All other chemicals were of reagent grade and were purchased from Merck AG (Darmstadt, Germany). Pumice was obtained from the Pumice Research Centre, Süleyman Demirel University (Isparta, Turkey). Water used in the experiments was purified by using a Barnstead (Dubuque, IA) ROpure LP® reverse osmosis unit.
Preparation of pumice particles (PPs)
The PPs were washed using distilled water to remove pollutant materials with potential to affect adsorption performance. After this step, the PPs were warmed up under 130 °C for 5 h to remove unwanted organic impurities potentially placed within structure and surface. Because of this heating process, the surface area of particles was enlarged, too.
Preparation of Cu2+-attached pumice particles (Cu2+-APPs)
Attachment of Cu2+ ions onto PPs was performed as described previously (Erzengin et al. Citation2011). Briefly, 0.150 g of PPs was processed with a Cu2+ solution [100 mg/l (pH 5.0), adjusted with HCl] at room temperature for 2 h. A Cu2+ solution with 1000-ppm was used as an atomic absorption standard solution. The concentration of the Cu2+ ions for an initial and final solution was studied with a graphite furnace atomic absorption spectrometer to determine the amount of attached Cu2+ ions onto PPs (GFAAS, Analyst 800/PerkinElmer, Waltham, MA). All studies were carried out in triplicate. The outflow amount of Cu2+ from the particles was also studied in the media having 1.0 M NaCl with pHs varied from 4.0 to 7.0.
Preparation of Cu2+-attached pumice particles embedded monolithic composite cryogel (Cu2+-APPsEMCC) column
Polymerization steps up to embedding process was described in our previous studies (Erzengin et al. Citation2011, Odabaşı et al. Citation2005). Briefly, 5 ml of monomer solution consisting of 50 mg MBAAm and 0.3 ml was degassed for 5 min to eliminate oxygen. After preparation of polymer solution, 10 mg of Cu2+-APPs was added to this solution, and the prepared solution was poured into a plastic syringe (5 ml, i.d. 0.8 cm). Then, 100 μl (10% (w/v) of APS (as a free radical producer) and 20 μl of TEMED (as a catalyst) were added to the prepared mixture, and it was frozen at −12 °C for 24 h and afterwards thawed at room temperature. After thawing, the prepared column was respectively washed with diluted HCl solution and water–ethanol mixture for removing possible impurities such as unconverted monomers and initiator. Clearance of the matrix was observed by changes of optical densities of washing solutions. After washing, the cryogel was stored in buffer containing 0.02% sodium azide at 4 °C until use.
Characterization of macroporous composite cryogel
Characterization of pumice particles
Mineralogical and chemical studies of pumice were done by using X-ray fluorescence spectrometer (XRF) (Thermo Scientific, Waltham, MA, ARL 9900) to identify the chemical composition. The specific surface area (m2/g) measurements of samples were carried out by N2 adsorption using BET method (Brunauer, Emmett, Teller) (Micromeritics, ASAP 2020, Norcross (Atlanta), GA). Before measurements, the samples were dried at 60 °C for 6 h.
Porosity of Cu2+-APPs embedded composite cryogel
The ϕ value was calculated for the determination of free water amount in the cryogel sample and the volume. The saturation of a piece of cryogel sample was provided with deionized water. As a next step, this piece was immersed in water of volume V1. Volume V2 was determined as the total volume of a cylinder. The volume difference, V0 = V2 − V1, was estimated to define the volume V0 defining the volume of water-saturated cryogel.
The water-saturated cryogel was weighed to determine the mass, mw. Free water placed within the large pores of the cryogel was removed by squeezing the cryogel sample. For the next step, the cryogel sample not containing free water was weighed to determine the mass of cryogel, ms, as given before (Yao et al. Citation2006). The EquationEquation (1)(1)
(1) was used to calculate the porosity of cryogels:
(1)
(1)
where ρw is the deionized water density. To obtain totally dried cryogel, it was placed in the oven at 60 °C for 12–24 h. md (dried cryogel mass) was obtained. EquationEquation (2)
(2)
(2) was used to estimate the total water fraction (TWF):
(2)
(2)
Surface morphology
The microstructure and morphology of composite cryogel were observed by SEM. 0.1 M sodium phosphate buffer (pH 7.0) containing 2.5% glutaraldehyde was used to fix the sample overnight. The same buffer solution was used three times for washing of the sample. The postfix process in 1% osmium tetroxide was performed for 1 h and the sample was washed one more time. Ethanol–water mixtures of 0–25–50–75–99.5% were used to perform the dehydration of sample. The sample obtained after dehydration process was coated with gold-palladium (40:60). JEOL JSM 5600 SEM (Tokyo, Japan) was used to examine surface morphology of cryogels.
Adsorption of HSA from aqueous solutions
Experiments on adsorption of HSA onto Cu2+-APPs embedded cryogel column was performed via the recirculating system. The temperature of this system was controlled by water jacket equipped onto the system. The water of 30 ml was used to wash the cryogel. The phosphate buffer of 0.02 M at pH 8.0 was used to equilibrate system. After this step, the HSA solution of 30 ml was given to the column for 1 h. The decrease in absorbance value obtained at 280 nm was monitored to determine HSA adsorption performance of cryogels. The HSA adsorption performance of cryogels was investigated for different conditions such as variable medium pH, initial HSA concentration, and temperature.
For all experiments carried out, acetate buffer of 0.05 M containing 1.0 M NaCl at pH 5.0 was used for HSA elution. Desorption agent of 30 ml was given to the system for a characteristic desorption process at a flow rate of 1.0 ml/min. The final concentration of HSA remained in desorption medium was designated by spectrophotometry at 280 nm. The ratio of HSA desorption from Cu2+-APPs embedded cryogel column was performed by comparing the amounts of protein eluted and adsorbed. After desorption process, NaOH of 1 M was used for the cleaning of the composite cryogel and phosphate buffer of 0.02 M at pH 8.0 was used for the re-equilibration. The ratio of the amount of HSA adsorbed to the amount of HSA in desorption medium was estimated as desorption ratio. The 20 adsorption–desorption cycle was accomplished for the separation experiments of HSA with the same cryogel column to test the reproducibility of Cu2+-APPs embedded composite cryogel.
Study with human plasma
Cu2+-APPsEMCC was also used for adsorption of HSA from human plasma. A significant amount of blood was obtained from a healthy person with his consent under the medical care, and centrifuged at 500 g for 3 min at room temperature. With the use of phosphate-buffered saline at pH 7.4, 10-fold dilution was performed to the freshly separated human plasma of 5 ml. After this step, 20 ml of human plasma was given to the column with a flow rate of 1 ml/min at room temperature. Estimation of the initial and final concentration of HSA within blood plasma provides the determination of HSA amount adsorbed onto the cryogels. The bromocresol green dye method applied at 628 nm was used for the determination of the concentration of HSA (Trivedi et al. Citation1997).
Electrophoresis analyses
To determine the purity of HSA desorbed from cryogels, the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses were conducted on 10% separating mini gels (9 × 6.5 cm) and 6% stacking gels for 120 min at 100 V. The staining and the de-staining process of gels were performed with 0.25% (w/v) Coomassie Brilliant R 250 in acetic acid:methanol:water mixture (1:3:6, v/v/v) and in ethanol:acetic acid:water mixture (1:4:6, v/v/v), respectively.
Results and discussion
Characterization studies
XRF shows that SiO2, Al2O3, and K2O are the main oxides in the pumice under investigation, confirming the mineralogical constituents, whereas TiO2, MnO, MgO, Fe2O3, and CaO analyzed in the pumice detected fewer than 3.0% (). BET-method was applied for the calculation of the specific surface area of pumice and found as 65.2 m2/g PPs. The incorporation of Cu2+ ions to the PPs was enabled with the surface functional hydroxyl groups of silica and alumina basically. The amount of Cu2+ ions chelated on PPs was found to be 1.75 mg/g PPs. The specific surface area of the non-embedded cryogel and Cu2+-APPsEMCC were found to be 38.7 and 56.3 m2/g, respectively, by BET method. Cryogels have low surface area due to the large pores. It is noted that PPs embedding in this study increased the surface area of the polymer up to 49%.
Table I. Chemical composition of pumice.
It was determined as a result of leakage experiments that there is no leakage in any of adsorption and desorption media. As a conclusion, it can be said that there is no non-specific adsorption of Cu2+ ions due to the successful removal of these ions from the PPs during the washing procedure.
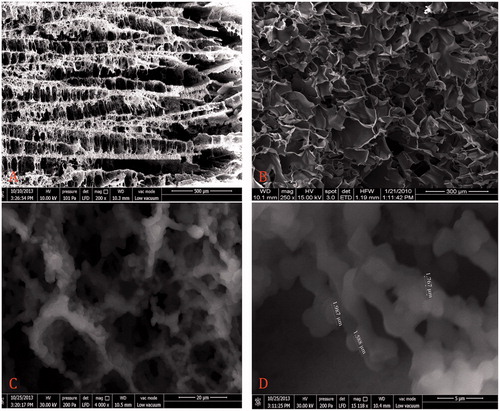
shows the SEM images of surface morphology and microstructures within the Cu2+-APPsEMCC. show the microstructures of cryogels with and without PPs, respectively. As seen clearly in these two figures that we obtained a new generation of cryogel having multi-floored caves with stalactites and stalagmites when we used PPs as a composite material. And also, as focused on , it is seen that the walls built by micro PPs (about 2 μm in size) were replaced thin leafy walls in the classical cryogels. Compared to classical composite cryogel structures (Ünlü et al. Citation2011), these new ones have more ligand binding sites, and therefore, more adsorption capacity. The possible reason for these structures may be the orientation of water crystals and monomer molecules by PPs. As seen in , the pore skeleton of Cu2+-APPsEMCC with size in the range from 10 to 80 μm in diameter is interconnected multi-floored caves with stalactites and stalagmites. And, Cu2+-APPs were uniformly distributed into the cryogel network (). It can be concluded that there is almost no mass transfer resistance because of the convective flow of the solution/human plasma through the pores.
Figure 1. SEM images of different cryogel structures. (A, B) Comparative representation of cryogels with and without pumice particles, respectively. (C, D) The cryogel walls built by micro pumice particles.
Deionized water was used for the measurement of the porosity, ϕ, and the total water content, of the Cu2+-APPsEMCC and these values were found as in the order of 68.3% and 90.1% (v/v). According to these results, it can be concluded that the small pores of polymer matrix has bounded to nearly 21.8% of the total water where there is almost no flowing liquid passed through. Nevertheless, free water occupies the larger pores that are 68.3% of the total pores. These large pores have most of the fluid flowing channels.
Aqueous solutions studies
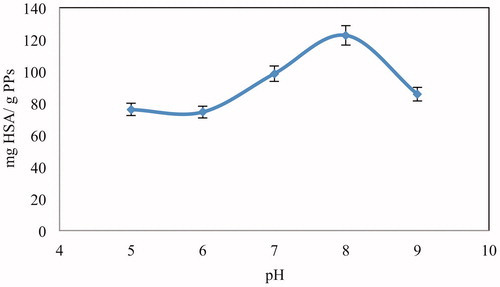
Effect of pH on adsorption
To control the efficiency and adsorption performance owned by the adsorbent, adjustment of pH is one of the important features for adsorption studies. The effect of pH on HSA adsorption is given in . The maximum adsorption of HSA was observed at pH 8.0. The HSA adsorption capacity decreased at above and below this pH value. The chelating bonding occurring between metal ions and amino acid residues is the base for the protein adsorption on IMAC. The reaction was favored at weakly alkaline pH by the ionization of amino acid residues and thus, the adsorption of protein on the metal-immobilized matrixes was brought by Wong et al. (Citation1991). The chelation between the metal ion and histidine residue of HSA has enabled the HSA adsorption in IMAC. For most of the proteins, the pKa values of histidine residue are varied from 6 to 7 (Arnold Citation1991, Gutiérrez et al. Citation2007, Porath and Olin Citation1983). For this reason, the optimum pH for adsorption of HSA onto Cu2+-APPs embedded cryogel was observed around 8.0.
Effect of initial concentration of HSA on adsorption
shows adsorption isotherm for adsorption of HSA onto the plain PPs embedded cryogel and Cu2+-APPs embedded cryogel. Adsorption isotherm of HSA on Cu2+-APPs embedded MCCs indicates that adsorption capacity increased when the equilibrium concentration of HSA increased.
Figure 3. Effect of concentration of HSA on adsorption; pH: 8.0; embedded Cu2+-APPs: 15 mg; flow rate: 1 ml/min; T: 25 °C.
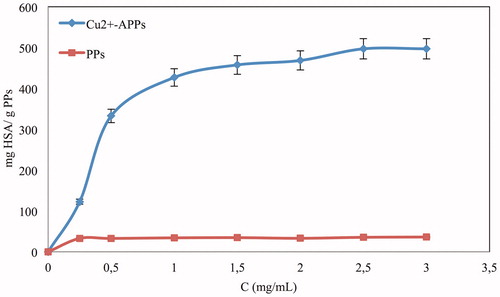
HSA adsorption on Cu2+-APPs embedded cryogel through Cu2+ ions was up to 496.9 mg/g Cu2+-APPs which very significant when compared with some adsorbents cited in . Maximum adsorption of HSA on plain PPs embedded cryogel was about 36.2 mg/g PPs which is quite low.
Table II. Comparison of adsorption capacities of some different adsorbents for HSA.
There are several crucial parameters, basically designated by the polymeric matrices, such as the existence of the cryogel walls built by micro PPs having high surface area and multi-floored caves with stalactites and stalagmites polymeric matrices which provide no mass transfer limitation and steric hindrance in terms of the convective flow of HSA molecules in adsorption process. Attachment of Cu2+ ions organized by PPs has raised adsorption capacity of PPs for HSA by creating specific binding sites. Coordination of Cu2+ ions with PPs enabled specific coordination sites for HSA molecules. Due to the lower diffusion resistance of Cu2+-APPs embedded cryogel, HSA molecules can easily go through composite cryogel network and then HSA molecules reach to the specific recognition sites (i.e., Cu2+ ions) in the cryogel structure and bind fast.
There are also the best characteristics owned by an adsorbent as a hydrophilic character and macroporous structures of the polymer (Kantipuly et al. Citation1990). In this experiment, good permeability and functionality were achieved for adsorption experiments of HSA molecules due to the porous and hydrophilic structure of the Cu2+-APPs embedded cryogel.
Adsorption isotherms
Langmuir adsorption model (Langmuir Citation1916) expressed by EquationEq. (3)(3)
(3) was applied to analyze the experimental data related to the binding behavior of HSA onto Cu2+-APPsECC. This equation can be linearized to EquationEq. (4)
(4)
(4) to obtain semi-reciprocal plot:
(3)
(3)
(4)
(4)
Here, Q is the adsorbed amount of HSA (mg/g), Ce is the equilibrium HSA concentration (mg/ml), K = k2/k1 is the dissociation constant of the system. Ce versus Ce/Qe data gave a linear plot with a correlation coefficient (R2) of 0.9963 for Cu2+-APPsEMCC. And it indicates that the Langmuir model assuming a monolayer adsorption takes place onto a surface could be applied to these affinity systems. From the slope and the intercept of the straight line obtained, the values of Kd and Qm are determined. In an ideal adsorbent, the Kd values should be small for the target molecules. The maximum adsorption capacity Qm and Kd were found to be in the order of 555.5 mg/g PPs and 4.15 × 10−6 M for adsorption of HSA onto Cu2+-APPsEMCC.
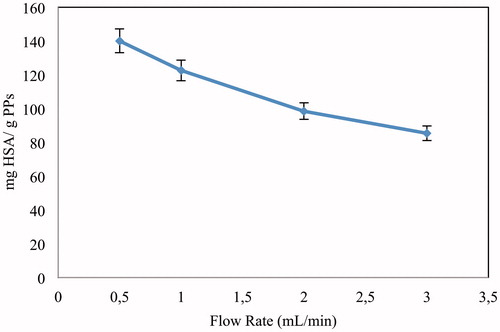
Effect of flow rate
shows the amounts of HSA adsorbed for the cases of different flow rates. From the results, it can be concluded that the HSA adsorption capacity of Cu2+-APPsEMCC was decreased scantily from 205.8 to 85.4 mg/g PPs with the increased flow rate (from 0.5 to 3.0 ml/min). The solution volume handled efficiently until progress point and thus the service time owned by cryogel column was decreased with the increase of flow rate. The reason for this result is that the interaction between the HSA molecules and Cu2+-APPsEMCC was taken place in a very short time at higher flow rates. The results obtained are extremely matched with the literature (Odabaşı et al. Citation2010, Valdman et al. Citation2001). In the case of low flow rates, the interaction between HSA molecules and the ligands was taken place in a longer time and thus adsorption performance of cryogels was better. Because of continuous interaction between the cryogel and fresh biologic material, the concentration of the solution in the given layer of cryogel was relatively constant.
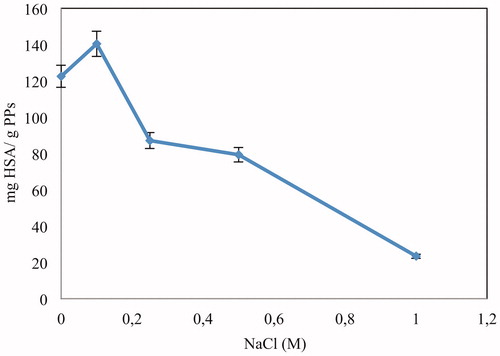
Effect of ionic strength
Investigation of ionic strength effect on the HSA adsorption was performed at different NaCl concentrations (0.0–1.0 M) as displayed in . As seen here, the maximum HSA adsorption was observed at 0.1 M NaCl concentration. Adsorption of HSA first increased with increasing ionic strength, and after passing through a maximum at 0.1 M NaCl, it decreased with increasing ionic strength. This might be due to the fact that the addition of NaCl first reduced the non-specific interactions between metal ions and protein molecules, and enhanced adsorption capacity. After 0.1 M NaCl addition, salt ions decreased adsorption capacity reducing the electrostatic interactions (Cömert and Odabaşı Citation2014). Consequently, it can be said that the donor–acceptor (coordination) interactions have dominant effect for HSA adsorption on Cu2+-APPsEMCC between 0.0 and 0.1 M NaCl concentrations while electrostatic interactions have dominant effects between 0.1 and 1.0 M NaCl.
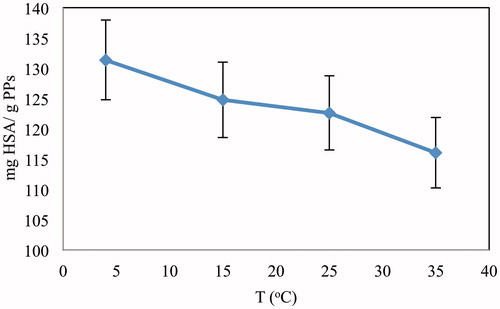
Effect of temperature
At the temperatures varying from 5 °C to 35 °C, adsorption of HSA onto cryogels was studied. Adsorption of HSA on Cu2+-APPsEMCC at equilibrium was decreased significantly with increasing temperature, and highest adsorption capacity was observed at 5 °C (). The adsorption capacity of the cryogel decreased about 11.7% for Cu2+-APPsEMCC from 5 °C to 35 °C. The explanation for this behavior can be the exothermic feature of adsorption process.
Desorption and reusability studies
Desorption of protein in mild and low cost conditions (i.e., salts) is an important advantageous for biochromatography systems. Due to economic restraints, there is a growing interest in the preparation and use of effective low-cost and reusable adsorbents (Odabaşı et al. Citation2005). Desorption of HSA from Cu2+-APPsEMCC was performed in column system and expressed in percentage of totally adsorbed HSA. To desorb HSA from cryogels acetate buffer of 0.05 M at pH 5.0 containing eluent agent (1.0 M NaCl) was used, and desorption performance of 97.1% was achieved. The aim of adding elution agent was to inhibit electrostatic interactions and thus to achieve the removal of the HSA molecules from cryogel. It must be pointed out that there was observed no leakage of Cu2+ ions from the adsorbent. As given before, it can be understood that the suitable desorption agent for this study was determined as acetate buffer of 0.05 M at pH 5.0 (containing 1.0 M NaCl). Therefore, it enables a good repeatability performance for the same affinity cryogel used. To determine the reproducibility of Cu2+-APPsEMCC, 20 adsorption–desorption cycles were performed using the same cryogel. At the end of the reusability experiments, it was observed that there was no notable decrease but only 4% for adsorption performance of the cryogel.
HSA adsorption from human plasma
Pretty higher adsorption values (529.5 mg/g Cu2+-APPs) were come true as the Cu2+-APPsEMCC was used. Whereas, very low adsorption amount (38.6 mg/g PPs) was happened on non-specific studies. It was not surprising the higher adsorption capacity of HSA studied from human plasma on Cu2+-APPsEMCC when compared with studies in aqueous solutions. Human plasma has a native environment with high HSA concentration. In such media, a high adsorption degree may be occurred because of high driving force between native environment and Cu2+-APPsEMCC.
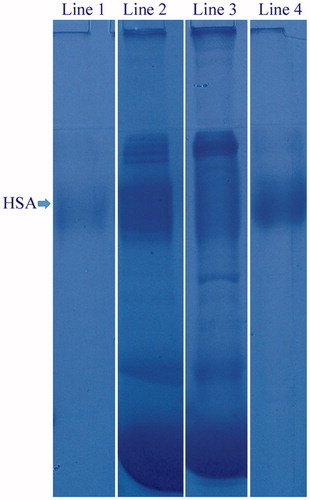
The samples obtained before and after adsorption studies with human plasma were analyzed using SDS-PAGE to affirm adsorption performance of Cu2+-APPsEMCC. As shown in , lane 1 belongs to trade HSA used as a marker, lane 2 belongs to diluted plasma before adsorption, lane 3 belongs to diluted plasma after adsorption, and lane 4 belongs to desorbed protein. The protein band (in lane 4) appeared across the trade HSA confirms that HSA was purified successfully from human plasma.
Conclusion
In the field of separation science, composite cryogels can be attributed as a new generation of stationary phases. The main disadvantages owned by cryogels are low adsorption capacity, whereas short diffusion path, having large pores, very short retention time and low-pressure drop can be given as remarkable advantages. In this study, in order to improve undesirable features of cryogels, Cu2+-attached PPs embedded composite cryogels were synthesized. Up to now, chelating agents used for IMAC matrices have mainly organic characters like iminodiacetic acid and nitrilotriacetic acid. This kind of chelating agent might be poisonous and expensive when they used for food and pharmaceutical purposes (Sun et al. Citation2011). Whereas, pumice is a natural, low cost, and has a high surface area for adsorption studies and was used as adsorbent first time in IMAC. We believe that this matrix (Cu2+-APPsEMCC) with large surface area prepared in this study will be all-purpose for the field of protein purification with high adsorption performance.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Alkan H, Bereli N, Baysal Z, Denizli A. 2009. Antibody purification with protein A attached supermacroporous poly(hydroxyethyl methacrylate) cryogel. Biochem Eng J. 45:201–208.
- Andaç M. 2015. Cibacron blue immobilized poly(glycidyl-methacrylate) nanobeads for albumin removal in proteome studies. Artif Cells Nanomed Biotechnol. 43:133–139.
- Arnold FH. 1991. Metal-affinity separations: a new dimension in protein processing. Biotechnology (N Y). 9:151–156.
- Arvidsson P, Plieva FM, Lozinsky VI, Galaev IY, Mattiasson B. 2003. Direct chromatographic capture of enzyme from crude homogenate using immobilized metal affinity chromatography on a continuous supermacroporous adsorbent. J Chromatogr A. 986:275–290.
- Bo T, Pawliszyn J. 2006. Characterization of bovine serum albumin-tryptophan interaction by capillary isoelectric focusing with whole column imaging detection. J Chromatogr A. 1105:25–32.
- Baydemir G, Odabaşı M. 2013. Microsphere-embedded cryogel for selective and efficient depletion of immunoglobulin G from human serum. Artif Cells Nanomed Biotechnol. 41:319–326.
- Ceylan Ş, Odabaşı M. 2013. Novel adsorbent for DNA adsorption: Fe3+ attached sporopollenin particles embedded composite cryogels. Artif Cells Nanomed Biotechnol. 41:376–383.
- Cömert ŞC, Odabaşı M. 2014. Investigation of lysozyme adsorption performance of Cu2+-attached PHEMA beads embedded cryogel membranes. Mate Sci Eng C. 34:1–8.
- Dainiak MB, Kumar A, Plieva FM, Galaev IY, Mattiasson B. 2004. Integrated isolation of antibody fragments from microbial cell culture fluids using supermacroporous cryogels. J Chromatogr A. 1045:93–98.
- Derazshamshir A, Ergün B, Peşint G, Odabaşı M. 2008. Preparation of Zn+2-chelated poly(HEMA-MAH) cryogel for affinity purification of chicken egg lysozyme. J Appl Polym Sci. 109:2905–2913.
- Erzengin M, Ünlü N, Odabaşı M. 2011. A novel adsorbent for protein chromatography: supermacroporous monolithic cryogel embedded with Cu2+-attached sporopollenin particles. J Chromatogr A. 1218:484–490.
- Gedikli M, Ceylan Ş, Erzengin M, Odabaşı M. 2014. A novel matrix for hydrophobic interaction chromatography and its application in lysozyme adsorption. Acta Biochim Pol. 61:731–737.
- Gökay Ö, Karakoç V, Andaç V, Türkmen D, Denizli A. 2015. Dye-attached magnetic poly(hydroxyethyl methacrylate) nanospheres for albumin depletion from human plasma. Artif Cells Nanomed Biotechnol. 43:62–70.
- Gurbuz F, Codd GA. 2008. Microcystin removal by a naturally-occurring substance: pumice. Bull Environ Contam Toxicol. 81:323–327.
- Gutiérrez R, Martín del Valle EM, Galán MA. 2007. Immobilized metal-ion affinity chromatography: status and trends. Sep Purif Rev. 36:71–111.
- Kantipuly C, Katragadda S, Chow A, Gesser HD. 1990. Chelating polymers and related supports for separation and preconcentration of trace metals. Talanta. 37:491–517.
- Langmuir I. 1916. The constitution and fundamental properties of solids and liquids. Part I. Solids J Am Chem Soc. 38:2221–2295.
- Odabaşı M. 2011. Magnetic dye-affinity beads for human serum albumin purification. Prep Biochem Biotechnol. 41:287–304.
- Odabaşı M, Baydemir G, Karataş M, Derazshamshir A. 2010. Preparation and characterization of metal-chelated poly(HEMA-MAH) monolithic cryogels and their use for DNA adsorption. J Appl Polym Sci. 116:1306–1312.
- Odabaşı M, Garipcan B, Denizli A. 2003. Preparation of a novel metal-chelate affinity beads for albumin isolation from human plasma. J Appl Polym Sci. 90:2840–2847.
- Odabaşı M, Ozkayar N, Ozkara S, Unal S, Denizli A. 2005. Pathogenic antibody removal using magnetically stabilized fluidized bed. J Chromatogr B Analyt Technol Biomed Life Sci. 826:50–57.
- Odabaşı M, Say R, Denizli A. 2007. Molecular imprinted particles for lysozyme purification. Mater Sci Eng C. 27:90–99.
- Persson P, Baybak O, Plieva FM, Galaev IY, Mattiasson B, Nilsson B, Axelsson A. 2004. Characterization of a continuous supermacroporous monolithic matrix for chromatographic separation of large bioparticles. Biotechnol Bioeng. 88:224–236.
- Porath J, Carlsson J, Olsson I, Belfrage G. 1975. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 258:598–599.
- Porath J, Olin B. 1983. Immobilized metal ion affinity adsorption and immobilized metal ion affinity chromatography of biomaterials. Serum protein affinities for gel-immobilized iron and nickel ions. Biochemistry. 22:1621–1630.
- Ribeiro MB, Vijayalakshmi M, Todorova-Balvay D, Bueno SM. 2008. Effect of IDA and TREN chelating agents and buffer systems on the purification of human IgG with immobilized nickel affinity membranes. J Chromatogr B Analyt Technol Biomed Life Sci. 861:64–73.
- Sharma S, Agarwal GP. 2001. Interactions of proteins with immobilized metal ions: role of ionic strength and pH. J Colloid Interf Sci. 243:61–72.
- Sun J, Rao S, Su Y, Xu R, Yang Y. 2011. Magnetic carboxymethyl chitosan nanoparticles with immobilized metal ions for lysozyme adsorption. Colloids Surf A Physicochem Eng Asp. 389:97–103.
- Trivedi VD, Saxena I, SIANBqui MU, Qasim MA. 1997. Interaction of bromocresol green with different serum albumins studied by fluorescence quenching. Biochem Mol Biol Int. 43:1–8.
- Tuzmen N, Kalburcu T, Uygun DA, Akgol S, Denizli A. 2015. A novel affinity disks for bovine serum albumin purification. Appl Biochem Biotechnol. 75:454–468.
- Ünlü N, Ceylan Ş, Erzengin M, Odabaşı M. 2011. Investigation of protein adsorption performance of Ni2+-attached diatomite particles embedded in composite monolithic cryogels. J Sep Sci. 34:2173–2180.
- Valdman E, Erijman L, Pessoa FLP, Leite SGF. 2001. Continuous biosorption of copper and zinc by immobilized waste biomass of Sargassum sp. Process Biochem. 36:869–873.
- Wang L, Shen S, He X, Yun J, Yao K, Yao S. 2008. Adsorption and elution behaviors of bovine serum albumin in metal-chelated affinity cryogel beds. J Biochem Eng J. 42:237–242.
- Wilkes MM, Navickis RJ. 2001. Patient survival after human albumin administration. A meta-analysis of randomized, controlled trials. Ann Intern Med. 135:149–164.
- Williams SL, Eccleston ME, Slater NK. 2005. Affinity capture of a biotinylated retrovirus on macroporous monolithic adsorbents: towards a rapid single-step purification process. Biotechnol Bioeng. 89:783–787.
- Wong JW, Albriqht RL, Wanq NHL. 1991. Immobilized metal ion affinity chromatography – chemistry and applications on bioseparations. Sep Purif Method. 20:49–106.
- Yao K, Yun J, Shen S, Wang L, He X, Yu X. 2006. Characterization of a novel continuous supermacroporous monolithic cryogel embedded with nanoparticles for protein chromatography. J Chromatogr A. 1109:103–110.
- Yavuz H, Odabaşı M, Akgöl S, Denizli A. 2005. Immobilized metal affinity beads for ferritin adsorption. J Biomater Sci Polym Ed. 16:673–684.
- Yip TT, Hutchens TW. 1994. Immobilized metal ion affinity chromatography. Mol Biotechnol. 1:151–164.