Abstract
Olanzapine (OLZ) is a typical anti-psychotic drug, which is highly lipophilic in nature, belongs to Biopharmaceutical Classification System (BCS) class II category. Though OLZ is an effective agent in the treatment of Schizophrenia, but it exhibits poor bioavailability (57%) due to extensive first-pass metabolism resulted in high dose is required to achieve therapeutic concentration in brain. Emerging evidences are indicating that high dose administration of OLZ may cause Extrapyramidal symptoms (EPS) in the psychotic patients. Hence, the present study is designed to develop Olanzapine solid lipid (OLZ-SLNs) using minimal dose of OLZ thereby enhancing the brain efficacy as well as to reduce the side effects associated with OLZ. OLZ-SLNs have been prepared by “solvent diffusion method” using lipids, such as glyceryl monostearate (GMS), tripalmitin (TP), Tween 80, and Stearyl amine as positive charge inducer. The prepared OLZ-SLNs were subjected to particle size analysis, zeta potential, and poly dispersity index measurement by using Malvern Zetasizer. Pharmacokinetics assessments of OLZ-SLNs were carried in conscious male Wistar rats through intravenous administration. Results have shown that average particle size and zeta potential of SLNs of GMS and TP were ranged from 165.1 ± 2.2 to 110.5 ± 0.5 and 35.29 ± 1.2 and 66.50 ± 0.7 mV, respectively. Relative bioavailability of OLZ in the brain was increased up to 23-fold and clearance was decreased when OLZ-SLNs while administrated intravenously. The area under the curve (AUC) and mean residence time (MRT) of OLZ-SLNs in brain were higher than OLZ suspension. These results indicate that SLNs are a promising drug delivery for OLZ. It may be an effective tool to enhance the bioavailability of OLZ in the brain with less dose administration, which could reduce the EPS associated with OLZ.
Introduction
Olanzapine (OLZ) is an anti-psychotic drug which is currently used in the treatment of schizophrenia and it is found to be reducing the positive as well as negative symptoms of schizophrenia (Conley and Mahmoud Citation2001); as also, reducing agitations in patients affected with bipolar disorder (Meehan et al. Citation2001) and post-traumatic stress disorder symptoms (Petty et al. Citation2001). The pharmacological responses of OLZ predominantly mediate through antagonism of central serotonin type 2 (5-HT2A) and dopamine type 2 (D2) receptors. The blockade of both dopamine and serotonergic receptors by OLZ mainly attributed for the therapeutic efficacy of OLZ against schizophrenia. Though OLZ is having high clinical value as anti-psychotic agent, but it exhibits poor bioavailability due to extensive hepatic first-pass metabolism before reaching the systemic circulation, i.e. 40% of the drug gets metabolized. The oral bioavailability of OLZ is 57% and it is mainly metabolized by direct glucuronidation and cytochrome P450-mediated oxidation (CYPIA2, CYP2D6) pathway. The less bioavailability in plasma may accounts for reduced drug availability in brain. The poor bioavailability of OLZ in brain can be overcome by increasing the initial dose administration, which may facilitate the side effects associated with drugs. Recent evidences are indicating that the patients taking OLZ were found to be affected with Extrapyramidal symptoms (EPS) like rigidity, bradykinesia, tremor, akathisia, and dyskinesia.
Delivering this drug to the brain posses a great challenge in crossing the blood brain barrier (BBB). Solid lipid nanoparticles (SLNs) have been exploited as a probable possibilities as carriers for tissue targeting. In addition, SLNs are composed of solid lipid matrix, which provides an effective means for control release of the drug. It also improves the drug bioavailability in the brain. Thus, the present study is designed to enhance the OLZ availability in brain by incorporating minimal dose of OLZ into SLNs, which act as a carrier. Fasciculately, OLZ-SLNs can be administered through intravenously to overcome the existing drug-related problems like poor bioavailability and untoward effects due to high dose.
Solid lipid nanoparticles are biocompatible/biodegradable lipid carriers size ranges from 10 nm to 1000 nm. Lipids such as mono-, di-, tri-glycerides, and hard waxes are widely used as a carrier to formulate SLNs. The choice of preferring emulsifiers and co-emulsifiers in SLNs based on the route of administration and SLNs are preparing by different methods like high pressure homogenization, microemulsion, and solvent evaporation/diffusion (Gasco Citation1993, Sjostrom and Bergenstahl, Citation1992, Zur Muhlen et al. Citation1998). Currently, newer drug-loaded solid lipid nanoparticles are emerged by many active researchers due to their wide applicability in drug delivery science. Moreover, SLNs avoid the disadvantages of the other colloidal carriers, such as fat emulsion, polymeric nanoparticles, liposomes (Heiati et al. Citation1996, Muller et al. Citation1995, Schwarz et al. Citation1994, Westesen et al. Citation1997). In the present study, OLZ-loaded SLNs were formulated by solvent diffusion method and the same were subjected to physico-chemical characterization, in vitro dissolution studies, pharmacokinetics parameters, and brain bioavailability assessment of OLZ upon intravenous administration in male Wistar rats. The evaluated parameters of novel OLZ-SLNs were compared with OLZ suspension to differentiate the merits of new formulation than conventional dosage forms.
Materials and methods
Chemicals
Olanzapine was obtained as a gift sample from Ind Swift Pvt. Ltd., Chandigarh, India. TP was purchased from Sigma Aldrich (Mumbai, India), Glycerymonoatearate was procured from Kemphasol (Mumbai, India). Tween 80 and Stearyl amine were purchased from Sisco (Mumbai, India), dialysis membrane was supplied from Himedia Labs, Mumbai, India. The other chemicals and reagents used in this study were of analytical grade unless mentioned.
Partition coefficient studies of OLZ in glyceryl monostearate and tripalmitate
Ten milligrams of OLZ was dispersed in a mixture of melted lipid (1 g) and 1 mL of hot phosphate buffer pH 7.4 (PB) and shaken for 30 min in a water bath shaker and maintained at above 10 °C to achieve the melting point of the lipid. Aqueous phase was separated from the lipids by centrifugation at 10,000 rpm for 20 min. The clear supernatant obtained was suitably diluted with 0.1 N HCl and OLZ content was determined in UV-Visible spectrophotometer at 258 nm against solvent blank.
Preparation of OLZ-SLN by solvent diffusion technique
Solid lipid nanoparticles were prepared based on the principal of “Emulsion Solvent Diffusion” technique. OLZ (5 mg), tripalmitate (30 mg), and 10 mg of stearylamine were dissolved in a mixture of acetone:ethanol (1:1 ratio) and heated to 60–70 °C. The resulting solution was poured into 50 mL of 1%w/v aqueous Tween 80 under the mechanical stirring drop by drop with a syringe and stirred for the required time. The formulated SLNs were centrifuged and freeze dried. Stearyl amine was used as a positive charge inducer and added along with the lipid and drug.
Entrapment efficiency
Entrapment efficiency of freeze dried SLNs was determined by weighing the required quantities of SLNs (5 mg) and it was dissolved in 0.1 N HCl under water bath at 70 °C for 30 min and then cooled to room temperature to preferentially precipitate the lipid. Drug content in the supernatant after centrifugation (4000 rpm for 15 min) was determined by UV visible spectroscopy at 258 nm using 0.1N HCl as blank. (Abou el Ela et al. Citation2014, Govender et al. Citation1999, Song et al. Citation2008).
Characterization of OLZ-SLNs
Particle size and zeta potential
The developed nanoparticles were washed with double distilled water (filtered through 0.22 L) several times before particle size analysis. Particle size and zeta potential were measured by photon correlation spectroscopy using Malvern Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, UK), which works based on the Mie theory. All size and zeta potential measurements were carried out at 25 °C using disposable polystyrene cells and disposable plain folded capillary zeta cells. (Betancourt et al. Citation2007).
Differential scanning calorimetry
Differential scanning calorimetry (DSC) analysis was performed using DSC Q200 (TA Instruments, New Castle, DE). The instrument was calibrated with indium for melting point and heat of infusion. A heating rate of 20 °C/min was employed throughout the analysis in the range of 25–200 °C. Standard aluminum sample pans were used for all the samples; an empty pan was used as reference. The thermal behavior was studied under a nitrogen purge of 40 mL/min; triplicate run were carried out on each sample to check reproducibility (Kashi et al. Citation2012).
Scanning electron microscopy
External morphology of nanoparticles was determined using scanning electron microscopy (SEM). Freeze-dried nanoparticles were re-suspended in distilled water and later dropped into a silicon grid and dried under vacuum. They were subsequently coated with gold (JFC 1200 Fine Coater, Tokyo, Japan). The surface morphology of the samples was observed under a scanning electron microscope (JEOL Ltd, Tokyo, Japan) operated at 15 keV pulse at different resolutions (Özbaßs-Turan and Akbugă Citation2011).
In vitro release studies
The release of OLZ from the SLNs was studied under sink conditions. Tripalmitate-SLN (TP-SLN) and Gleceryl monostearate (GMS-SLN), which showed higher drug content and entrapment efficiency were evaluated for in vitro release. 5 mL of SLNs equivalent to 1 mg were put in dialysis bags (MWCO 12000, HiMedia). The dialysis bags were placed in 50 mL of dissolution medium and stirred under magnetic stirring at 37 °C. Aliquots of the dissolution medium were withdrawn at each time interval and the same volume of fresh dissolution medium was added to maintain a constant volume. Samples withdrawn from phosphate buffer saline (pH 7.4) were analyzed for OLZ content spectrophotometrically at 258 nm against solvent blank (Asadi Citation2014, Seju et al. Citation2011).
Release kinetics
In vitro dissolution has been recognized as an important element in drug development. Under certain conditions it can be used as a surrogate for the assessment of bioequivalence. Several theories/kinetic models describe the drug dissolution from immediate and modified release dosage forms. There are several models to represent the drug dissolution profiles, where ft is the function of t (time) related to the amount of drug dissolved from the pharmaceutical dosage system. To compare dissolution profiles between two drug products model dependent (curve fitting), statistic analysis, and model independent methods can be used.
In vivo pharmacokinetic evaluation in plasma
The required amount of OLZ was dissolved in 0.3% w/v CMC (carboxy methyl cellulose). Male Wistar rats weighing 200 ± 20 g were used for pharmacokinetics and bio distribution studies. All the rats were fasted for overnight before the experiments and had free access to water and 6 animals were taken in each group. All animal experiments procedures were approved by the Institutional Animal Ethical Committee, J.S.S. College of Pharmacy, Udhagamandalam (Proposal no. JSSCP/IAEC/M.PHARM/PH.CEUTICS/03/2010–11). Drug suspension (0.3% w/v CMC) and TP-SLNs were administered intravenously by caudal vein at a dose of 0.18 mg (calculated based on human dose of 10 mg, conversion factor = 0.018). Blood (0.5 mL) was collected by the tail vein at 0, 0.5, 1, 2, 3, 4, 6, 8, 12 and 24 h after administration separately. Three animals were kept for each time intervals, respectively. Blood samples were placed into Eppendorf tubes containing 0.3 mL of anticoagulant (citrate) solution and centrifuged immediately. After centrifugation, the plasma obtained was stored at –20 °C until further analysis by HPLC.
Brain distribution study
After blood samples withdrawn, euthanasia given for all the animals and the brain samples were collected for distribution study at each time intervals. Brain samples were washed with normal saline solution to remove blood or other contents and dried with tissue paper. The tissue samples were homogenized ice cold 10% KCl and then centrifuged at 10,000 rpm for 10 min and the supernatant solutions were collected and stored at –70 ± 2 °C until further analysis by HPLC.
Chromatographic condition
The sample is analyzed using Hiber C18 column (250 × 4.6mm i.d., 5 μ) with Shimadzu LC – 10AT solvent delivery gradient pump equipped with rheodyne sample injector with 20 μL loop and SPD-10A VP PDA detector (Shimadzu Analytical Pvt Ltd, Chennai, India). The mobile phase consisted of acetonitrile:Potassium dihydrogen orthophosphate (40:60% v/v), pH is adjusted to 4.5 with orthophosphoric acid and was used at a flow rate of 1 mL/min and the elute was monitored at 254 nm.
Statistical analysis
Data are expressed as mean ± SEM (standard error of mean). The statistical significance between the groups in pharmacokinetic parameters was analyzed by unpaired Student’s t-test. P values less than 0.05 were considered statistically significant. Statistics was carried out using Graph pad Prism software, V.5.01, La Jolla, CA.
Results and discussion
Partition coefficient studies
Olanzapine is a hydrophobic drug with log P values of 2.199. The partition coefficient was found with respect to Glyceryl tripalmitate (TP) (282.5) and Glyceryl monostearate (GMS) (54.86). Since, TP is more lipophilic than GMS; hence, it had more affinity towards OLZ. It is suspected to have more imperfections in its lipid matrix to accommodate the drug. Hence, an initial study of the partitioning nature of the drug between the melted lipid and aqueous media can provide some clues about the entrapment of OLZ in SLN formulation.
Effect of formulation process variables
Solid lipid nanoparticles were prepared by solvent diffusion technique by varying the amount of the lipid. In this study, 16 different batches were prepared separately using both GMS and TP as lipids. It was observed that the particle size of SLNs was increased while increasing the lipid concentration (60 mg). The possible reason for increased particle size of SLNs may be attributed by the presence of more lipid concentration when compared to surfactants. Interestingly, surfactant concentration was not sufficient to envelop the lipid nanoparticles hence, larger particles were observed.
Thus, due to lower particle size (165.1 nm) in the batch 8 with GMS and TP batch 13 with particle size (110.5 nm) were considered to be an optimized batch. The effect of stirring time and stirring speed on particle size was in the range of 165–541 nm as in GMS and 110.5–361 nm as in TP, respectively. It is obvious that with an increase in the stirring time up to 4 h and stirring speed up to 5000 rpm there was a decrease in the particle size. There was no significant change in the particle size while increasing the surfactant concentration, thus, 1.5% is considered as the optimized surfactant concentration. Generally, the effective size range of the SLNs in brain targeting is 50 to 300 nm. Our optimized batch having the average particle size (110.5 nm) in TP-SLN and (165.1 nm) GMS-SLN which fall in that range might be useful to cross BBB.
Entrapment efficiency
The lipid core was found to affect the extent of entrapment efficiency which is represented in . As observed with TP-SLN and GMS-SLN, the maximum entrapment efficiency was found to be 96.3% with TP-SLN and 67.2% with GMS-SLN, respectively. The entrapment efficiency of TP-SLN was higher than GMS-SLN because TP is more lipophilic in comparison to GMS, which can accommodate more drug in the lipid matrix. Since, TP-SLN has shown higher entrapment efficiency; therefore it was taken for the further studies.
Table 1. particle size, zeta potential, and entrapment efficiency.
Measurement of particle size and zeta potential
Polydispersity index (PDI) values of all the formulations indicate that particle size distribution was unimodel. The optimized batch was having least particle size (110.5 nm) and had a PDI of 0.340 in TP-SLN, whereas GMS-SLN having low particle size (165.1 nm) with PDI of 0.726. It was found that batch prepared with stearylamine had a zeta potential of 66.50 mV and batch with tripalmitate has shown zeta potential of 35.29 mV in the optimized batch. It has been reported that positively charged SLNs have a better uptake by brain than neutral or negatively charged particles. Stearylamine contains lipophilic hydrocarbon chain (18 carbons), which is accommodated in the lipid core that is projecting the amine group into the aqueous phase, and induces positive charge.
Differential scanning colorimetry
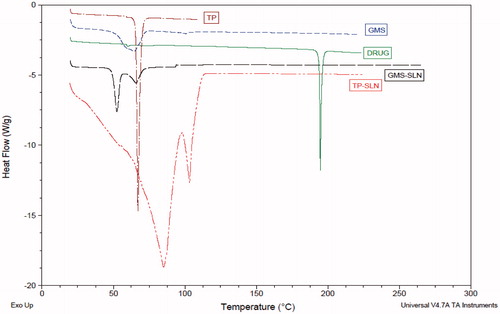
The DSC curve of OLZ () has shown melting endotherm at 194.78 °C. The peak intensity corresponding to the melting of OLZ has decreased in thermograms of OLZ-loaded TP and GMS SLNs. These results indicate that only a small fraction of the drug substance existed in the crystalline state. Reduction in the melting point and the enthalpy of the melting endotherm was observed when the lipid was formulated as SLNs. Incorporation of OLZ inside the lipid matrix resulted in increased number of defects in the lipid crystal lattice, and hence decreased the melting point of the lipid in the final SLNs formulations. Freitas and Muller also observed the crystalline behavior of compritol SLNs differed distinctly from that of the bulk lipid. Small particle size of SLNs leads to high surface energy, which creates an energetically suboptimal state that decreased the melting point.
Scanning electron microscopy
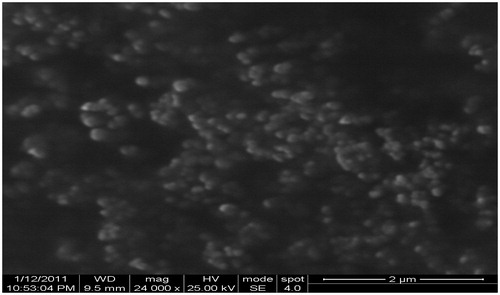
The external morphological studies using scanning electron microscopy (SEM) have revealed that maximum nanoparticles were found to be spherical in shape (). The nanoparticle size observed by SEM correlated well with the particle size measured by Zetasizer (Malvern instrument).
In vitro kinetics of OLZ-SLN
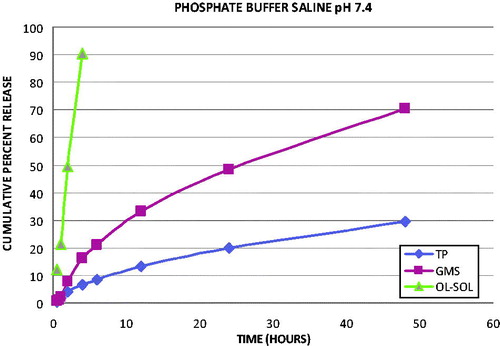
In vitro dissolution studies were carried out using phosphate buffer pH 7.4 saline. The release profiles indicate that SLN formulations were retarded the release of the drug from the lipid matrix when compared with plain OLZ solution. The in vitro release graph of SLN formulations and OLZ solution in phosphate buffer is depicted in . It was observed that OLZ solution showed 90.24% release in 4 h, whereas 6.62 and 16.68% release for TP-SLN and GMS-SLN, respectively, at the end of same time. GMS-SLN release rate was nearly 50% higher than that of TP-SLN at the end of 48 h.
Release kinetics
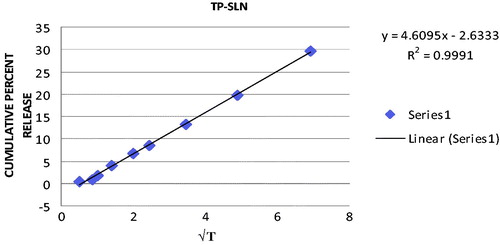
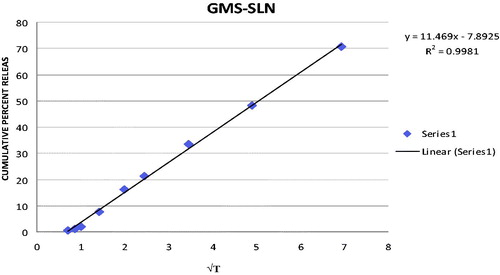
In order to elucidate mode and mechanism of drug release, the in vitro data were transformed and interpreted at graphical interface constructed using various kinetic models. The in vitro release data obtained for SLN formulations were fitted into various kinetic models. The results are summarized in . The best linearity was obtained in Higuchi’s plot for both SLN formulations indicating the release from matrix as a square root of time-dependent process. The Higuchi plot for TP-SLN and GMS-SLN are shown in and , respectively.
Table 2. Regression value for various kinetic models.
Release mechanism
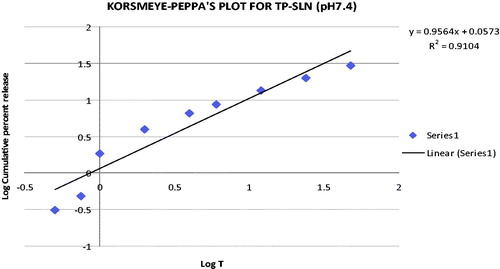
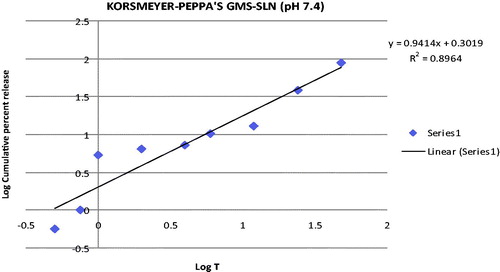
Through incorporating the release data in Korsmeyer–Peppa’s equation, the mechanism of the drug release can be indicated from the value of release exponent “n”. Peppa’s plot for TP-SLN and GMS-SLN are given in and , respectively. The release exponent values “n” for TP-SLN and GMS-SLN was found to be 0.9564 and 0.9414, respectively. Since, the release exponent “n” values were between 0.5 and 1, it indicates that the SLN formulations undergo anomalous diffusion.
Pharmacokinetic evaluation
Pharmacokinetic parameters of OLZ after i.v. administration are represented in . Peak concentration (Cmax) and time of peak concentration (Tmax) were obtained directly from the individual plasma-concentration time profiles. The area under the concentration–time curve from time zero to time t (AUC0→t) was calculated using the Wagner Nelson method. The area under the curve (AUC) determines the bioavailability of the drug.
Table 3. Pharmacokinetic parameters for OLZ in the plasma after i.v. administration.
The relative bioavailability presented in shows the concentration–time curve of Glyceryl tripalmitate solid lipid nanoparticles (TP-SLN) and OLZ suspension. After the injection of OLZ-SLNs, the OLZ concentration was still present after 6 h, in case of OLZ suspension concentration was reached minimum after 3 h. Following i.v. administration of the OLZ suspension, the mean measured peak serum concentration achieved was 25.83 ng/mL, whereas with OLZ SLNs is 55.92 ng/mL.
After intravenous administration of OLZ-suspension, free OLZ is available for solvation and might have solubilized in plasma, subsequently solution of OLZ distributed rapidly compared to distribution of OLZ entrapped in SLN. The initial serum concentration (at 30 min) was observed is higher for OLZ suspension than OLZ-SLN, following intravenous administration. A difference in OLZ concentrations between the SLNs and OLZ suspension was observed, OLZ-TP showed higher concentrations than OLZ suspension ().
In vivo bio distribution studies
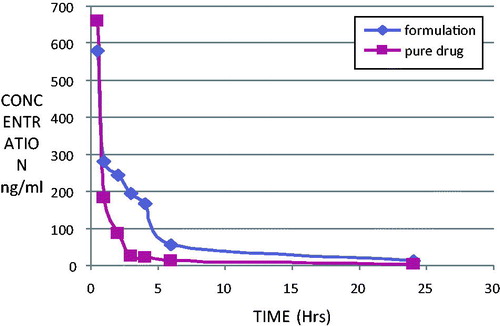
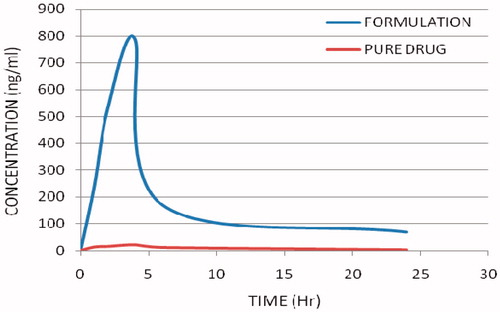
In order to evaluate the enhanced distribution pattern in the brain following i.v. administration, the control aqueous suspension and the SLN formulations containing OLZ were intravenously administered to conscious male Wistar rats. shows the brain drug concentration versus time profile of OLZ following i.v. administration in aqueous OLZ suspension and OLZ-SLNs. Interestingly, SLNs were more effective in enhancing bioavailability of OLZ in the brain. In brain, the concentrations of OLZ were significantly higher for OLZ-SLN when compared to OLZ suspension. Also, the concentration of OLZ was present in the brain was lasting until 24 h after administration of OLZ-SLNs.
AUC(0–t), MRT(0–t), and Fr values of the brain for OLZ formulations are given in . When compared with OLZ-suspension, the relative bioavailability of OLZ-TP in the brain was 23.66. AUC(0–t) and MRT(0–t) values for the brain in SLNs were found higher than OLZ-suspension. SLN of OLZ releases the drug at slow rate. The highest Fr value was observed with OLZ-TP in brain. Entertainingly, the Fr value was increased about 23 times compared with OLZ–suspension.
Table 4. Pharmacokinetic parameters for formulation and pure drug in brain.
In non-RES organ, such as brain, OLZ-SLN has shown enhanced brain concentrations of OLZ and maintained high drug concentration at 24 h (). The AUC of OLZ-SLNs in brain were found to be higher than OLZ-suspension. These results indicate that OLZ-SLNs improve brain OLZ level as well as therapeutic efficacy of the OLZ in the treatment of schizophrenia. The possible mechanism of SLNs in considering the factor of drug penetration in BBB might be the over coating of drug-loaded SLNs with polysorbate 80 led to the adsorption of apolipoprotein E of plasma onto the nanoparticle surface. This coated particle behaves as LDL particles and could interact with LDL receptors, initiating their uptake by endothelial cells. Subsequently, either released drug might diffuse into brain cells or the particles may be transcytosed. Polysorbate 80 appears to act as an anchor for apolipoprotein E showed that ability of BBB passage by a sufficient amount of olanzapine. Solid lipid nanoparticles of olanzapine in brain may be the result of transport of intact olanzapine nanoparticles through the BBB by endocytosis.
Conclusion
The solvent diffusion technique was found to be a suitable method for producing SLNs and lipophilic drugs like OLZ can be successfully incorporated into the lipids by using this method. In vitro release study has revealed that OLZ-SLNs were exhibited sustained release. The formulated SLNs with OLZ shown a significant increase in relative bioavailability, i.e. 23-fold in the brain compared to pure OLZ suspension. Higher relative bioavailability of OLZ in the brain may be attributed through transport of intact OLZ nanoparticles across the BBB by endocytosis mechanism. Moreover, incorporation of low dose of OLZ in SLNs could be an effective tool to achieve the optimum concentration of OLZ in brain. SLNs have provided sustained release of the OLZ, and enhanced the drug delivery potential in the brain. Hence OLZ-SLNs formulation can be a novel approach for effective delivery of OLZ in brain. The development of SLNs not only intensifies the BBB permeation of OLZ, also it could enhance the free OLZ availability to antagonize the 5-HT2A and D2 receptors in brain cells thereby enhancing the therapeutic efficacy. In addition, the minimizing the OLZ dose level can reduce the drug-related side effects like EPS in schizophrenic patients due to neurotransmitters imbalance. Hence, this study can be concluded that the OLZ-SLNs is an effective approach to enhance the brain drug delivery system and it can be prototyped for other drugs which are having poor brain bioavailability.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Abou el Ela AESF, Hassan MA, El-Maraghy DA. 2014. Ketorolac tromethamine floating beads for oral application: characterization and in vitro/in vivo evaluation. Saudi Pharm J. 22:349–359.
- Asadi A. 2014. Streptomycin-loaded PLGA-alginate nanoparticles: preparation, characterization, and assessment. Appl. Nanosci. 4:455–460.
- Betancourt T, Brown B, Brannon-Peppas L. 2007. Doxorubicin- loaded PLGA nanoparticles by nanoprecipitation: preparation, characterization and in vitro evaluation. Nanomedicine. 2:219–232.
- Conley RR, Mahmoud R. 2001. A randomized double-blind study of risperidone and Olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry. 158:765–774.
- Gasco MR. 1993. Methods for producing solid lipid microparticles having a narrow size distribution. United States Patents, USS 188837.
- Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. 1999. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Release. 57:171–185.
- Heiati H, Phillips NC, Tawashi R. 1996. Evidence for phospholipid bilayer formation in solid lipid nanoparticles formulated with phospholipid and triglyceride. Pharm Res. 13:1406–1410.
- Kashi TSJ, Eskandarion S, Esfandyari-Manesh M, Marashi SMA, Samadi N, Fatemi SM, et al. 2012. Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method. Int J Nanomed. 7:221–234.
- Meehan K, Zhang F, David S, Tohen M, Janicak P, Small J, et al. 2001. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 21:389–397.
- Muller RH, Mehnert W, Lucks JS, Schwarz C, zur Mühlen A, Weyhers H, Freitas C, Rühl D. 1995. Solid lipid nanopaticles (SLN) – an alternative colloidal carrier system for controlled drug delivery. Eur J Pharm Biopharm. 41:62–69.
- Özbaßs-Turan S, Akbugă J. 2011. Plasmid DNA-loaded chitosan/TPP nanoparticles for topical gene delivery. Drug Deliv. 18:215–222.
- Petty F, Brannan S, Casada J, Davis LL, Gajewski V, Kramer GL, et al. 2001. Olanzapine treatment for post-traumatic stress disorder: an open-label study. Int Clin Psychopharmacol. 16:331–337.
- Schwarz C, Mehnert W, Lucks JS, Muller RH. 1994. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J. Control Release. 30:83–96.
- Seju U, Kumar A, Sawant KK. 2011. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: in vitro and in vivo studies. Acta Biomater. 7:4169–4176.
- Sjostrom B, Bergenstahl B. 1992. Preparation of submicron drug particles in lecithin-stabilized w/o emulsions: I. Model studies of the precipitation of cholesteryl acetate. Int J Pharm. 88:53–62.
- Song X, Zhao Y, Hou S, Xu F, Zhao R, He J, et al. 2008. Dual agents loaded PLGA nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm. 69:445–453.
- Westesen K, Bunjes H, Koch MHJ. 1997. Physicochemical characterization of lipid nanoparticles and evaluation of drug loading capacity and sustained release potential. J Control Release. 48:223–236.
- Zur Muhlen A, Schwarz C, Mehenert W. 1998. Solid lipid nanoparticles (SLN) for controlled drug delivery-drug release and release mechanisms. Eur J Pharm Biopharm. 45:149–155.