Abstract
Surface modification of medical polymers is carried out to improve biocompatibility. In this study, conventional polymers (chitosan and polypropylene) were modified to laser at different features (oriented and non-oriented) to create a vast range of physicochemical characteristics on the surface of polymers and investigate their effects on biocompatibility of treated surfaces. Atomic force microscope (AFM) was applied to study the morphology of treated samples in comparison with those of the untreated PS. Contact angle analyses were used to evaluate the wettability and surface energy of the treated films. AFM studies showed that after laser treatment, some distinctive nanostructures are created on the surface of polymers. The data from contact angle measurements demonstrated that laser irradiation created surfaces with a vast range of properties in the wettability point of view. The cellular results revealed that after surface modification by laser irradiation, biocompatibility of polymeric films, especially oriented films was enhanced.
Introduction
Control of surface properties is very important for high-performance adhesion. Biomaterial wettability is an important factor in the surface modification of materials. Surface modification of hydrophobic polymer surfaces can be achieved by wet (acid, alkali), dry (plasma), or radiation treatments (ultraviolet radiation and laser) (Biazar et al. Citation2010, Biazar et al. Citation2011, Heidari et al. Citation2011). It is well known that interactions between the surface of an artificial biomaterial and biological environment are the key factors to determine the biocompatibility (Ai et al. Citation2011, Biazar et al. Citation2011, Biazar et al. Citation2012, Biazar and Heidari Citation2013, Biazar and Heidari Citation2014, Biazar and Sahebalzamani Citation2014, Baradaran-Rafii et al. Citation2015, Heidari et al. Citation2011, Hosseinkazemi et al. Citation2015, Majdi et al. Citation2011, Sahebalzamani et al. Citation2015, Zeinali et al. Citation2014) of materials. Various studies have shown that the interfacial interactions are related to surface properties, such as surface chemical composition (Jung et al. Citation2009), surface wettability (Ikada et al. Citation1981), physical surface modification, and its effects on wettability are an interesting field for surface engineers. It should be noted that there has been a great deal of scientific research on molecularly smooth or modeled “simply rough” surfaces, but little work has been done on wettability and spreading phenomena of real engineering surfaces. From a practical point of view, a simple methodology is needed to account for the heterogeneous rough surface influence on wetting and contact angle measurements. The first attempt at establishing a methodology was made by Wenzel (Wenzel Citation1936). His simple model was based on the assumption that rough surfaces extend the solid–liquid interface area in comparison with a projected smooth surface. Among these techniques which have been developed for this purpose, laser irradiation (Chu Citation2007, Mochizuki et al. Citation2011) can be applied to create new functional groups, micro- and nanostructures, and change in surface wettability.
The purpose of this study was to provide the polypropylene (hydrophobic) and chitosan (hydrophilic) surfaces with a wide range of wettability and morphological properties using excimer laser irradiation, followed by studies on their eventual physicochemical characteristics with respect to AFM, contact angle, and cellular analyses.
Materials and methods
The solid materials used as substrates in the wetting experiments were prepared from Sigma Company (Sigma-Aldrich Inc., St. Louis, MO) (high-density polypropylene and chitosan; 2%W/V, degree of deacetylation >75%, medium molecular weight). Samples (20 × 20 mm2 with a thickness of 5 mm), after washed with deionized water and dried, were exposed to laser. KrF excimer laser (Zeiss MEL 80, Germany) with λ of 248 nm, laser fluence of 5 J/cm2, and repetition rate of 1 Hz was applied to treat the surface of polymeric films at two features by oriented and non-oriented irradiations.
An atomic force microscope (AFM; nanosurf easy scan 2 flex, Switzerland) was used to investigate morphological changes of the polymeric surfaces after laser modification. Sessile drop method was applied to measure static contact angles using Kruss G10 equipment (Hamburg, Germany).
For cellular analysis, aliquots of cell suspension in RPMI medium including 300,000 SW742 epithelial cells (Pasteur Institute, Iran) were seeded on a six-multiwell cell culture plate (Orange County Industrial Plastics, Anaheim, CA), which was precoated with modified and non-modified samples. The films were put in an incubator (37 °C, CO2) over three hours for cell attachment, followed by rinsing of the loosely attached cells with a phosphate buffer solution (pH 7.2), and adding 2 mL of fresh medium to the cell culture in the incubator for seven days. Proliferation of cells was determined from measurement of viable cell numbers by MTT assay. The MTT tetrazolium compound was reduced by living cells into a colored formazan product that was soluble in a tissue culture medium. The quantity of formazan product was directly proportional to the number of viable cells in the culture. The assays were performed by adding 1 mL of MTT solution and 9 mL of fresh medium to each sample after aspirating the medium, and incubating at 37 °C for four hours protecting from light. The colorimetric measurement of formazan dying was performed at a wavelength of 570 nm using a microplate reader.
Results and discussion
AFM results () of laser-irradiated PP and chitosan revealed that laser irradiation created some nanostructures on the polymers surfaces. It has been reported that the laser irradiation of polymers with high adsorption results in ablation of the surface, which subsequently leads to induction of some specific morphological structures on the surface (Knittel et al. Citation1997, Srinivasan et al. Citation1990). Surface roughness of polymers increased after exposure to laser irradiation ( shows that contact angle of water on the surface of the laser-irradiated samples decreased. Although the oxygen-based functional groups were observed in all laser-treated samples, their wettability differs significantly. The initial decrease in water contact angle could be related to production of polar functional groups on the polymers’ surface.
Figure 1. Topography of polymers modified by laser radiation. Chitosan (A: non-modified surface, B: modified & non-oriented surface, C: modified & oriented surface), and polypropylene (D: non-modified surface, E: modified & non-oriented surface, F: modified & oriented surface).
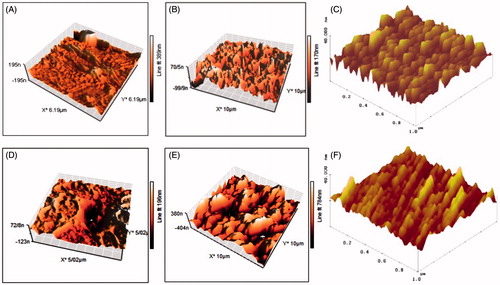
Table 1. The amount of RMS (roughness) for polymeric samples.
Table 2. Contact angles of normal and irradiated samples (average).
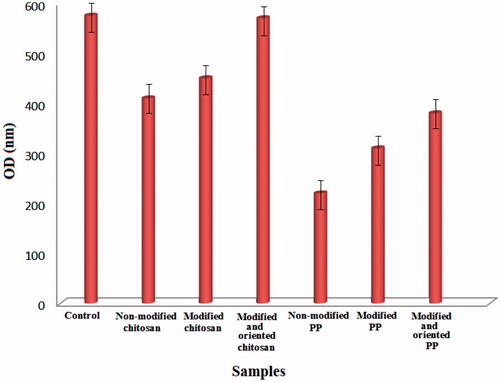
The cellular results for laser-irradiated samples are depicted in . After laser irradiation, the viability for the both polymers increased. On orientation of polymer surfaces by laser radiation, the polymers showed more viability than non-oriented surfaces. Excimer laser irradiation of polymers in air results in chemical reactions, e.g. oxidation of their surfaces. The chemical structure of irradiated polymers shows that carbonyl (C = O) or hydroxyl (–OH) groups are formed. It is known that the presence of these strongly polar groups on polymer surfaces can improve adhesion properties, depending on the laser characteristics.
Conclusions
Laser treatment was shown to induce a vast range of physiochemical properties onto the surface of PP and chitosan by regulating the treatment conditions; it is possible to achieve surfaces with hydrophobic and hydrophilic characteristics. The cellular results demonstrated that after laser irradiation, especially oriented irradiation viability of the polymers increased. The best biocompatible surfaces were for oriented laser irradiation of chitosan and polypropylene.
Acknowledgements
We are grateful to the Tonekabon University for providing the experimental equipments.
Disclosure statement
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- Ai J, Heidari S, Ghorbani F, Ejazi F, Biazar E, Asefnejad A, Pourshamsian K, Montazeri M. 2011. Fabrication of coated-collagen electrospun PHBV nanofiber film by plasma method and its cellular study. J Nanomater. 2011:123724. doi:10.1155/2011/123724.
- Baradaran-Rafii A, Biazar E, Heidari S. 2015. Oriented nanofibrous silk as a natural scaffold for ocular epithelial regeneration. J Biomater Sci Polym Ed. 26:1139–1151.
- Biazar E, Heidari M, Asefnezhad A, Montazeri N. 2011. The relationship between cellular adhesion and surface roughness in polystyrene modified by microwave plasma radiation. Int J Nanomed. 6:631–639.
- Biazar E, Heidari S, Baghermanesh B, Rezaei M. 2011. Design of modified electro-spun poly urethane nanofiber film using chemical method. Orient J Chem. 27:953–958.
- Biazar E, Heidari S. 2013. The healing effect of stem cells loaded in nanofibrous scaffolds on full thickness skin defects. J Biomed Nanotechnol. 9:1471–1482.
- Biazar E, Heidari S. 2014. Design of an oriented porous polymeric guide for neural regeneration. Int J Polym Mater Polym. 63:753–757.
- Biazar E, Roveimiab Z, Shahhosseini G, Khataminezhad M, Zafari M, Majdi A. 2012. Biocompatibility evaluation of a new hydrogel dressing based on polyvinylpyrrolidone/polyethylene glycol. J Biomed Biotechnol. 2012:343989. doi:10.1155/2012/343989.
- Biazar E, Sahebalzamani A. 2014. Modification of poly caprolactone nanofibrous mat by laminin protein and its cellular study. J Biomater Tissue Eng. 4:20–27.
- Biazar E, Zeinali R, Montazeri N, Pourshamsian K, Jabarvand BM, Asefnejad A, et al. 2010. Cell engineering: nanometric grafting of poly-N-isopropylacrylamide onto polystyrene film by different doses of gamma radiation. Int J Nanomed. 5:549–556.
- Chu PK. 2007. Plasma surface treatment of artificial orthopedic and cardiovascular biomaterials. Surf Coat Technol. 201:5601–5606.
- Heidari S, Azhdadi SN, Asefnezhad A, Sadraeian M, Montazeri M, Biazar E. 2011. The relationship between cellular adhesion and surface roughness for polyurethane modified by microwave plasma radiation. Int J Nanomed. 6:641–647.
- Heidari S, Zeinali R, Biazar E, Ghazvini A, Baniahmad F, Sahebalzamani A. 2011. Effect of plasma radiation on intelligent surface grafted to NIPAAm with chemical initiator under UV radiation. Orient J Chem. 27:1457–1464.
- Hosseinkazemi H, Biazar E, Bonakdar S, Ebadi MT, Shokrgozar MA, Rabiee M. 2015. Modification of PCL electrospun nanofibrous mat with Calendula officinalis extract for improved interaction with cells. Int J Polym Mater Polym. 64:459–464.
- Ikada Y, Iwata H, Horii F. 1981. Blood compatibility of hydrophilic polymers. J Biomed Mater Res. 15:697–718.
- Jung IK, Bae JW, Choi WS, Choi JH, Park KD. 2009. Surface graft polymerization of poly(ethylene glycol) methacrylate onto polyurethane via thiol-ene reaction: preparation and characterizations. J Biomater Sci Polym Ed. 20:1473–1482.
- Knittel D, Kesting W, Schollmeyer E. 1997. Surface structuring of synthetic fibres by UV laser irradiation, part II: mechanism and models. Polym Int. 43:240–250.
- Majdi A, Biazar E, Heidari S. 2011. Fabrication and comparison of electro-spun poly hydroxy butyrate valrate nanofiber and normal film and its cellular study. Orient J Chem. 27:523–528.
- Mochizuki A, Ogawa T, Okamoto K, Nakatani T, Nitta Y. 2011. Blood compatibility of gas plasma-treated diamond-like carbon surface – effect of physicochemical properties of DLC surface on blood compatibility. Mater Sci Eng C. 31:567–573.
- Sahebalzamani M, Biazar E, Shahrezaei M, Hosseinkazemi H, Rahiminavaie H. 2015. Surface modification of PHBV nanofibrous mat by laminin protein and its cellular study. Int J Polym Mater Polym. 64:149–154.
- Srinivasan R, Casey KG, Braren B, Yeh M. 1990. The significance of a fluence threshold for ultraviolet laser ablation and etching of polymers. J Appl Phys. 67:1604–1606.
- Wenzel RN. 1936. Resistance of solid surfaces to wetting by water. Ind Eng Chem. 28:988–994.
- Zeinali R, Biazar E, Heidari S, Rezaei M, Asadipour K. 2014. Regeneration of full-thickness skin defects using umbilical cord blood stem cells loaded into modified porous scaffolds. Asaio J. 60:106–114.