Abstract
In this work, poly(HEMA-MAPA) membranes were prepared by UV-polymerization technique. These membranes were characterized by SEM, FTIR, and swelling studies. Synthesized membranes had high porous structure. These membranes were used for controlled release of curcumin which is already used as folk remedy and used as drug for some certain diseases and cancers. Curcumin release was investigated for various pHs and temperatures. Optimum drug release yield was found to be as 70% at pH 7.4 and 37 °C within 2 h period. Time-depended release of curcumin was also investigated and its slow release from the membrane demonstrated within 48 h.
Introduction
Curcumin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-hepta-1,6-dien-3,5-dione] is a hydrophobic polyphenolic compound derived from turmeric which is a common spice from Indian region obtained from rhizome of the Curcuma longa (Anand et al. Citation2008, Johnson and Mukhtar Citation2007). Yellowish orange color of turmeric comes from the active compounds of curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin). Curcumin plays a important role as a folk remedy in India and is still used as a paste form called Ayurveda to treat eye infections, dress wounds, treat bites, burns, acne and skin diseases (Hatcher et al. Citation2008). Numerous studies have been carried out for the health effects of curcumin, such as antioxidant activity, treatment for Alzheimer’s disease, inflammation, chemoprevention, antimicrobial activity, antidiabetic, hypoglycemic and cholesterol-lowering effects, and chemotherapy. Its anticancer potential was also demonstrated with in vitro studies (Aggarwal et al. Citation2003, Anand et al. Citation2008, Johnson and Mukhtar Citation2007, Prasad et al. Citation2014).
Drug delivery is a process which releases a specific bioactive agent to the target region with a constant rate (Bhardwaj et al. Citation2015, Langer Citation1990, Citation1994, Orive et al. Citation2003, Parveen et al. Citation2012, Sharma et al. Citation2016). New class of drugs are still discovering and testing for human use. However, most of these drugs have some limitations, such as poor solubility, high toxicity, high dosage, poor stability, non-specific targeting, short live, and so on. Drug delivery systems have been used to overcome these limitations (Bhardwaj et al. Citation2015, Orive et al. Citation2003, Sharma et al. Citation2016). By using controlled drug delivery systems, drugs can be used for defined periods of time with determined rates (Qui and Park Citation2012, Yoshida et al. Citation1993).
For controlled drug delivery purposes, the most attractive materials are hydrogels. Hydrogels are hydrophilic supports that hold large amount of water inside their polymeric network systems prepared by polymerizable cross-linkers (Kamath and Park Citation1993). The most important behavior of hydrogelic materials is protecting the loaded drugs from environments (Park and Park Citation1999).
New chromatographic support materials have been synthesized and applied for bioseparation processes, because conventional chromatographic techniques are diffusion based and have some drawbacks, such as compressibility of bead packaged column, fouling, and slow flow rates (Arıca et al. Citation1998, Bamford et al. Citation1992, Denizli et al. Citation1998, Guo et al. Citation1994, Kubato et al. Citation1996, Kubota et al. Citation1996, Langlotz and Kroner Citation1992). Recently, porous membrane-based polymeric materials have been produced as an alternative to the traditional bead-based columns and used for separation of proteins, enzymes and even whole cells, and also applied for biotechnological purposes, such as drug release, heavy metal adsorption, and immobilization (Arica et al. Citation2001, Denizli et al. Citation1997, Gebauer et al. Citation1997; Kim et al. Citation1991, Klein et al. Citation1997, Langlotz and Kroner Citation1992, Sarfert and Etzel Citation1997, Wolpert Citation1997). These membranes have high porosity and their internal surface areas are very large. They also demonstrate high chemical, biological, and mechanical stabilities (Bamford et al. Citation1992).
Herein, we describe the curcumin release from membrane-based polymeric carrier synthesized by UV polymerization of HEMA and MAPA monomers. Synthesized membranes were characterized by SEM, FTIR, and swelling studies and release conditions for curcumin were optimized and best conditions were determined.
Materials and methods
Materials
Curcumin was supplied from Sigma (Steinheim, Germany). HEMA, PVA, and EGDMA were obtained from Aldrich (Steinheim, Germany). KPS was provided from Merck (Darmstadt, Germany). All other chemicals used in this study were of analytical grade. Ultrapure water was provided by using MiliQ RioD13 (Bedford, MA) and the conductivity of water was 18 mΩ/cm.
Synthesis of MAPA
N-methacryloyl-l-phenylalanine (MAPA) was used as a functional monomer for membrane synthesis. For synthesis of MAPA, l-phenylalanine and sodium nitrite (NaNO2) were dissolved in solution of potassium carbonate (K2CO3). Reaction mixture was first cooled down to 0 °C and then mixed magnetically under nitrogen atmosphere. Methacryoyl chloride was added to this initial solution and further mixed for 2 h at room temperature. At the end of the reaction period, pH of the reaction medium was adjusted to 3.0 and product was extracted with chloroform (CHCl3). Organic phase was dried with magnesium sulfate (MgSO4) and chloroform was evaporated. Product (methacryloylamido phenylalanine) was crystallized by using ether-cyclohexane mixture. Obtained viscous liquid was diluted with ethanol (Öztürk et al. Citation2007).
Preparation of poly(HEMA-MAPA) membrane
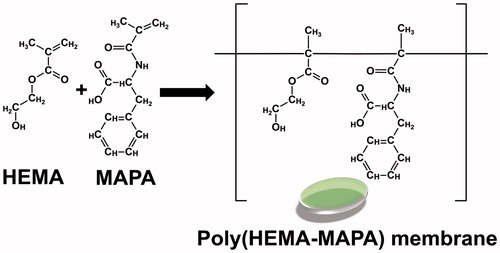
Poly(HEMA-MAPA) membrane was synthesized by UV-initiated photo polymerization technique. Briefly, 225 μL of HEMA (2-hydroxyethyl methacrylate), 25 μL of MAPA, and 10 μL of EGDMA (ethylene glycol dimethacrylate) were sonicated in a polymerization reactor. Then, 2.5 mg of DMPA (2,2-Dimethoxy-2-phenylacetophenone) was added to this initial monomer solution as a photo-initiator. Curcumin dissolved in isopropanol (0.1 mg/mL) was added to the polymerization solution and homogenized by a sonicator. After removing the dissolved oxygen by nitrogen bubbling, polymerization mixture was transferred to a glass reactor and polymerized under the UV-light for 6 min. In order to remove the unreacted monomers, membranes were rinsed with distilled water by immersing for 3 s, five times. Washed membranes were dried in an oven for 24 h at 30 °C and stored until use.
Characterization of poly(HEMA-MAPA) membrane
Fourier transform infrared spectroscopy (FTIR) spectrum pf poly(HEMA-MAPA) membrane was obtained by using a FTIR spectrophotometer (Varian FTS7000, Palo Alto, CA). For this, polymeric membranes were dried and grounded first, and then mixed with IF grade KBr. Well mixed sample was pressed into a pellet form and FTIR spectrum of the membrane was recorded.
Surface morphologies of the synthesized polymeric membranes were determined by using SEM device (Quanta 250 S FEG, The Netherlands). For this initially, surface of the polymeric membrane was covered with a thin layer of gold and then mounted in the SEM device and SEM photographs were taken.
Another characterization parameter for polymeric matrices is swelling test. For swelling studies, synthesized polymeric membranes were dried in oven and weighed. Dried membrane pieces were immersed in 50 mL of water for 48 h at room temperature. Swelled mass of polymeric membranes were determined by weighing the membranes for different time intervals removing the surface bonded water by filter paper. Swelling ratio was calculated by using the following formula (Akgöl et al. Citation2005):
where, MF is swelled mass of membrane and Mİ is mass of the dried membrane.
Optimization of curcumin release conditions
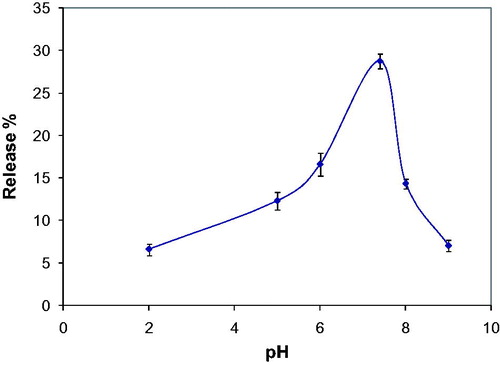
Effect of medium pH on curcumin release from poly(HEMA-MAPA) membrane was studied by using different buffer systems and the pH range of 2–8 (100 mM Hydrochloric acid/Potassium chloride buffer, for pH 2; 100 mM acetate buffer, for pH 4.0–5.0; 100 mM phosphate buffer, for pH 6.0–8.0). For this, poly(HEMA-MAPA) membrane was placed in 1.0 mL of buffer solution and curcumin release was monitored for 24 h by using a spectrophotometer at 420 nm.
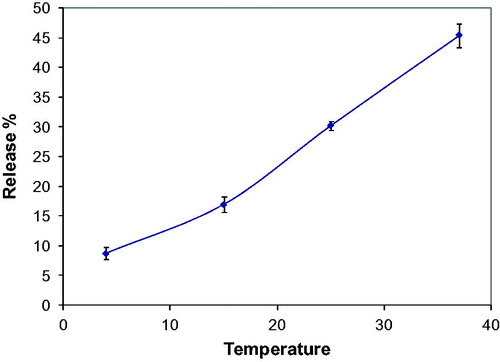
Curcumin release from poly(HEMA-MAPA) membrane was also investigated for various temperatures. For this purpose, the polymeric membrane was placed in pH 7.4 phosphate buffer solution (100 mM) and drug release profile of the membrane was followed for three different temperatures (4, 25 and 37 °C).
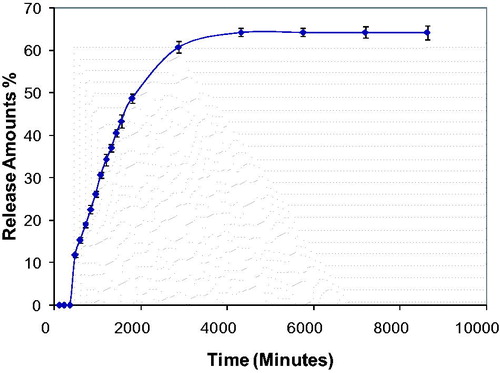
Another important parameter for drug release systems is effect of time. In order to draw the time profile for curcumin release, releasing medium was prepared by placing the membrane in pH 7.4 phosphate buffer solution at 37 °C and released amount of curcumin was determined for different time internals (0, 20, 40, 60, 90 and120 min, and 24 and 48 h).
Results and discussion
Characterization of poly(HEMA-MAPA) membranes
In the present work, poly(HEMA-MAPA) membranes were synthesized by UV polymerization technique and used as a releasing system for controlled release of curcumin. A typical preparation steps for the membrane is summarized in . Synthesized membranes were of amorphous and could hold huge amount of water inside its porous structure because of its high water bind capability.
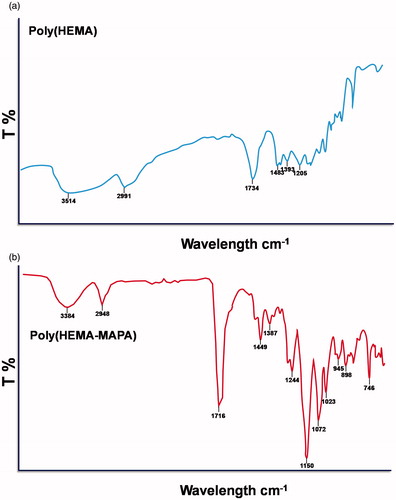
FTIR spectrum of poly(HEMA) and poly(HEMA-MAPA) membranes are demonstrated in . As clearly seen in this figure, stretching vibration band of hydrogen bonded alcohol and carbonyl groups are located at around 3400 and 1720 cm−1, respectively. Stretching vibrations of C–N is also located at around 1250 cm−1 and sharp peak at around 750 cm−1 came from bending vibrations of N–H groups. These findings contributed the incorporation of the MAPA monomer into the polymeric backbone.
Surface morphology and characteristics of poly(HEMA-MAPA) membrane is demonstrated in . As seen in this figure, synthesized membranes have quite rough surface with spherical structures and surface of the membrane was also quite non-porous.
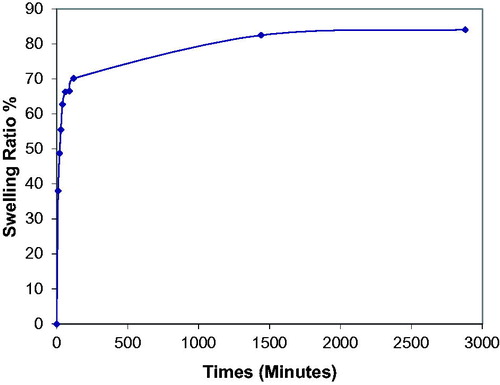
Results of swelling studies for poly(HEMA-MAPA) membrane is shown in . As demonstrated in this figure, swelling degree of the membrane was found to be as 70% at the end of 2 h. After 24 h incubation, membrane reached its equilibrium swelling degree as 85%.
Optimization of release conditions
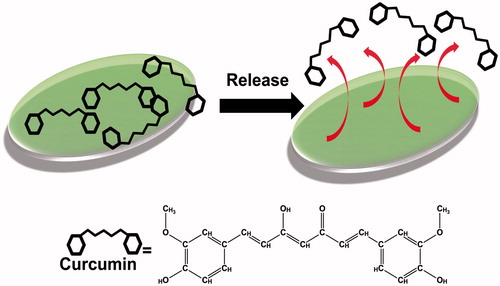
The present work demonstrates the curcumin release from the poly(HEMA-MAPA) membranes and some parameters were changed in order to reach the maximal drug release. A schematic presentation for drug release from membrane is shown in .
The effect of medium pH on the curcumin release from the poly(HEMA-MAPA) membrane is demonstrated in and maximum curcumin release was observed by using pH 7.4 phosphate buffer. After this pH, released amount of curcumin decreased highly. Curcumin is a high H atom donor within the pH values of 3–7. On the contrary, enol form of curcumin is dominant at higher pHs than 8. At high pHs, curcumin plays as an electron donor (and this behavior is also contributes to its antioxidant activity). Also at basic pHs, curcumin is considerably instable and converted to its reduced form (Sharma et al. Citation2005).
It was predicted that, the main interaction between curcumin and comonomer MAPA was a combination of ionic and hydrophobic interaction. At acidic and basic pHs, ionic interactions became dominant and curcumin bond to the membrane tighter with the contribution of hydrophobic interactions. At neutral pH, only interaction between the membrane and curcumin was hydrophobic and this weak interaction allowed the release of curcumin.
Effect of temperature for curcumin release was investigated up to the body temperature (37 °C) and it was found that, maximum curcumin release was demonstrated at body temperature (. It was concluded from this result that porosity of the membrane increased with increasing temperature and by this way curcumin release was simplified. Also, increasing temperature enhances the kinetic energy and molecular velocity of curcumin and this also contributed the accelerated release of curcumin.
Time-depended release profile of curcumin from poly(HEMA-MAPA) membrane is demonstrated in . As seen at this figure, release amount of curcumin increased with time. Also, at the end of the 24th and 48th h, curcumin released from membrane more efficiently. It can be concluded from this result that, interaction of curcumin with membrane weakened with time. Also, pore structure of membrane was affected physically with time and this matter also contributed the release characteristic of curcumin from membrane.
Conclusion
Herein, polymeric membrane-based drug release strategy was developed for curcumin. This membrane was highly biocompatible because of the monomers used for polymeric membrane synthesis and these monomers can be metabolized in body. This is a very important behavior for the usage of the designed release system for in-body applications. In this work, the membrane-based curcumin release system was designed for epidermal usage of curcumin to cure some skin diseases. By using the controlled release of curcumin, some toxic adverse effects of the treatment can be eliminated. In this study, optimal pH and temperature were found to be 7.4 and 37 °C, respectively. These optimum values were very compatible with in-body usage without any extreme release factors. This polymeric membrane and developed controlled release system for curcumin may play an important role for drug release studies and may be used as a model system for other release investigations. Also, these membrane-based release strategy can be adapted as implanted form for efficient and on-site release of drugs to treat various in-body diseases.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Aggarwal BB, Kumar A, Bharti AC. 2003. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 23:363–398.
- Akgöl S, Bereli N, Denizli A. 2005. Magnetic dye affinity beads for the adsorption of beta-casein. Macromol Biosci. 5:786–794.
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. 2008. Curcumin and cancer: disease with an “age-old solution”. Cancer Lett. 267:133–164.
- Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. 2008. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 76:1590–1611.
- Arica MY, Kaçar Y, Ergene A, Denizli A. 2001. Reversible immobilization of lipase on phenylalanine containing hydrogel membranes. Process Biochem. 36:847–854.
- Arıca MY, Testereci HN, Denizli A. 1998. Dye-ligand and metal chelate poly(2-hydroxyethylmethacrylate) membranes for affinity separation of proteins. J Chromatogr A. 799:83–91.
- Bamford CH, Al-Lamee A, Purbrick MD, Wear TJ. 1992. Studies of a novel membrane for affinity separations: I. Functionalisation and protein coupling. J Chromatogr A. 606:19–31.
- Bhardwaj A, Kumar L, Mehta S, Mehta A. 2015. Stimuli-sensitive systems-an emerging delivery system for drugs. Artif Cell Blood Sub. 43:299–310.
- Denizli A, Şenel S, Arıca MY. 1998. Cibacron Blue F3GA and Cu (II) derived poly(2-hydroxyethylmethacrylate) membranes for lysozyme adsorption. Colloid Surface B. 11:113–122.
- Denizli A, Tanyolaç D, Salih B, Aydinlar E, Özdural A, Pişkin E. 1997. Adsorption of heavy-metal ions on Cibacron Blue F3GA-immobilized microporous polyvinylbutyral-based affinity membranes. J Membrane Sci. 137:1–8.
- Gebauer KH, Thömmes J, Kula MR. 1997. Breakthrough performance of high-capacity membrane adsorbers in protein chromatography. Chem Eng Sci. 52:405–419.
- Guo W, Shang Z, Yu Y, Zhou L. 1994. Membrane affinity chromatography of alkaline phosphatase. J Chromatogr A. 685:344–348.
- Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. 2008. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 65:1631–1652.
- Johnson JJ, Mukhtar H. 2007. Curcumin for chemoprevention of colon cancer. Cancer Lett. 255:170–181.
- Kamath KR, Park K. 1993. Biodegradable hydrogels in drug delivery. Adv Drug Deliv Rev. 11:59–84.
- Kim M, Saito K, Furusaki S, Sugo T, Ishigaki I. 1991. Protein adsorption capacity of a porous phenylalanine-containing membrane based on a polyethylene matrix. J Chromatogr A. 586:27–33.
- Klein E, Yeager D, Seshadri R, Baurmeister U. 1997. Affinity adsorption devices prepared from microporous poly(amide) hollow fibers and sheet membranes. J Membrane Sci. 129:31–46.
- Kubato N, Miura S, Saito K, Sugita K, Watanabe K, Sugo T. 1996. Comparison of protein adsorption by anion-exchange interaction onto porous hollow-fiber membrane and gel bead-packed bed. J Membrane Sci. 117:135–142.
- Kubota N, Nakagawa Y, Eguchi Y. 1996. Recovery of serum proteins using cellulosic affinity membrane modified by immobilization of Cu2+ ion. J Appl Polym Sci. 62:1153–1160.
- Langer R. 1990. New methods of drug delivery. Science. 249:1527–1533.
- Langer R. 1994. Immobilization biotechnology and drug delivery. Artif Cell Blood Sub Biotechnol. 22:15–17.
- Langlotz P, Kroner KH. 1992. Surface-modified membranes as a matrix for protein purification. J Chromatogr A. 591:107–113.
- Orive G, Hernandez RM, Gascon AR, Dominguez-Gil A, Pedraz JL. 2003. Drug delivery in biotechnology: present and future. Curr Opin Biotechnol. 14:659–664.
- Öztürk N, Akgöl S, Arısoy M, Denizli A. 2007. Reversible adsorption of lipase on novel hydrophobic nanospheres. Sep Purif Technol. 58:83–90.
- Park H, Park K. 1999. Smart hydrogels. In: Salamone JC, Ed. Concise Polymeric Materials Encyclopedia. Boca Raton, FL: CRC Press, pp. 1476–1478.
- Parveen S, Misra R, Sahoo SK. 2012. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 8:147–166.
- Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. 2014. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 32:1053–1064.
- Qui Y, Park K. 2012. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliver Rev. 64:49–60.
- Sarfert FT, Etzel MR. 1997. Mass transfer limitations in protein separations using ion-exchange membranes. J Chromatogr A. 764:3–20.
- Sharma A, Garg T, Aman A, Panchal K, Sharma R, Kumar S, Markandeywar T. 2016. Nanogel – an advanced drug delivery tool: current and future. Artif Cell Blood Sub. 44:165–177.
- Sharma RA, Gescher AJ, Steward WP. 2005. Curcumin: the story so far. Eur J Cancer. 41:1955–1968.
- Wolpert S. 1997. Aldehyde activated microporous membranes. J Membrane Sci. 132:23–32.
- Yoshida R, Sakai K, Okano T, Sakurai Y. 1993. Pulsatile drug delivery systems using hydrogels. Adv Drug Deliv Rev. 11:85–108.