Abstract
The application of hemoglobin-based oxygen carriers (HBOCs) remains stagnant because a large number of reactive oxygen species (ROS) will be produced in organisms when HBOCs are used. Ferulic acid (FA) has a known effect on quenching oxygen free radicals and relieving vasospasm. Thus a kind of FA modified hemoglobin (BAEGF-Hb) is expected to produce the anti-oxidation effect on HBOCs spontaneously, and reduce toxicity. Isoelectric focusing electrophoresis, UV–vis wavelength scanning, and oxygen affinity curve showed that hemoglobin (Hb) was successfully modified and this modification had no effect on the structure of Hb and P50. The modification degree of Hb is 4.
Introduction
Development of a stabilized hemoglobin (Hb) complex used as a blood substitute has been vigorously pursued for many years. Possibly the best option is to produce an artificial red cell which has the properties of human red cell. However, this vision was never close to fruition (Bonaventura et al. Citation2013, Sakai Citation2014). While it is possible to create a liposome that encapsulates Hb, the red cell is far more sophisticated, containing cytoskeleton, enzymes, and transport systems, that keep Hb in the reduced state and maintain extraordinary flexibility for its 120-d lifespan (Sakai Citation2012, Sato et al. Citation2009). Finally, cell-free Hb carries O2, but it should be modified to prolong its vascular retention and reduce renal toxicity. To overcome these obstacles, Hb can be cross-linked to stabilize the tetramer thereby preventing its dissociation. For instance, Hb cross-linked with bis(3,5-dibromosalicyl) fumarate (DBBF) stabilizes the protein (Bobofchak Kevin et al. Citation2008, Vandegriff et al. Citation1989, Walder et al. Citation1994). Professor Chang’s research of submicron polymer membrane Hb nanocapsules is also an important approach (Wang et al. Citation2005, Yu and Chang Citation1996). Although there are other problems with Hb, including participation in oxidative toxicity, the most vexing problem is vasoconstriction (Gould and Moss Citation1996, Walter and Chang Citation1990). Vasoconstriction mainly occurs in the arterial or arteriolar circulation and a serious consequence of vasoconstriction is capillary collapse. A series of studies were assumed that nitric oxide (NO) scavenging by Hb was the mechanism of vasoconstriction and hypertension (Tsai et al. Citation2012).
The catabolism of large amounts of Hb production inevitably results in large amounts of free hemin, bilirubin, and ultimately free iron, known to catalyze various oxidative and peroxidative reactions. In tissues, various oxidant and antioxidant systems are in the delicate and critical balance, but non-physiological amounts of free radicals can easily disrupt it. As a result, increasing the production of harmful oxygen species and creating a catalytically active environment are favorable to peroxidation of lipids.
As the lack of superoxide dismutase (SOD) and catalase (CAT) in hemoglobin-based oxygen carriers (HBOCs), proper control of these complexes and interrelated redox phenomena is difficult (Gu and Chang Citation2009, Quebec and Chang Citation1995). The redox potential of Fe3+/Fe2+ in Hb is such that O2 will reduce to superoxide O2•− before Met-Hb was reduced to ferrous Hb. These various active species, as well as Hb itself and its catabolic products, can damage proteins, carbohydrates, nucleic acids, stimulate the peroxidation of unsaturated lipids, and promote the production of a range of toxic breakdown products (Nagababu et al. Citation2002, Zhang et al. Citation2014, Zhu et al. Citation2010). Free radicals and iron-derived reactive oxygen species (ROS) are implicated in the pathogenesis of numerous diseases and vascular disorders, including reperfusion injury, myocardial infarction, and hemorrhagic shock and cancer, and may be a factor in post-traumatic damage to the central nervous system (Cashon and Alayash Citation1995). As we know, ferulic acid (FA) has the function to eliminate free radical. Meanwhile, it may also reduce free radical damage and the recent literatures show the above-mentioned advantages (Alam et al. Citation2014, Raat and Ince Citation2007).
In this study, as FA is able to scavenge the free radical, we synthesized a novel chemical reagent, bromoacetylethyleneglycol ferulate (BAEGF), which was then reacted with Hb to form a modified Hb (BAEGF-Hb). BAEGF-Hb will be safe due to the function of FA (Mathew and Abraham Citation2004, Nicholson et al. Citation2008). Its stability, oxygen binding curve, and auto-oxidation were analyzed. There are definite trends in the stability and auto-oxidation rates. These results should help choose the best starting material for polymerization so that the products might serve as therapeutic oxygen carriers.
Experimental procedures
Reagents and instruments
Human umbilical cord blood was obtained from Tianjin Stem Cell Gene Engineering Company. Vitamin C (Vc) was obtained from Tianjin Fine Chemical Engineering Development Center (Tianjin, China). Other chemicals used were of analytical grade and the solutions were prepared with deionized water. Spectrophotometry analysis was conducted using SHIMADZU UV-2550 and the ultrafiltration membrane was used with 10 kDa ultrafiltration centrifugal tubes (Nanosep, Omega™). The NMR test was performed by NanoBay 400 MHz (Avance™ III HD, Bruker) and the oxygen affinity of the Hb samples was measured on a Hemox analyzer (TCS Scientific, PA).
Methods
(1) Preparation of purified Hb from human placentas referred to the methods described by Walder et al. (Citation1994). The final product in the retentate was collected and stored at −80 °C.
(2) Determination of the Hb concentration by the method of Evelyn and Malloy (referring to Snell’s paper; Snell and Marini Citation1988).
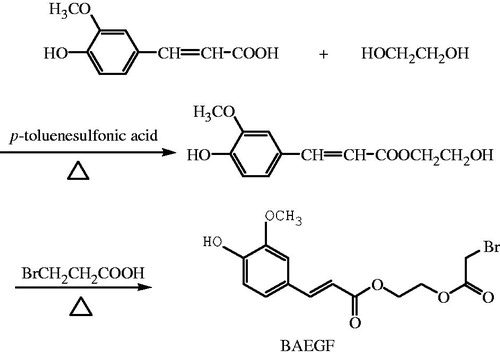
(3) Synthesis of BAEGF ().
p-Toluenesulfonic acid (0.46 g, 0.0024 mol) and FA (7.77 g, 0.04 mol) were weighed into 100 mL three-necked flask and stirred with 29.8 g of ethylene glycol (0.48 mol). Water separator and thermometer were installed, and then the flask was heated up to 120 °C under the protection of nitrogen gas. After 25 min, the mixture was cooled to room temperature and neutralized with 1 M NaOH to pH 7.Then 40 mL of saturated saline solution was added. With ethyl acetate extraction three times, the washings were combined and evaporated to dryness, yielding 11.68 g of monoethyleneglycol ferulate.
A mixture of monoethyleneglycol ferulate (2.38 g, 0.01 mol), bromoacetic acid (4.17 g, 0.03 mol), and p-toluenesulfonic acid (0.19 g, 0.001 mol) in 10 mL dioxane was stirred and heated to reflux under nitrogen gas for 4 h. The combined extract was washed with brine and dried over anhydrous sodium sulfate. The solvent was removed by rotary evaporation and then the crude product was purified by column chromatography on silica gel (eluent petroleum ether/ethyl acetate 70/30) to give the product BAEGF as a yellow oil in 43% yield, 1H NMR (CDCl3, ppm) δ 7.65 (d, 1H, J=15.9), 7.08 (t, 2H, J=8.1, J=9.9), 6.93(d, 1H, J=8.1), 6.31(d, 1H, J=15.9), 4.46–4.35(m, 4H), 3.94(s, 3H), 3.90(s, 2H).
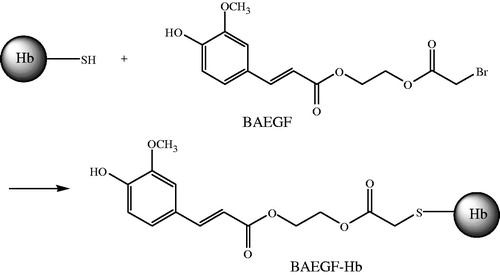
(4) Procedure of BAEGF modified Hb ()
Thiol modification of Hb was carried out with BAEGF, as the chart below shows. The reaction of the amino group is similar to that of thiol group.
The stroma-free hemoglobin (SFH) solution was under the protection of nitrogen at 1 mM (tetramer) in PBS for 1 h at 4 ± 2 °C, followed by reaction with BAEGF, molecular weight 359, at a 1-in-10 molar excess for 4 h at 4 ± 2 °C with gentle stirring. BAEGF-Hb was purified as the retentate after 10 kDa ultrafiltration centrifugal tube (Nanosep, Omega™) at 4000 r/min, 15 min later, and the final product was exchanged into 2 mM PBS. The product was stored frozen at −20 °C.
(5) Isoelectric focusing electrophoresis
According to Awdeh's method (Hovanessian and Awdeh Citation1976).
(6) Standard curve of BAEGF.
Gradient concentration of BAEGF solution of 2–20 μg/dL was measured at absorbance of 327 nm. Then the standard curve was obtained.
(7) UV–vis wavelength scanning
UV–vis full wavelength scanning of the Hb solution, Vc solution, DMSO solution, BAEGF solution, and the BAEGF-Hb solution were recorded in 200–700 nm range.
(8) Degree of modification
In the process of obtaining BAEGF-Hb, the filtrate was collected after the first centrifugation. UV–vis spectrum measured the absorption of the filtrate in 327 nm, after the solution was diluted 200 times. The amount of free BAEGF was easy to be known. The BAEGF modified on Hb equals the difference between total and free BAEGF. The molecular number of BAEGF modified on Hb divided by the molecular number of Hb is the degree of modification.
(9) Measurements of oxygen affinity
The BAEGF-Hb was bulk-filtered with a 0.2 μm filter followed by two sequential 0.2 μm sterile filtration steps. The product was concentrated to 6.4 ± 0.2 g/dL into sterile glass bottles.
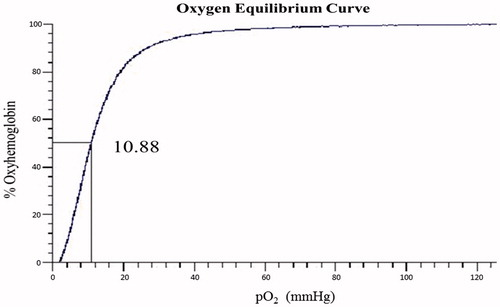
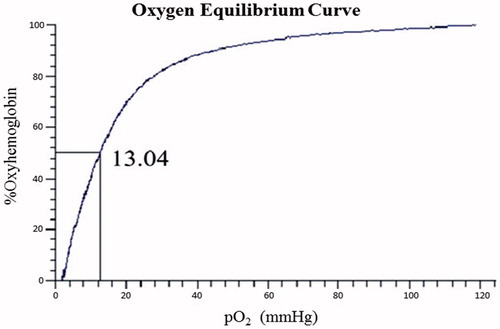
Oxygen equilibrium measurements of samples were carried out using Hemox analyzer at 37 °C in PBS buffer (pH 7.4). Triplicate measurements for each sample were performed to obtain the average values of P50 (oxygen affinity). Values for P50 (the O2 pressure at which Hb is half saturated) were obtained from the oxygen equilibrium curves.
Results and discussion
Isoelectric focusing electrophoresis
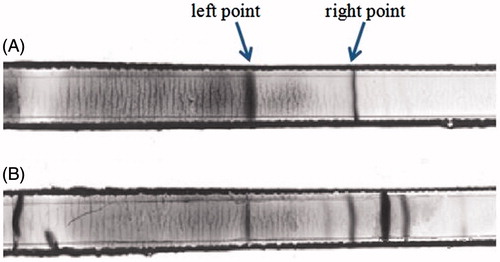
It can be found in that pure blood shows two isoelectric points, the left point is oxyhemoglobin (oxyHb) and the right one is deoxygenated Hb (deoxyHb) (). It is obvious that deoxyHb has higher location of the migration. These results are consistent with Walder’s conclusion (Walder et al. Citation1994). The content of oxyHb reduced after the modification with BAEGF due to deoxygenation. The deoxidized Hb is divided into several strips and the location of isoelectric point changes obviously because of various degree modifications by FA (). This result indicates that the deoxidized Hb was modified by BAEGF successfully. Isoelectric point of BAEGF-Hb is higher than pure Hb.
Standard curve of BAEGF
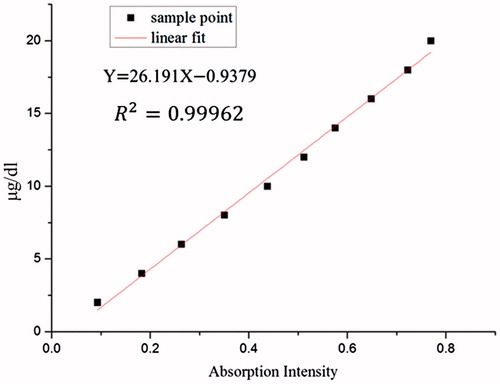
We can see from that the absorbance of BAEGF shows a good linear relationship in the concentration range of 2–20 μg/dL. This can be used to calibrate the actual content and further measure the modification degree of Hb.
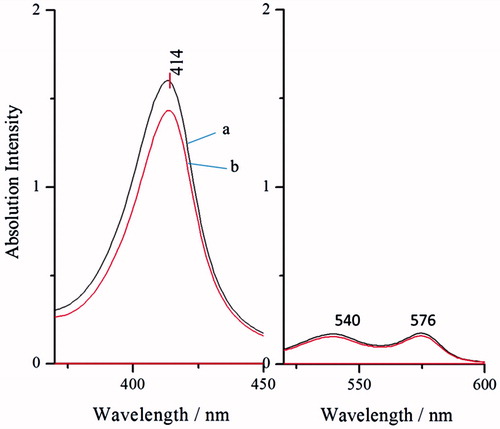
UV–vis wavelength scanning
Native Hb (, line b) was used as a control. It is evident from that the absorption intensity of Hb before and after modification was not changed. As we know, the absorption peak of heme is in 415 nm. This demonstrated that there is no change in the spatial conformation around heme. Meanwhile, the absorption peaks, which are at 540 nm and 577 nm, show a good oxygenation state. This indicates that modification (, line a) has no effects on the function of carrying oxygen.
Degree of modification
Spectrophotometric measurements were carried out in a UV–visible spectrophotometer. The concentration of BAEGF is 3.995 mM combining the standard curve of FA, the Hb concentration is 1 mM, and therefore the degree of modification is 4. This result was verified after oft-repeated experimentation.
Oxygen affinity
Natural red blood cells (RBCs) regulate their oxygen affinity through the action of 2,3-diphosphoglycerate (2,3-DPG), a molecule present inside the cells. The lack of 2,3-DPG in cell-free Hb results in unfavorably high oxygen affinity, which affects oxygen release when needed. The oxygen affinity (P50) of Hb and BAEGF-Hb are shown in and , respectively. Due to the absence of 2,3-DPG, the P50 values of both Hb and BAEGF-Hb are lower than that of natural RBCs (26 mmHg). After the modification of BAEGF, the P50 value of Hb becomes a little higher than that before modification, which may be owed to the coordination effect of BAEGF on oxygen release. The consistency in P50 value of BAEGF-Hb is more significant than that of Hb; the P50 value of BAEGF-Hb is generally low, which makes them suitable for the oxygenation of ischemic tissues. In contrast, the recent findings call for the biotechnology development of stable, low-affinity BAEGF-Hb with low NO dioxygenase reactivity (Cole Russell and Vandegriff Citation2011, Sakai et al. Citation2011).
Conclusion
The purpose of this study is that FA is used to modify Hb with reduction. First, the modifier, BAEGF, was successfully synthesized and characterized by NMR. Furthermore, it was ensured that the modification is successful by Gel Isoelectric Point technology.
The most important step of this study is to confirm the degree of modification. By the deduction of the absorbance of UV–vis spectra, we determine that the exact degree of modification is 4. UV–vis scanning spectrum was used and the results showed that the modification with BAEGF did not change the structure of Hb. The P50 detection showed that oxygen affinity of Hb had no significant effect after modification.
In the future, we will focus on the safety and efficacy of the Hb modified with BAEGF. The key point to be solved is to scavenge ROS. Furthermore, with BAEGF modified PEG-Hb and/or other poly-Hb, it will be ensured that whether the HBOCs have better half-life and prolong the effective time in vivo.
Acknowledgements
We appreciate Professor Liu Jiaxin and Wang Hong, for helpful discussions and Li Shen for communicating unpublished results.
This report would not have been possible without the support and substantive contributions of the Senior Research Scientists at Institute of Blood Transfusion, Chinese Academy of Medical Sciences, Chengdu, P.R. China. I would like to thank Li Shen and Zhou Wentao who helped me a lot in the experiment.
Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Funding information
Our work has been supported by grants from The National Science Foundation of China (No. 21202117/B020703) and The National High Technology Research and Development Program (“863” Program) of China (No. 2012AA021903).
References
- Alam F, Yadav N, Ahmad M, Shadan M. 2014. Blood substitutes: possibilities with nanotechnology. Indian J Hematol Blood Transfus. 30:155–162.
- Bobofchak Kevin M, Tarasov E, Olsen Kenneth W. 2008. Effect of cross-linker length on the stability of hemoglobin. Biochim Biophys Acta. 1784:1410–1414.
- Bonaventura C, Henkens R, Alayash AI, Banerjee S, Crumbliss AL. 2013. Molecular controls of the oxygenation and redox reactions of hemoglobin. Antioxid Redox Signal. 18:2298–2313.
- Cashon RE, Alayash AI. 1995. Reaction of human hemoglobin hba0 and two cross-linked derivatives with hydrogen peroxide: differential behavior of the ferryl intermediate. Arch Biochem Biophys. 316:461–469.
- Cole Russell H, Vandegriff KD. 2011. MP4, a vasodilatory pegylated hemoglobin. Adv Exp Med Biol. 701:85–90.
- Gould SA, Moss GS. 1996. Clinical development of human polymerized hemoglobin as a blood substitute. World J Surg. 20:1200–1207.
- Gu J, Chang TMS. 2009. Extraction of erythrocyte enzymes for the preparation of polyhemoglobin-catalase-superoxide dismutase. Artif Cells Blood Substit Immobil Biotechnol. 37:69–77.
- Hovanessian AG, Awdeh ZL. 1976. Gel isoelectric focusing of human-serum transferrin. Eur J Biochem. 68:333–338.
- Mathew S, Abraham T. 2004. Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit Rev Biotechnol. 24:59–83.
- Nagababu E, Ramasamy S, Rifkind JM, Jia Y, Alayash AI. 2002. Site-specific cross-linking of human and bovine hemoglobins differentially alters oxygen binding and redox side reactions producing rhombic heme and heme degradation. Biochemistry. 41:7407–7415.
- Nicholson SK, Tucker GA, Brameld JM. 2008. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc Nutr Soc. 67:42–47.
- Quebec EA, Chang TMS. 1995. Superoxide dismutase and catalase cross-linked to polyhemoglobin reduces methemoglobin formation in vitro. Artif Cells Blood Substit Immobil Biotechnol. 23:693–705.
- Raat NJH, Ince C. 2007. Oxygenating the microcirculation: the perspective from blood transfusion and blood storage. Vox Sang. 93:12–18.
- Sakai H. 2014. Biocompatibility of a highly concentrated fluid of hemoglobin-vesicles as a transfusion alternative. Regener Med Artif Cells Nanomed. 3:133–147.
- Sakai H, Okuda N, Takeoka S, Tsuchida E. 2011. Increased viscosity of hemoglobin-based oxygen carriers retards no-binding when perfused through narrow gas-permeable tubes. Microvasc Res. 81:169–176.
- Sakai H. 2012. Present situation of the development of cellular-type hemoglobin-based oxygen carrier (hemoglobin-vesicles). Curr Drug Discov Technol. 9:188–193.
- Sato T, Sakai H, Sou K, Medebach M, Glatter O, Tsuchida E. 2009. Static structures and dynamics of hemoglobin vesicle (HBV) developed as a transfusion alternative. J Phys Chem B. 113:8418–8428.
- Snell SM, Marini MA. 1988. A convenient spectroscopic method for the estimation of hemoglobin concentrations in cell-free solutions. J Biochem Biophys Methods. 17:25–33.
- Tsai AG, Intaglietta M, Sakai H, Delpy E, La Rochelle CD, Rousselot M, Zal F. 2012. Microcirculation and NO-CO studies of a natural extracellular hemoglobin developed for an oxygen therapeutic carrier. Curr Drug Discov Technol. 9:166–172.
- Vandegriff KD, Medina F, Marini MA, Winslow RM. 1989. Equilibrium oxygen binding to human hemoglobin cross-linked between the α chains by bis(3,5-dibromosalicyl) fumarate. J Biol Chem. 264:17824–17833.
- Walder RY, Andracki ME, Walder JA. 1994. Preparation of intramolecularly cross-linked hemoglobins. Meth Enzymol. 231:274–280.
- Walter SV, Chang TMS. 1990. Chronotropic effects of in vitro perfusion with albumin, stroma-free hemoglobin, and polyhemoglobin solutions. Biomater Artif Cells Artif Organs. 18:283–298.
- Wang MY, Yu YT, Chang TMS. 2005. New method for preparing more stable microcapsules for the entrapment of genetically engineered cells. Artif Cells Blood Substit Immobil Biotechnol. 33:257–269.
- Yu WP, Chang TMS. 1996. Submicron polymer membrane hemoglobin nanocapsules as potential blood substitutes: preparation and characterization. Artif Cells Blood Substit Immobil Biotechnol. 24:169–183.
- Zhang R, Xiang Y, Ran Q, Deng X, Xiao Y, Xiang L, Li Z. 2014. Involvement of calcium, reactive oxygen species, and atp in hexavalent chromium-induced damage in red blood cells. Cell Physiol Biochem. 34:1780–1791.
- Zhu H, Du Q, Chen C, Chang TMS. 2010. The immunological properties of stroma-free polyhemolysate containing catalase and superoxide dismutase activities prepared by polymerized bovine stroma-free hemolysate. Artif Cells Blood Subst Biotechnol. 38:57–63.