Abstract
Cranial bone repair and regeneration via tissue engineering principles has attracted a great deal of interest from researchers during last decade. Here, within this study, 6 mm critical-sized bone defect regeneration via genetically modified mesenchymal stem cells (MSC) were monitored up to 4 months. Cranial bone repair and new bone formations were evaluated by histological staining and real time PCR analysis in five different groups including autograft and bone morphogenetic protein-2 (BMP-2) transfected MSC groups. Results presented here indicate a proper cranial regeneration in autograft groups and a prospering regeneration for hBMP-2 encoding mesenchymal stem cells.
Introduction
Cranial bone defects can be occurred due to trauma, congenital deformations or tumor resections. Autologous bone grafts are still the gold standard for the reconstruction. However, these grafts have several limitations such as; limited source availability, donor site morbidity. In some cases, allografts can be used as an alternative to autografts but allografts are also have some drawbacks like; immunogenicity, minor risk of disease transmission, relative cost for use (Betz Citation2002). During the last decade, researches tried to find alternative methods. Tissue engineering can be a key solution for the regeneration and reconstruction of bone defects. It is a field of regenerative medicine that consist alone or the combination of three important elements: cells, a proper biomaterial or so called scaffold to support cell attachment and growth, and stimulating factors to induce cellular activity (Griffith and Naughton Citation2002, Khademhosseini et al. Citation2009, Nerem and Sambanis Citation1995). As a cell source, mesenchymal stem cells are crucial for tissue engineering applications and mostly used cell type for the regeneration of bone defects (Bajada et al. Citation2008, Bianco and Robey Citation2001, Caplan Citation2007, Jones and Yang Citation2011, Steinert et al. Citation2012). Moreover, several different types of scaffolds were used for reconstruction of not only the cancellous bones like cranium but also cortical bones like extremities (Bose et al. Citation2012, Szpalski et al. Citation2010). As mentioned before, researches also use stimulating factors to induce cellular activity. For the bone reconstruction, these factors can be calcium phosphate ceramics which have osteoconductive properties or can be growth factors or cytokines that induce cell propagation and differentiation (Boden Citation1999, Bose and Tarafder Citation2012, Khan et al. Citation2012).
Bone morphogenetic proteins (BMPs) are the member of Transforming Growth Factor (TGF) superfamily which acts critical roles in tissue development and cellular regulations for reconstruction (Chen Citation2004, Wozney Citation1989). Their activity was first discovered in mid 60s and today there are almost 15 BMPs were identified. Several BMPs have different physiological roles in tissue formation, development and healing. Therefore, there are several studies were reported the use of BMPs in tissue engineering applications (Bessa et al. Citation2008, Capra and Conti Citation2009, Inci et al. Citation2014, Odabas et al. Citation2013). Among others, BMP-2 is one the most important growth factor for the bone regeneration. It plays a key role in bone and cartilage formation via cell differentiation (Bragdon et al. Citation2011, Rozen Citation2009). During last decade, researchers use recombinant BMP-2 via several methods, such as controlled release from the scaffold, direct administration into defect area (Agrawal and Sinha Citation2016, Inci et al. Citation2014, Schliephake Citation2010). However, there were some undesirable consequences reported after using recombinant BMP-2. Recent investigations revealed that overuse of BMP-2 could lead to several complications such as osteolysis, hematoma, most generally extopic bone formation due to hyper-growth of the osteoblasts (Tannoury and An Citation2014). Moreover, cost-related concerns and dosage uncertainties are also important issues that may directly affect the clinical use of BMPs.
Using BMP-2 encoding cells may be a possible way for overcome all these drawbacks. These can be achieved by using viral and non-viral vector systems which had reported in several studies with various different vector alternatives (Balmayor and van Griensven Citation2015, Seeherman and Wozney Citation2005). In this study, we present the comparative results of using hBMP-2 encoding mesenchymal stem cells for the repair a critical size cranium defect.
Materials and methods
Reagents were purchased from Sigma–Aldrich (Münich, Germany) and used without any modifications unless otherwise stated.
Transfection studies
The transfection of hBMP-2 plasmids (kindly donated by Ludwig Boltzman Institute, Austria) was performed via a well-known in vitro transfection kit (Turbofect, Fermentas, Waltham, MA) to rat bone marrow mesenchymal stem cells (passage between 2nd and 5th). A standard condition medium consisting DMEM/F12, FBS 10%, l-glutamine 1% and 0.5% antibiotic/antimycotic solution was used for the culture of the cells.
Animal model
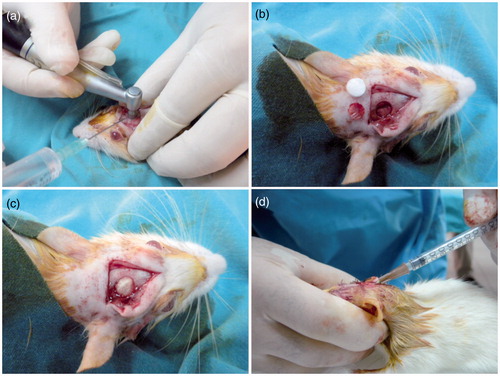
About 60 female Wistar-Albino adult rats, weighing between 250–300 g, were used for in vivo animal studies (H.Ü Animal Experimentations Local Ethics Board – 2011/52-1). All animals were caged in a controlled environment. Food and water were administered ad libitum during whole study. The animals were divided randomly into five groups. About 12 rats were used in the following groups: (i) “Group C”; named as control group or defect only group, (ii) “Group A” refer to Autograft group which, (iii) “Group S” refer to “scaffold only” group; a well-known commercially available Gel-Foam (Pfizer, NY) gelatin sponge were used as scaffold, (iv) “Group N” refer to scaffold seeded with normal cells and (v) “Group T” refer to scaffold seeded with hBMP-2 transfected cells. In a typical procedure, the animals were anesthetized intra-peritoneally by using Ketamine HCl (50 mg/ml) and Alfasime (2%). The critical size cranial defect (r = 6 mm) was created by using a rotary round-headed saw as reported elsewhere (Bölgen et al. Citation2014). During all procedures, sagittal sinus and perichondrium were kept intact. After implantations, the defect was closed with 3.0 Caprosyn sutures (Syneture, Minneapolis, MN).
Normal or transfected cells were trypsinized, were washed with buffered saline and were suspended in PBS (pH 7.4) prior to use and injected onto implantation area (onto scaffold) after defect closure ().
Histology and histomorphometry
The methodology for histologic and histomorphometric evaluations were adapted from earlier reports (Aydın et al. Citation2011, Bölgen et al. Citation2014). Bones were fixed in 10% neutral buffered formalin at room temperature and decalcified in De Castro solution (chloral hydrate, nitric acid, distilled water) and embedded in paraffin by using an automated tissue processor with vacuum. About 3- to 5-μm thick serial sections were stained with Hematoxylin and Eosin (HE), Masson’s trichrome (MT). MT produces high contrast images with red bone, green osteoid-cartilage and purple cell cytoplasm. Photomicrographs of each calvarial defect area were generated by a light microscope (Leica DMR) attached computerized digital camera (Model DFC 480, Leica Westlar, Germany). The entire defect area was visible at the lowest magnification. Bright-field images were captured and analyzed quantitatively by image processing program (LAS and Qwin Plus, Leica Inc. Westlar, Germany). Number of pixels corresponding to new trabecular bone area in each image was quantified, divided by the total number of pixels corresponding to total defect area and converted to μm2 in each specimen; the final percentage was noted.
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from cranial tissues using TriReagent (peqGOLD TriFastTM, peqlab, Erlangen, Germany). Total RNA was isolated using High Pure RNA Isolation Kit (Roche Diagnostics, Mannheim, Germany) according to manufacturer’s instructions. The RNA-containing pellets were treated with RNase-free DNase (DNaseI; Roche Diagnostics, Mannheim, Germany) to prevent genomic DNA contamination. The quantity and quality of the isolated RNA of each sample was measured spectrophotometrically at 260 and 280 nm (NanoDrop 2000, Thermo Scientific, Waltham, MA) and total RNA of 1 μg from each sample was reverse transcribed with random hexamers using Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Germany) according to the manufacturer’s protocol. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis was performed by using Light-Cycler 480 instrument (Roche Diagnostics, Mannheim, Germany) for Runx2, ALP, Col1a1 and OC mRNA levels, according to the manufacturer’s instructions. The expression levels of genes of interest were normalized to mRNA level of beta actin (ACTB) gene. Gene-specific intron spanning primers and probes for each gene assay were designed for the transcripts of Runx2, ALP, Col1a1 and OC genes with the online Universal Probe Library (UPL) Assay Design Center. The sequence of the primers and UPL numbers are described in . The qRT-PCR conditions were as follows: initial denaturation at 95 °C for 10 min followed by 50 cycles of 95 °C for 10 s and 60 °C for 20 s, and then the samples were cooled to 40 °C. Each sample was analyzed in triplicate.
Table 1. The gene-specific primer and probe sequences.
Statistical analysis
A prospective randomized-controlled double-blinded in vivo study was designed. Independent variables were groups (n = 10) and time (n = 2). For statistical analysis of histological evaluations, the independent variable was the groups and the dependent variables were histomorphometric measurements and biochemical ALP results. The normality of distribution and the homogeneity of variances of the sample were established using the Shapiro–Wilk test. All parameters were analyzed by non-parametric Kruskal–Wallis for multiple comparisons and the Dunn test for post hoc analysis. Descriptive statistics were expressed as the median, the minimum and the maximum. The differences were considered significant when P < 0.05.
For gene expression studies, the mRNA expression levels of Runx2, ALP, Col1a1 and OC were compared by relative expression software tool (REST©, 2009 v2.013, Qiagen, Hilden, Germany) using “Pair-wise Fixed Reallocation Randomization” statistical analysis test (Pfaffl et al. Citation2002). The values of P < 0.05 were considered as statistically significant.
Results and discussion
Critical-sized calvarial defects represent a non-union defect which cannot spontaneously heal during the lifetime of the organism and need an intervention for the healing. The critical size defect for an adult rat can be determined as over 5–6 mm in diameter (Bosch et al. Citation1998, Li et al. Citation2015). In this study, possible inducing effect of hBMP-2 encoding MSCs in cranial regeneration were evaluated up to 4 months.
Histomorphometric analysis
HE and MT histological staining were performed in order to evaluate bone tissue formation and tissue regeneration. All results were also analyzed quantitatively by means of new bone tissue amount and the percentage of new bone tissue in all defect area. Results were depicted in and .
Figure 2. Basic tissue structures within the cellular levels of all groups at months 1 and 4. Scaffold particles have been degraded and left empty places during the histologic process. With MT staining, compact bone stains with red, osteoids stains with green while cell cytoplasm stains with violet. CB: compact bone; TB: trabecular bone; FT: fibrous tissue; S: scaffold; O: osteoid; BR: brain; HE: hematoxylin eosin; MT: Masson’s trichrome.
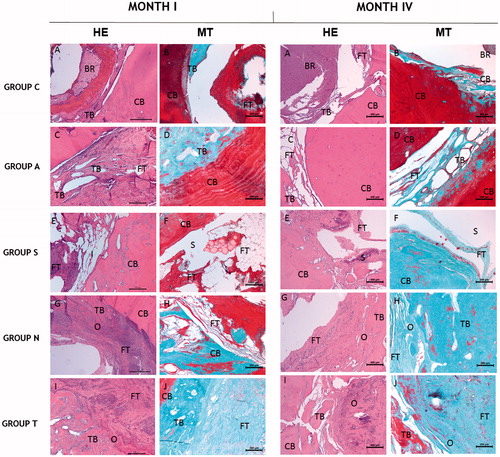
Figure 3. The overall results of histomorphometric and blood ALP analysis. (a and b) 1st and 4th month evaluations of blood ALP results in all groups. (c and d) 1st and 4th month evaluations of the percentage of new bone tissue amount in the defect. (e and f) 1st and 4th month evaluations of the newly formed and regenerated bone tissue area results throughout all groups.
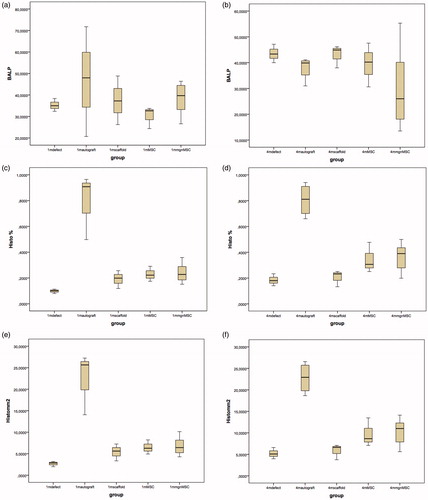
New bone per total defect area ratio (P = 0.016) and the new bone area in mm2 (P = 0.016) exhibited significant difference in control and experiment groups at 1 month. New bone area and the new bone per total defect area ratio were significantly higher in BMP-2 transfected MSC (Group T) (P = 0.034 and P = 0.034, respectively) and autograft group (Group A) (P = 0.001 and P = 0.001, respectively) when compared to that of the control, Group C (P = 0.001 and P = 0.001, respectively) at 1 month. New bone area and the new bone per total defect area ratio were significantly better in Group A comparing to Group T (P = 0.039 and P = 0.039, respectively) ( and ).
Significant differences were noted for new bone area in mm2 (P = 0.005) and the new bone per total defect area ratio (P = 0.005) between experiment and the control groups at month 4. Autograft group exhibited higher new bone area in mm2 and the new bone per total defect area ratio comparing to those of control (empty defect) group (P = 0.001 and P = 0.001), control scaffold only applied group (Group S) (P = 0.002 and P = 0.002) and Group T (P = 0.041 and P = 0.041). New bone area was higher in Group T when compared to that of control (Group C) (P = 0.045) ( and ).
In all scaffold applied groups (Group S, Group N and Groups T), a mild tissue reaction was noted without any necrosis but a few number of lymphocyte and macrophages around in early phase of regeneration. However, the scoring was not performed because the scaffold itself has already been used in clinic as a biocompatible matrix. Note that the cavities are filled with better organized connective tissue and new bone is higher in autograft in months I and IV. Histomorphometric results differed between control and experiment groups from the beginning of month I and a significant difference by means of blood ALP level in same time point. The elevation of blood ALP is expected due to early regeneration phase of the defect (Golub and Boesze-Battaglia Citation2007).
According to these evaluations, active bone reconstruction in autograft group was ahead among other groups. BMP-2 transfected MSC applied group have higher results in terms of healing and bone reconstruction. On the other hand, there was no total healing of critical-sized defects in all groups including autograft.
Quantitative real-time PCR (qRT-PCR) analysis
Early expressions of Runx2, ALP, Col1a1 and OC were investigated by qRT-PCR. Here, we omit scaffold group due to technical inconveniences which we believe do not affect overall results. As shown in , there were no significant changes for Runx2, ALP, Col1a1 and OC mRNA levels in 7th day of MSC, autograft and modified (transfected) MSC groups when compared to control group.
Figure 4. The fold differences in mRNA level of (a) Runx2, (b) ALP, (c) Col1a1 and (d) OC gene in 7th day and 14th day applied groups in comparison. Relative mRNA expression of (a) Runx2, (b) ALP, (c) Col1a1 and (d) OC genes compare between control, autograft, normal MSC and genetically modified (transfected) MSC groups. Bars represent expression levels normalized to ACTB as the housekeeping gene and relative to control group. *P < 0.05. mRNA, messenger RNA; PCR, polymerase chain reaction.
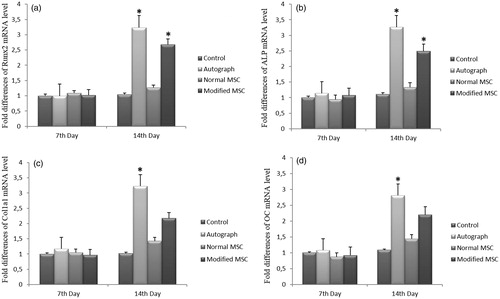
On the other hand, Runx2, ALP, Col1a1 and OC mRNA levels were increased in 14th day of autograft group when compared to control group (P < 0.05) (). However, no significant changes were found for Col1a1 and OC mRNA expressions in 14th day of autograft group when compared to control group (P > 0.05) ().
Furthermore, modified (transfected) cell treatment significantly increased the Runx2 and ALP mRNA expressions when compared to control group in 14th day (P < 0.05) (). However, normal MSC and modified MSC treatments did not significantly change the Col1a1 and OC levels in 14th day (P > 0.05) ().
In addition, there were no significant changes for Runx2, ALP, Col1a1 and OC mRNA expressions of 14th day when compared 7th day in normal MSC group (P > 0.05). However, the expression of Runx2, ALP, Col1a1 and OC mRNA levels of 14th day were significantly higher than those of the 7th day in autograft group (P < 0.05). As shown in , the relative mRNA levels of Runx2 and ALP genes of 14th day were significantly higher than those of the 7th day in modified MSC group (P < 0.05). As compared to 7th day, similar increase expression patterns for Runx2 and ALP mRNAs were obtained for 14th day in modified MSC group (P < 0.05). However, for the modified MSC group, the expression of Col1a1 and OC mRNA levels were also increased in 14th day when compared to 7th day insignificantly (P > 0.05).
Using genetically modified cells is a new approach for tissue repair and regeneration. These genetically modified cells can be primary cells which gain to synthesize exogenous growth factors or can be stem cells which also have the ability to self-renewal and differentiation to local cells besides secreting exogenous factors (Alessandri et al. Citation2004, Sheyn et al. Citation2010). Mesenchymal stem cells in particular, have several advantages as a cell sources such as high regeneration and differentiation potential, immunomodulatory effect over surrounding tissues and have tendency to damaged tissue sites. Similar to our findings, several studies reported that using mesenchymal stem cells alone or in combination with scaffold can improve the rate of healing and lead better regeneration (Aydın et al. Citation2011, Bölgen et al. Citation2014, Hodgkinson et al. Citation2010, Piskin et al. Citation2009, Wei et al. Citation2013).
Bone morphogenetic proteins play a key role in cell differentiation and self-renewal of stem cells in a mediated microenvironment and support the regeneration at defect sites. Besides, the concentration of BMP-2 defines whether the cells undergo self-renewal or differentiation (Riley et al. Citation1996). However, even with using gene therapy strategies, it is still difficult to adjust the secretion of exogenous growth factors. Therefore, although the genes related to bone regeneration is up-regulated; the inadequate secretion of exogenous growth factors could not provide a complete regeneration of the bone tissue when we compare with autograft treatment (Menendez et al. Citation2011).
Within this study, we also evaluated the new bone formation by a basic radiomorphometric analysis throughout the selected radiographs of each group. Regretfully, due to unexpected decomposition of the samples during the storage we cannot reach an applicable number of animals for reliable results. However, we can report that initial findings had a good correlation with histologic and Real Time PCR evaluations.
Conclusion
Using genetically modified cells can be promising technique to overcome significant drawbacks in tissue engineering applications. Here, we demonstrated that, genetically modified BMP-2 encoding mesenchymal stem cells can improve the healing of critical size cranial bone defect and can compete particularly with autograft treatment. We believe that with some progressive improvements, this technique can take the place of auto or allo-grafting.
Funding information
Erhan Piskin was supported by the Turkish Academy of Sciences (TUBA) as a full member. This study is partially supported by Kırıkkale University Scientific Research Projects Coordination Unit.
Acknowledgements
The authors would like to express their thanks to Dr. Georg Feichtinger and Prof Dr Heinz Redl for the kind gift of BMP-2 encoding plasmids.
Disclosure statement
The authors declare no competing interests in relation to this article. In addition, the authors are alone responsible for the content and writing of this paper.
References
- Agrawal V, Sinha M. 2016. A review on carrier systems for bone morphogenetic protein-2. J Biomed Mater Res B Appl Biomater. [Epub ahead of print]. doi: 10.1002/jbm.b.33599.
- Alessandri G, Emanueli C, Madeddu P. 2004. Genetically engineered stem cell therapy for tissue regeneration. Ann N Y Acad Sci. 1015:271–284.
- Aydın HM, Korkusuz P, Vargel I, Kılıç E, Güzel E, Çavusoğlu T, et al. 2011. A 6-month in vivo study of polymer/mesenchymal stem cell constructs for cranial defects. J Bioact Comp Pol. 26:207–221.
- Balmayor ER, van Griensven M. 2015. Gene therapy for bone engineering. Front Bioeng Biotechnol. 3:9. doi: 10.3389/fbioe.2015.00009.
- Bajada S, Mazakova I, Richardson JB, Ashammakhi N. 2008. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2:169–183.
- Bessa PC, Casal M, Reis RL. 2008. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J Tissue Eng Regen Med. 2:81–96.
- Betz RR. 2002. Limitations of autograft and allograft: new synthetic solutions. Orthopedics. 5:561–570.
- Bianco P, Robey PG. 2001. Stem cells in tissue engineering. Nature. 414:118–121.
- Boden SD. 1999. Bioactive factors for bone tissue engineering. Clin Orthop Relat Res. 367:84–94.
- Bölgen N, Korkusuz P, Vargel İ, Kılıç E, Güzel E, Çavuşoğlu T, Uçkan D, Pişkin E. 2014. Stem cell suspension injected HEMA-lactate-dextran cryogels for regeneration of critical sized bone defects. Artif Cells Nanomed Biotechnol. 42:70–77.
- Bosch C, Melsen B, Vargervik K. 1998. Importance of the critical-size bone defect in testing bone-regenerating materials. J Craniofac Surg. 9:310–316.
- Bose S, Roy M, Bandyopadhyay A. 2012. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 10:546–554.
- Bose S, Tarafder S. 2012. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 8:1401–1421.
- Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. 2011. Bone morphogenetic proteins: a critical review. Cell Signal. 4:609–620.
- Caplan AI. 2007. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 213:341–347.
- Capra P, Conti B. 2009. The role of bone morphogenetic proteins (BMPs) in bone tissue engineering: a mini review. Sci Acta. 3:25–32.
- Chen D, Zhao M, Mundy GR. 2004. Bone morphogenetic proteins. Growth Factors. 22:233–241.
- Griffith LG, Naughton G. 2002. Tissue engineering-current challenges and expanding opportunities. Science. 295:1009–1014.
- Golub EE, Boesze-Battaglia K. 2007. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 18:444–448.
- Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. 2010. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 11:1513–1526.
- Inci I, Odabas S, Vargel I, Guzel E, Korkusuz P, Cavusoglu T, et al. 2014. Gelatin-hydroxyapatite cryogels with bone morphogenetic protein-2 and transforming growth factor beta-1 for calvarial defects. J Biomater Tissue Eng. 4:624–631.
- Li Y, Chen SK, Li L, Qin L, Wang XL, Lai YX. 2015. Bone defect animal models for testing specific substitute biomaterials. J Orthop Translat. 3:94–104.
- Jones E, Yang X. 2011. Mesenchymal stem cells and bone regeneration: current status. Injury. 42:562–568.
- Khademhosseini A, Vacanti JP, Langer R. 2009. Progress in tissue engineering. Sci Am. 300:64–71.
- Khan WS, Rayan F, Dhinsa BS, Marsh D. 2012. An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopaedic surgery: how far are we? Stem Cells Int. 2012:236231.
- Menendez MI, Clark DJ, Carlton M, Flanigan DC, Jia G, Sammet S, et al. 2011. Direct delayed human adenoviral BMP-2 or BMP-6 gene therapy for bone and cartilage regeneration in a pony osteochondral model. Osteoarthritis Cartilage. 8:1066–1075.
- Nerem RM, Sambanis A. 1995. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1:3–13.
- Odabas S, Feichtinger GA, Korkusuz P, Inci I, Bilgic E, Yar AS, et al. 2013. Auricular cartilage repair using cryogel scaffolds loaded with BMP-7-expressing primary chondrocytes. J Tissue Eng Regen Med. 7:831–840.
- Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for groupwise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36.
- Piskin E, Isoglu IA, Bölgen N, Griffiths S, Vargel I, Çavusoglu T, et al. 2009. In vivo performance of simvastatin-loaded electrospun spiral-wound polycaprolactone scaffolds in reconstruction of cranial bone defects in the rat model. J Biomed Mater Res. A90:1137–1151.
- Riley EH, Lane JM, Urist MR, Lyons KM, Lieberman JR. 1996. Bone morphogenetic protein-2: biology and applications. Clin Orthop Relat Res. 324:39–46.
- Rozen V. 2009. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 20:475–480.
- Schliephake H. 2010. Application of bone growth factors–the potential of different carrier systems. Oral Maxillofac Surg. 14:17–22.
- Seeherman H, Wozney JM. 2005. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 3:329–345.
- Sheyn D, Mizrahi O, Benjamin S, Gazit Z, Pelled G, Gazit D. 2010. Genetically modified cells in regenerative medicine and tissue engineering. Adv Drug Deliv Rev. 62:683–698.
- Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. 2012. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 1:237–247.
- Szpalski C, Barr J, Wetterau M, Saadeh PB, Warren SM. 2010. Cranial bone defects: current and future strategies. Neurosurg Focus. 29:E8.
- Tannoury CA, An HS. 2014. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 14:552–559.
- Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. 2013. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 34:747–754.
- Wozney JM. 1989. Bone morphogenetic proteins. Prog Growth Factor Res. 1:267–280.