Abstract
In vitro coculture system provides a powerful tool for tissue engineering. In this study, we evaluated the gene expressions of human adipose-derived stem cells (ASCs) on polycaprolactone (PCL) scaffold in coculture model with fetal chondrons. Electrospun PCL scaffolds (900 nm fiber diameter) were created and human infrapatellar fat pad-adipose-derived stem cells (IPFP-ASCs) were seeded on these scaffolds. Scanning electron microscopy (SEM) showed attachment of human IPFP–ASCs to scaffold. IPFP-ASCs on scaffolds were cocultured with fetal chondrons in transwell. Gene expressions were investigated using real-time polymerase chain reaction (real-time PCR). In comparison with control group, the expression level of collagen type 2 and aggrecan were significantly decreased but Indian Hedgehog(IHH) significantly increased (P < 0.05).These findings may interpreted that IPFP-ASCs seeded on PCL scaffold, in cocultures with fetal chondrons are tending toward osteogenesis rather than chondrogenesis.
Introduction
Articular cartilage (AC) that covers the ends of the long bones is a hyaline cartilage with load-bearing and wear-resistant property. AC is avascular tissue so it has a limited capacity for intrinsic repair (Hunziker Citation2009), and for this reason, injuries can lead to advanced damage, degeneration, pain, and disability of joint. The goal of tissue engineering is to restore the functional cartilage in articular disorders (Caplan and Goldberg Citation1999). Three factors are involved in cartilage engineering: cells, scaffolds, and signaling factors, alone or in combination. Viable cell source can be chondrocytes, adult stem cells, and genetically modified cells. Many cartilage tissue engineering researchers utilize chondrocytes as the cell source (Byers et al. Citation2008, Freed et al. Citation1993). However, this approach is challenged by limited supply of chondrocytes and their de-differentiation during in vitro culture, or morbidity during chondrocytes harvest (Lee et al. Citation2000). Therefore, other studies apply mesenchymal stem cells (MSCs) instead of chondrocyte (Caplan Citation2000, Martin et al. Citation2001). MSCs have been demonstrated to exist in adult human tissue types, such as bone marrow, subcutaneous adipose tissue, and infrapatellar fat pad (IPFP) (Dragoo et al. Citation2003, Johnstone et al. Citation1998, Wickham et al. Citation2003, Zuk et al. Citation2001) and have been used to repair defects (Bölgen et al. Citation2014). The advantage of IPFP compared with bone marrow is that according to previous studies IPFP gives higher yield of stem cells and there is decrease pain and morbidity with the yield of cells in treated patients (Dragoo et al. Citation2003). There are no specific cellular markers for the recognition of MSCs, so their identification is achieved by their ability to adhere to plastic or flask in vitro, and their multilineage differentiation potential in vitro and also through a combination of positive expression or lack of defined cell surface markers (Dominici et al. Citation2006). Typical types of surface marker proteins expressed in MSCs are as follows: receptor molecules, such as hyaluronate (CD44), surface enzymes (CD73), extracellular matrix proteins (CD90), vascular adhesion molecules (CD106) (Gimble and Guilak Citation2003).
Different scaffolding materials, such as poly(ɛ-caprolactone) (PCL), have been used for cartilage regeneration and soft tissue engineering (Elamparithi et al. Citation2015). The PCL was chosen because it is a FDA-approved, biodegradable polymers (Sinha et al. Citation2004) that supports chondrogenesis (Li et al. Citation2003) and degrades slowly and entirely cleared by the body (Huang et al. Citation2006, Sun et al. Citation2006). Scientists have turned to nanotechnology, specifically nanofibers because these types of scaffolds can mimic the architecture of the native extracellular matrix, which prepare the basic space for regeneration of new tissue (Wei and Ma Citation2008). The PCL is hydrophobic and this character has a negative effect on cell adhesion and proliferation. In recent years, some surface treatments of polyesters have been tried to increase their surface hydrophilicity to improve the cell–scaffold interfaces (Arufe et al. Citation2010, Diekman et al. Citation2009). Alkaline hydrolysis and plasma treatment of polymeric scaffolds were proved to significantly improve adhesion of cell and increase cell proliferation (Fan et al. Citation2009, Huang et al. Citation2010, Wang et al. Citation2009). Moreover, surface modification methods (e.g. plasma exposure) were used for different types of scaffolds can increase the above-mentioned properties of cells.
Many methods, such as applying of conditioned media from chondrocytes, are used to provide natural mediators for chondrogenic differentiation of MSCs. The results of these studies suggest that these factors have stimulatory impact on chondrogenesis (Aung et al. Citation2011, Di Nino et al. Citation2001, Lee and Im Citation2010, Solursh and Meier Citation1973). Here we used the fetal chondrons instead of growth factors in coculture systems. Chondron is composite of a chondrocyte and its surrounding pericellular matrix (PCM), which is histological and metabolic unit of cartilage (Poole et al. Citation1987, Poole, Citation1997). PCM is a slight layer of matrix that at once surrounds the chondrocyte and it is where chondrocytes and matrix interchange signals (Guilak et al. Citation2006). The greatly expressed genes of growth factors were identified in fetal cartilage by computing their relative expressed sequence tags (EST) frequency levels (Zhang et al. Citation2003). Here, we wanted to investigate whether the chondrocyte’s native pericellular matrix has a positive effect on differentiation of IPFP-ASCs to chondrogenic cells. Outcomes were analyzed using semi-quantitative genes expression of aggrecan, collagen 2 and Indian Hedgehog (IHH).
Materials and methods
Cell isolation, culture
IPFP was obtained from patients (aged 24, 25, and 46 years, n = 3) undergoing anterior cruciate ligament (ACL) surgery. Briefly, the tissue was washed 3 times with phosphate-buffered saline (PBS, Sigma) and diced finely and then digested with 0.1% collagenase 1 (Gibco Brigg, UK) for 50–55 min at 37 °C. Enzyme activity was neutralized with Dulbecco’s modified Eagle’s medium (DMEM-low glucose, Gibco, UK), containing 10% fetal bovine serum (FBS, Gibco, E.U. Approved (South American)) and centrifuged at 1400 rpm for 10 min. Then, the pellet was resuspended, washed 2 times with medium, and seeded on culture flask. Medium of culture flask containing DMEM, 10% FBS, 1% penicillin–streptomycin (Sigma-Aldrich) and maintained in incubator at 37 °C, 5% CO2, and 97% humidity (Kabiri et al. Citation2012). At the time of different passage, cell viability was determined by trypan blue staining. For freezing, after culturing through passage 1 or 2, the cells were suspended in a cryopreservation medium containing 90% FBS and 10% dimethylsulfoxide (DMSO, Sigma-Aldrich).
Enzymatic isolations of chondrons
Human fetal cartilages for cell isolation were obtained in accordance with the Ethics Committee of Clinical Research of the Tabriz University of Medical Sciences. Cartilage samples were dissected within 1 h from aborted fetuses (aged: 12–16 weeks; n = 3) to make sure that there was any visual contamination of other tissues. Chondrons isolation was accorded on a previously published method (Lee et al. Citation1997) with slight modification. The minced cartilage pieces were treated with 0.3% dispase (Gibco Brigg, UK) and 0.2% collagenase 2 (Gibco Brigg, UK) in PBS for 4 h. Enzymes activity were neutralized and then centrifuged at 1400 rpm for 10 min. The pellet washed and seeded on culture flasks in medium containing DMEM, 10% FBS, 1% penicillin–streptomycin, and 25 μg/mL ascorbic acid (Sigma-Aldrich St. Louis, MO). For freezing, after 24 h, floating cells were washed and suspended in a cryopreservation medium containing 90% FBS and 10% DMSO.
Scaffold characterization
PCL scaffold was obtained from a Stem Cell Technology Company (Iran). The nanofibrous PCL sheet was treated with plasma.
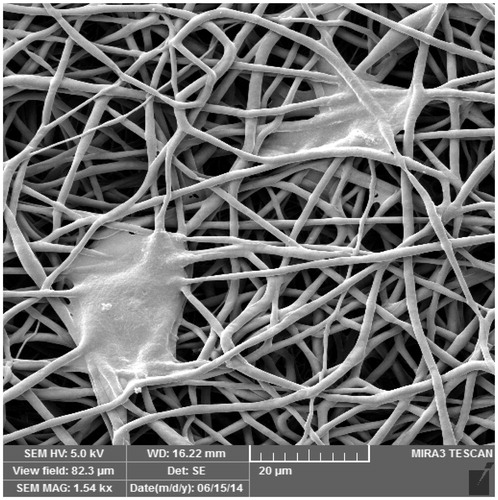
Before using, scanning electron microscope (MIRA3 FEG-SEM) was used for the observation of the structural morphology of scaffold. The samples of scaffold were cut from the nanofiberous sheet using a 7 mm dermal punchand coated with gold by a sputter-coater. Diameter of fibers in the electrospun scaffold we measured on scanning electron micrographs. The average diameter of fibers were determined from measurements of perpendicular to the long axis of the fibers within representative microscopic fields (Heydarkhan-Hagvall et al. Citation2008).
Cell morphology
First SEM was performed to investigate the cell–scaffold interaction between the IPFP-ASCs and PCL scaffolds. After 2 days of seeding, the scaffolds were fixed with 10% buffered formaldehyde for 30 min at room temperature, dried in vacuum and gold-sputtered.
Cell surface epitope characterization and flowcytometry
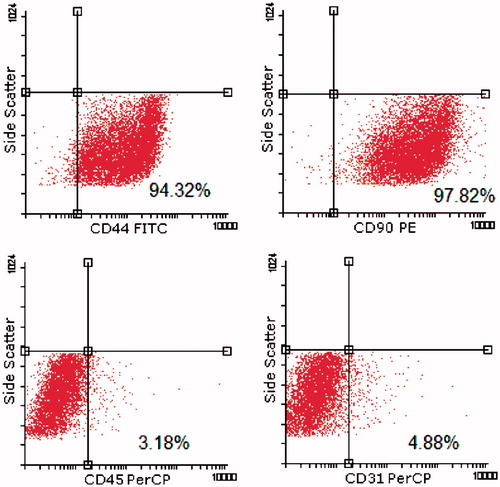
IPFP-ASCs from passage 2 were characterized for mesenchymal stem cell surface protein expression by flowcytometry. The monoclonal antibodies used were PE-labeled anti-CD90 and FITS-labeled anti-CD44 (BD Bioscience), PerCP-labeled anti-CD31 (R&D systems) and CD45 (Abcam, UK). The cells (3 × 105 cells) were washed and incubated with antibodies for 25 min at 4 °C temperature in dark environment. At least 10,000 events were acquired on BD caliber (BD ebioscience), and the data were analyzed using flowing software (PerttuTerho, Version: 2.5.1, Turku centra for Biotechnology, Turku, Finland).
Seeding of IPFP-ASCs into PCL scaffolds and coculture
The scaffolds were sterilized under ultraviolet (UV) light for 2 h on each side. The sterilized scaffold disks were placed into 24-well culture plate. Each scaffold was seeded with IPFP-ASCs at concentration of 5 × 105 cells/specimen and then incubated for 2 h to allow the cells to attach. Then, 1 mL (mL) medium [DMEM, 1% FBS, 1% penicillin–streptomycin, and 25 μg/mL ascorbic acid] was added to each well. For coculturing transwells (0.4 μm pore size, polyester membrane, Greiner, Germany) were placed into the 24-well plates in a way that were not in contact with scaffold but were suspended in the medium that had IPFP-ASCs/scaffold. The chondrons were suspended in chondrogenic medium [DMEM, 1% FBS, 1% penicillin–streptomycin, and 25 μg/mL ascorbic acid] at a concentration of 5 × 105 cell/300 μL. Then, chondrons suspension were added to the transwell. The 24-well culture plate was incubated at 37 °C in 5% CO2 for 21 days. About 100 μL medium was removed from the lower chambers and equal volume fresh medium were added to upper chambers every 2 days.
Real-time RT-PCR
After 21 days, each IPFP-ASCs seeded scaffold was homogenized under liquid nitrogen using a mortar. RNA was isolated from the samples by using RNX-Plus (Sinaclon, IRAN) according to the manufacturer's instructions. The concentration of RNA was estimated spectrophotometrically with NanoDrop 1000 Spectrophotometer (Wilmington, DE) at A260/280. The RNA samples were reverse transcribed into first-strand cDNA using the AccuPower® RT PreMix (Bioneer, Korea). Real-time polymerase chain reactions (RT-PCR) were performed using the SYBR Green PCR Mastermix (Applied Biosystems) according to the manufacturer’s instructions. These gene primers were purchased from (Bioneer, Korea). The used cartilage-specific oligonucleotide primers, were aggrecan (forward 5′-AGGGCGAGTGGAATGATGTT-3′; reverse 5′-GGTGGCTGTGCCCTTTTTAC-3′), and collagen type 2a1 (forward 5′-ATGCCACACTCAAGTCCCTCAA-3′; reverse 5′-CGCAAGTCTCGCCAGTCTCC-3′), (IHH) (forward 5′-CACTTCTGCCTGGTCCTGTT-3′; reverse 5′-GGGCTGAACTGCTTGTAGG-3′), housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward, 5′-CAAGATCATCAGCAATGCCTCC-3′; reverse, 5′-GCCATCACGCCACAGTTTCC-3′). All experiments were performed in triplicate for each sample. Interpretation of the results was performed using the Pfaffle method and the CT values were normalized with respect to GAPDH expression.
Statistical analysis
Results are presented as mean ± standard deviation. T-test method was used for statistical analysis. P < 0.05 were considered statistically significant.
Results
Scaffold characterization
SEM imaging was first performed to observe the structural morphology of fibers in the electrospun PCL scaffold. The surface of plasma-treated scaffold was smooth. The average diameter of nanofibers was approximately 900 nm. The orientation of the fibers was random ().
Cell morphology
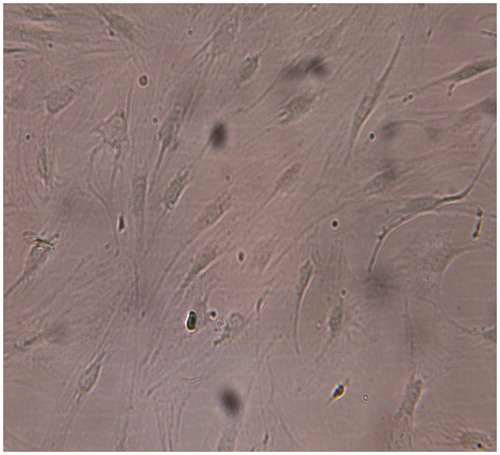
IPFP-ASCs were able to adhere to tissue culture flasks, whereas non adherent cells, such as red blood cells, were removed by media change. At the second passage, IPFP-ASCs appeared to adopt a more uniform fibroblast-like shape ().
Figure 2. Morphology of IPFP-ASCs in culture as observed with an inverted phase-contrast microscope at passage2, 10×.
To investigate the state of the pericellular matrix after isolation, toluidine blue staining was performed. Toluidine blue staining was strongly positive for glycosaminoglycans when observed with inverted phase-contrast microscope ().
Figure 3. A photograph showing PCM in chondron as a bluish surrounding. Stained with toluidine blue, 40×.
IPFP-ASCs cultured in vitro on the PCL scaffold were observed by SEM. The IPFP-ASCs attached to the surface and inside the pores of the PCL scaffold after 2 days ().
Cell surface epitope characterization and flowcytometry
IPFP-ASCs expressed the mesenchymal stem cell markers, such as CD44 (94.32%), CD90 (97.82%). However, few cells expressed hematopoietic marker such as CD45 (3.18%), or the endothelial marker CD31 (4.88%) ().
Real-time RT-PCR
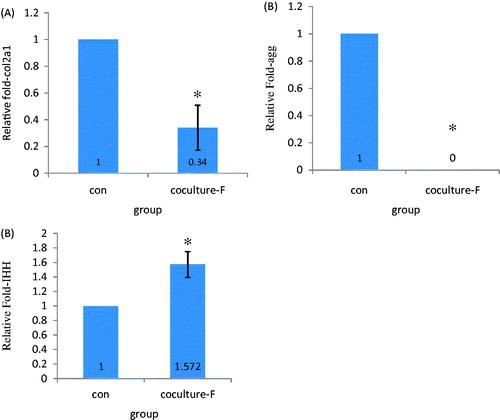
To evaluate the effects of coculture on cartilage-specific gene expression of IPFP-ASCs species primers were used to quantify the gene expression of aggrecan (agg), type-2 collagen (col2a1), and Indian Hedgehog (IHH). For the coculture group, IPFP-ASCs receiving conditioned medium of fetal chondrons, showed markedly decrease of col2a1 () and agg () compared with their corresponding control cultures. By contrast, coculture increased the mRNA levels of IHH (). All of the changes were statistically significant (P < 0.05).
Discussion
In this study, we used the fat from the infrapatellar fat pad, because previous studies have shown that infrapatellar fat pad-derived stem cells are able to differentiate to chondrogenic and osteogenic cells. Moreover, ASCs from the IPFP encapsulated in fibrin, express collagen type 2 and aggrecan and its matrix sulfatedglycosoaminoglycan (sGAG) content reached 50% of native cartilage (Dragoo et al. Citation2003). We observed the IPFP-ASCs showed plastic-adherent property in culture, and about 94.32% of IPFP-ASCs expressed the MSCs-positive CD markers: CD 44 and 97.82% of ASCs expressed the CD90. While slightly above of 2% of IPFP-ASCs expressed negative cell surface marker (CD31: platelet endothelial cell adhesion molecule and CD45: leukocyte common antigen), at second passage. So the cell surface epitope characterization and flowcytometry of IPFP-ASCs population showed similar staining pattern to that of bone marrow-derived stem cells. Our result showed the percentage of negative markers is high, but it seems to decrease with increasing of passage numbers because cells became more homogeneous. Taken together, the results suggest that the ASCs from the IPFP is a relatively homogeneous population of mesenchymal cells with low contamination by endothelial and leukocyte cells.
The goal of the present study was to produce extracellular matrix mimics so we used nanofibrous scaffolds. Because nanofibrous scaffolds composed of nanofibers similar to extracellular matrix molecules, such as collagen, laminin, and fibronectin (with 5–500 nm in size) and proteoglycans (e.g., hyaluronic acid, 450–1000 nm), which have been found to increase cell adherence, proliferation, and differentiation (Li et al. Citation2005).
SEM was performed to investigate diameter of fibers and the cell–scaffold interaction between the ASCs and PCL scaffolds. The results of SEM showed that the diameter of the fibers were at the nanometer size. Another interesting finding is that after 48 h the cells attached to the scaffolds. May be expression of adhesion molecules involved in it.
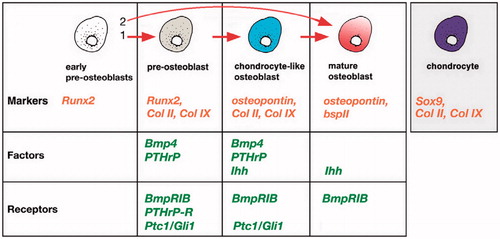
The transwell coculture system described herein allowed for cell–cell communication in the form of soluble factors without direct cell–cell contact. In this study, the coculture condition was insufficient to induce chondrogenic differentiation of the human infrapatellar fat pad-derived cells because did not express aggrecan gene and showed down regulation of type-2 collagen, compared with control group. The human IPFP-ASCs cocultured with fetal chondrons exhibited higher levels of IHH compared with control cultures. There are four transitional cell types during dermal bone formation (), and with respect to genes expression, it seems that ASCs differentiated into the chondrocyte-like osteoblasts (CLO) cells (Abzhanov et al. Citation2007). Finally, these cells could differentiate into mature osteoblasts.
Figure 7. Regulation of osteoblastic differentiation in cranial dermal bone. Chondrocyte-like osteoblast (CLO) cells expressing a unique combination of collagen 2,9 (but not Sox9 or aggrecan) as well as Indian hedgehog (Ihh), Ptc1, Gli1, Bone morphogenic protein4 (Bmp4) and parathyroid hormone-related protein (PTHrP). As these cells develop into mature osteoblasts, they downregulate collagen 2, 9 expression.
Some of in vitro cell culture models have shown that cartilage tissue or chondrocytes affect other chondrocytes or MSCs via soluble paracrine factors (Ahmed et al. Citation2007, Gan and Kandel Citation2007, Jikko et al. Citation1999). In contrast to the previous reports, our finding suggested that the coculture of IPFP-ASCs with fetal chondrons developed toward osteogenesis. This could be attributed to various reasons such as nanofiber scaffold and concentration of secreted factors. Based on previous studies, nanotopography can influence cellular behavior through known mechanisms such as the regulation of shape of cell and surface protein absorption but perhaps it is because of the unknown effects of nanotopographies. Also, four components involved in behavior of cells on biomaterial surfaces: (a) adsorption of serum components, (b) components of extracellular matrix secreted by the cell, (c) cell adherence molecules, and (d) cytoskeleton mechanics (Matsuzaka et al. Citation1999). This report is supported by the literature as there are experiments that nanofiber scaffolds promoted osteogenesis (Ruckh et al. Citation2010, Smith et al. Citation2009a, Citation2009b). The current studies suggest that the osteoinductive effects of nanofibers come from their ability to force cells into an osteogenic morphology. It is well established that morphology of cell and its function are strongly linked (Chen et al. Citation1997, Folkman and Moscona Citation1978) and that this principle uses to human bone marrow stromal cells (hBMSC) osteogenesis (McBeath et al. Citation2004, Rodriguez et al. Citation2004, Treiser et al. Citation2010). It is reported that mitogens, other than vascular endothelial growth factor (VEGF), such as fibroblast growth factor (FGF), are also present in human fetal epiphyseal conditioned media (Garcia‐Ramirez et al. Citation2000). In the present study, we expect that nanofibrous constructs could absorb these factors because previous studies have shown that the nanofibrous constructs were found to selectively increase the adsorption of specific proteins, such as fibronectin and vitronectin (Woo et al. Citation2007). It appears this happened and FGF showed its effect. Because FGF2, 4, 9 are expressed in sutural mesenchyme in early craniofacial skeletogenesis during intramembranous bone formation, and this shows that they may be involved in regulating calvarial osteogenesis (Britto et al. Citation2001, Kim et al. Citation1998, Mehrara et al. Citation1998, Rice et al. Citation2000). So it seems that the nanofibrous scaffolds and soluble factors have led to cell differentiation. However, future studies are necessary to elucidate.
Conclusion
The data presented have demonstrated that IPFP-ASCs from the infrapatellar fat pad had same CD marker of MSCs. We also have shown that the differential effects of fetal chondrons in indirect coculture model did not enhance chondrogenic differentiation of human IPFP-ASCs. Furthermore, the results of the present study demonstrate that both cell-secreted factors and nanofibrous PCL scaffold are involved on the differentiation pattern of human IPFP-ASCs. The culture condition-dependent differentiation of IPFP-ASCs provides new insights into how tissue-specific soluble factors may be directed to engineer complex tissues from stem cells that exhibit a differentiation potential toward multiple tissues.
Acknowledgements
This work was supported by Research department, Tabriz University of Medical Sciences, Tabriz, Iran. project number:5/4/7684-92-9-18.
References
- Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. 2007. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 134:3133–3144.
- Ahmed N, Dreier R, Göpferich A, Grifka J, Grässel S. 2007. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem Physio. 20:665–678.
- Arufe M, De la Fuente A, Fuentes I, De Toro F, Blanco F. 2010. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J Cell Biochem. 111:834–845.
- Aung A, Gupta G, Majid G, Varghese S. 2011. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum. 63:148–158.
- Bölgen N, Korkusuz P, Vargel İ, Kılıç E, Güzel E, Çavuşoğlu T, Uçkan D, Pişkin E. 2014. Stem cell suspension injected HEMA-lactate-dextran cryogels for regeneration of critical sized bone defects. Artif Cells Nanomed Biotechnol. 42:70–77.
- Britto JA, Evans RD, Hayward RD, Jones BM. 2001. From genotype to phenotype: the differential expression of FGF, FGFR, and TGFbeta genes characterizes human cranioskeletal development and reflects clinical presentation in FGFR syndromes. Plast Reconstr Surg. 108:2026–2039. discussion 2040-2026.
- Byers BA, Mauck RL, Chiang IE, Tuan RS. 2008. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Pt A 14:1821–1834.
- Caplan AI. 2000. Tissue engineering designs for the future: new logics, old molecules. Tissue Eng. 6:1–8.
- Caplan AI, Goldberg VM. 1999. Principles of tissue engineered regeneration of skeletal tissues. Clin Orthop Relat R. 367:S12–S16.
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. 1997. Geometric control of cell life and death. Science. 276:1425–1428.
- Di Nino DL, Long F, Linsenmayer TF. 2001. Regulation of endochondral cartilage growth in the developing avian limb: cooperative involvement of perichondrium and periosteum. Dev Biol. 240:433–442.
- Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. 2009. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 16:523–533.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8:315–317.
- Dragoo JL, Samimi B, Zhu M, Hame SL, Thomas BJ, Lieberman JR, Hedrick MH, Benhaim P. 2003. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 85:740–747.
- Elamparithi A, Punnoose AM, Kuruvilla S, Ravi M, Rao S, Paul SF. 2015. Electrospun polycaprolactone matrices with tensile properties suitable for soft tissue engineering. Artif Cell Nanomed Biotechnol. 22:1–7.
- Fan J, Varshney RR, Ren L, Cai D, Wang D-A. 2009. Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng Part B Rev. 15:75–86.
- Folkman J, Moscona A. 1978. Role of cell shape in growth control. Nature. 273:345–349.
- Freed LE, Marquis JC, Nohria A, Emmanual J, Mikos AG, Langer R. 1993. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 27:11–23.
- Gan L, Kandel RA. 2007. In vitro cartilage tissue formation by co-culture of primary and passaged chondrocytes. Tissue Eng. 13:831–842.
- Garcia‐Ramirez M, Toran N, Andaluz P, Carrascosa A, Audi L. 2000. Vascular endothelial growth factor is expressed in human fetal growth cartilage. J Bone Miner Res. 15:534–540.
- Gimble J, Guilak F. 2003. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 5:362–369.
- Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, Setton LA, Haider MA. 2006. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann NY Acad Sci. 1068:498–512.
- Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, et al. 2008. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 29:2907–2914.
- Huang AH, Farrell MJ, Mauck RL. 2010. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 43:128–136.
- Huang MH, Li S, Hutmacher DW, Coudane J, Vert M. 2006. Degradation characteristics of poly (ε‐caprolactone)‐based copolymers and blends. J Appl Polym. 102:1681–1687.
- Hunziker EB. 2009. The elusive path to cartilage regeneration. Adv Mater Weinheim Mater Deerfield. 21:3419–3424.
- Jikko A, Kato Y, Hiranuma H, Fuchihata H. 1999. Inhibition of chondrocyte terminal differentiation and matrix calcification by soluble factors released by articular chondrocytes. Calcified Tissue Int. 65:276–279.
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. 1998. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 238:265–272.
- Kabiri A, Esfandiari E, Hashemibeni B, Kazemi M, Mardani M, Esmaeili A. 2012. Effects of FGF-2 on human adipose tissue derived adult stem cells morphology and chondrogenesis enhancement in Transwell culture. Biochem Biophys Res Commun. 424:234–238.
- Kim HJ, Rice D, Kettunen PJ, Thesleff I. 1998. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 125:1241–1251.
- Lee CR, Grodzinsky AJ, Hsu HP, Martin SD, Spector M. 2000. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J Orthop Res. 18:790–799.
- Lee GM, Poole CA, Kelley SS, Chang J, Caterson B. 1997. Isolated chondrons: a viable alternative for studies of chondrocyte metabolism in vitro. Osteoarthr Cartilage. 5:261–274.
- Lee JS, Im GI. 2010. Influence of chondrocytes on the chondrogenic differentiation of adipose stem cells. Tissue Eng Part A. 16:3569–3577.
- Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. 2005. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 26:5999–6008.
- Li WJ, Danielson KG, Alexander PG, Tuan RS. 2003. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 67:1105–1114.
- Martin I, Shastri VP, Padera RF, Yang J, Mackay AJ, Langer R, Vunjak-Novakovic G, Freed LE. 2001. Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. J Biomed Mater Res. 55:229–235.
- Matsuzaka K, Walboomers X, De Ruijter J, Jansen J. 1999. The effect of poly-L-lactic acid with parallel surface micro groove on osteoblast-like cells in vitro. Biomaterials. 20:1293–1301.
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 6:483–495.
- Mehrara BJ, Mackool RJ, McCarthy JG, Gittes GK, Longaker MT. 1998. Immunolocalization of basic fibroblast growth factor and fibroblast growth factor receptor-1 and receptor-2 in rat cranial sutures. of Fibroblast Factor Fibroblast Factor -and -in Cranial. Plast Reconstr Surg. 102:1805–1817.
- Poole CA. 1997. Review. Articular cartilage chondrons: form, function and failure. J Anat. 191:1–13.
- Poole CA, Flint MH, Beaumont BW. 1987. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res. 5:509–522.
- Rice D, Aberg T, Chan Y, Tang Z, Kettunen PJ, Pakarinen L, Maxson R, Thesleff I. 2000. Integration of FGF and TWIST in calvarial bone and suture development. Development. 127:1845–1855.
- Rodriguez JP, Gonzalez M, Rios S, Cambiazo V. 2004. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J Cell Biochem. 93:721–731.
- Ruckh TT, Kumar K, Kipper MJ, Popat KC. 2010. Osteogenic differentiation of bone marrow stromal cells on poly(epsilon-caprolactone) nanofiber scaffolds. Acta Biomater. 6:2949–2959.
- Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. 2004. Poly-epsilon-caprolactone microspheres and nanospheres: an overview. Int J Pharm. 278:1–23.
- Smith LA, Liu X, Hu J, Ma PX. 2009a. The influence of three-dimensional nanofibrous scaffolds on the osteogenic differentiation of embryonic stem cells. Biomaterials. 30:2516–2522.
- Smith LA, Liu X, Hu J, Wang P, Ma PX. 2009b. Enhancing osteogenic differentiation of mouse embryonic stem cells by nanofibers. Tissue Eng Part A. 15:1855–1864.
- Solursh M, Meier S. 1973. A conditioned medium (CM) factor produced by chondrocytes that promotes their own differentiation. Dev Biol. 30:279–289.
- Sun H, Mei L, Song C, Cui X, Wang P. 2006. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 27:1735–1740.
- Treiser MD, Yang EH, Gordonov S, Cohen DM, Androulakis IP, Kohn J, Chen CS, Moghe PV. 2010. Cytoskeleton-based forecasting of stem cell lineage fates. Proc Natl Acad Sci USA. 107:610–615.
- Wang L, Tran I, Seshareddy K, Weiss ML, Detamore MS. 2009. A comparison of human bone marrow–derived mesenchymal stem cells and human umbilical cord–derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Pt A. 15:2259–2266.
- Wei G, Ma PX. 2008. Nanostructured biomaterials for regeneration. Adv Funct Mater. 18:3568–3582.
- Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. 2003. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat R. 412:196–212.
- Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, et al. 2007. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 28:335–343.
- Zhang H, Marshall K, Tang H, Hwang D, Lee M, Liew C. 2003. Profiling genes expressed in human fetal cartilage using 13,155 expressed sequence tags. Osteoarthr Cartilage. 11:309–319.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. 2001. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7:211–228.