Abstract
Silver nanoparticles were prepared through green route with the aid of Aeromonas sp. THG-FG1.2 as reductant. Visual observation, ultraviolet–visible spectroscopy, transmission electron microscopy, elemental mapping, energy dispersive X-ray spectroscopy, selected area diffraction pattern (SAED), and X-ray diffraction (XRD) were used to characterize the synthesized silver nanoparticles. UV visible studies indicated the surface plasmon resonance at 400 nm which depicts the formation of silver nanoparticles. The TEM images show spherical silver nanoparticles of 8–16 nm. XRD and SAED fringes revealed the structure of silver nanoparticles as face centered cubic (fcc). These silver nanoparticles also tested for their antimicrobial potential and showed effective antimicrobial activity against tested pathogens and thus applicable as potent antimicrobial agent. Furthermore, the nanoparticles potential has been reconnoitered for their enhanced synergistic effect with antibiotics against multidrug resistant bacteria. Thus, the silver nanoparticles synthesized by Aeromonas sp. THG-FG1.2, were effective in inhibition of pathogenic microbes and also show enhanced antibacterial activity with antibiotics.
Introduction
Nanotechnology is a method for the synthesis of various kinds of nanoparticles. It is one of the important fields with many applications in the revolutionary medicine (Song and Kim Citation2009). On the other hand, nanobiotechnology deals with the use of biological system for the synthesis of nanoparticles and it is an emerging and promising area in the modern medical and agricultural science. Nanoparticles are gaining importance and are being sufficiently applied in areas such as mechanics, biomedical sciences, magnetics, catalysis, optics, and energy science. An important and exciting matter is the development of a consistent approach for the synthesis of nanoparticles over a range of shapes, sizes, and chemical compositions, and with high monodispersity (Singh et al. Citation2015a). Chemical and physical methods for the synthesis of metal nanoparticles are well known, but the methodologies are expensive and not environmental friendly, thus limiting the applications of metal nanoparticles in biological and medical platforms. To overcome the limitation of physiochemical methodologies, the simple, low-cost, and eco-friendly technologies are needed whereby these nanoparticles can be synthesized while avoiding the use of toxic and expensive chemicals and solvents (Singh et al. Citation2015b). As a result, the green synthesis has received considerable attention due to the growing need to develop eco-friendly techniques for nanoparticles synthesis (Singh et al. Citation2015a). A great deal of effort has been put into the biosynthesis of inorganic materials, especially metal nanoparticles using microorganisms, plants, yeast, fungi, etc. (Singh et al. Citation2015a). Biosynthesis of nanoparticles using microorganisms or plants is one of the eco-friendly, biocompatible, nontoxic, and clean approaches. The microbial enzymes or the plant phytochemicals with anti-oxidant or reducing properties are usually responsible for reduction of metal compounds into their respective nanoparticles (Ankamwar et al. Citation2005). Furthermore, biologically synthesized nanoparticles often exhibit water soluble and biocompatible properties, which are essential for many pharmaceutical and biomedical applications (Nithya et al. Citation2011).
Among various kinds of nanoparticles, silver nanoparticles are one of the most important commercialized nanoparticles because of its potential applications in different fields such as optics, optoelectronics, drug delivery, gene therapy, biomedicine, biosensor, and oxidative catalysis (Darroudi et al. Citation2010, Nam et al. Citation2003, Parak et al. Citation2003, Schultz Citation2003, Wang et al. Citation2015a). On the other hand, in recent years, most of these pathogenic microorganisms have acquired resistance to antimicrobial agents (Gajbhiye et al. Citation2009, Shahverdi et al. Citation2007). It is well known, that silver is an effective antimicrobial agent and possesses a strong antibacterial activity against bacteria, viruses, and fungi, although the mechanism and the manner of action are still not well known (Sharma et al. Citation2009, Singh et al. Citation2015a). Several main mechanisms underlie the biocidal properties of silver nanoparticles against microorganisms. For instance, disruption of cell membrane or cell wall (Marambio-Jones and Hoek Citation2010, Nel et al. Citation2009) or by damaging DNA, proteins, and other phosphorus- and sulfur-containing cell constituents (AshaRani et al. Citation2009, Marambio-Jones and Hoek Citation2010, Nel et al. Citation2009).
The study focused on silver nanoparticles, due to their utmost applicability. In the present study, biological route for the synthesis of silver nanoparticles was adopted since it is ecofriendly, facile, and cost effective. Furthermore, the synthesized silver nanoparticles were evaluated for their applicability in antimicrobial activities against pathogens. Recently, silver nanoparticles were demonstrated enhanced antimicrobial activity with antibiotics (Naqvi et al. Citation2013, Singh et al. Citation2015d). For this reason, we also explored the enhanced activity of silver nanoparticles in combination with antibiotics against drug resistant bacteria.
Materials and methods
Materials
All the media were purchased from Difco, MB Cell, Seoul, Korea. Analytical grade silver nitrate (AgNO3) was obtained from Sigma-Aldrich Chemicals, St. Louis, MO. The standard antibiotics discs: erythromycin (E15) 15 μg/disc, novobiocin (NV30) 30 μg/disc, lincomycin (MY15) 15 μg/disc, penicillin G (P10) 10 μg/disc, vancomycin (VA30) 30 μg/disc, and oleandomycin (OL15) 15 μg/disc were purchased from Oxoid Ltd., Basingstoke, England. The pathogenic microorganisms Salmonella enterica [ATCC 13076], Pseudomonas aeruginosa [ATCC 6538], Escherichia coli [ATCC 10798], Vibrio parahaemolyticus [ATCC 33844], Bacillus cereus [ATCC 14579], Bacillus subtilis [KACC 14741], Staphylococcus aureus [ATCC 6538], Candida albicans [KACC 30062], and Candida tropicalis [KCTC 7909] were obtained from Korean Agricultural Culture Collection (KACC) and Korean Collection for Type Cultures (KCTC). The bacterial strains were cultured on nutrient agar (NA) media at 28 °C and preserved as a suspension in nutrient broth (NB) with glycerol (25%, w/v) and stored at −80 °C for further study. C. albicans and C. tropicalis were cultured on Sabouraud dextrose agar (SDA) at 28 °C and preserved at −80 °C in glucose yeast peptone broth glycerol stock vials.
Isolation and identification of bacterial strain
Soil sample was obtained in sterile bag from Suwon Fortress, South Korea. To obtain isolated strain, 1 g of soil sample was suspended in 10 mL of 0.85% (w/v) saline solution, serially diluted, and spread on NA plates. For further screening, the individual colonies were streaked on NA plate supplemented with 1 mM filter-sterilized silver nitrate (AgNO3) solution. The plates were incubated at 28 °C for 48 h and observed for bacterial growth. The colonies resistant to silver nitrate were subculture and obtained in the pure form. Molecular identification of the isolated strain was carried out using 16S rRNA sequencing based method. Genomic DNA was extracted and purified using a commercial Genomic DNA extraction kit (Solgent, Daejeon, Korea). The 16S rRNA gene was amplified using the universal bacterial primer sets including 27F/1492R (Lane Citation1991) and 518F/800R (Weisburg et al. Citation1991). The purified PCR products were sequenced by Solgent Co. Ltd. (Daejeon, Korea). The 16S rRNA gene sequences of related taxa were obtained from the GenBank database and EzTaxon-e server (Kim et al. Citation2012).
Biosynthesis of silver nanoparticles
Silver nanoparticles was synthesized following the method already described by Jo et al. (Citation2015) and Singh et al. (2015b). Briefly, NB medium was prepared, sterilized, and inoculated with a fresh growth of bacterial isolate. The cultured flasks were incubated in a rotating shaker set at 120 rpm for 24 h and 28 °C. After the incubation time, the culture was centrifuged at 10,000 rpm for 10 min to remove the bacterial pellet. The supernatant was used for the synthesis of silver nanoparticles. The culture supernatant was separately added with 1 mM filter-sterilized AgNO3 solution. Further, the culture supernatant with 1 mM AgNO3 was incubated in an orbital shaker for 48 h, at 120 rpm and 28 °C. The bioreduction of the Ag+ ions in the solution was monitored by color change. After completion of the incubation period, the mixture was first centrifuged at 2000 rpm for 5 min to remove any components of the medium and then the silver nanoparticles were collected by high speed centrifugation at 14,000 rpm for 20 min. The resulting silver precipitate was washed four to five times with deionized water. Finally, the silver nanoparticles were collected in the form of a pellet and were air dried and kept for future experiments.
Characterization
Absorption spectra was recorded on an ultraviolet–visible spectrophotometer (UV–Vis) (Ultrospec 2100 Pro, Amersham, Biosciences, UK). Transmission electron microscopy (TEM), elemental mapping, energy dispersive X-ray spectroscopy (EDX), and selected area diffraction pattern (SAED) measurements were made on a high resolution TEM JEM-2100F (JEOL) operated at an accelerating voltage of 200 kV. The samples for FE-TEM characterization were prepared by placing a drop of silver nanoparticles on carbon coated copper grid and dried at room temperature. The X-ray diffraction (XRD) analyses were performed on X-ray diffractometer, D8 Advance, Bruker, Germany, operated at 40 kV, 40 mA, with CuKα radiation, at a scanning rate of 6°/min, step size 0.02, over the 2θ range of 20–80°. The XRD sample was prepared by drying the silver nanoparticles collected after frequent washing with water.
Analysis of antimicrobial activity
The antimicrobial activity of the silver nanoparticles was measured against pathogenic microorganisms such as B. cereus, B. subtulis, S. aureus, S. enterica, E. coli, P. aeruginosa, V. parahaemolyticus on Mueller-Hinton agar (MHA) plates using the disc diffusion method. SDA plates were used for C. albicans and C. tropicalis. Overnight log culture of each pathogenic strain (100 μL, optical density of 0.5 at 620 nm) was spread evenly on MHA and SDA plates and dried properly. Then, 50 μL (500 ppm) of the purified silver nanoparticle solution in water was added over each disc and kept for incubation at 28 °C for 24 h. After incubation, the zones of inhibition were measured around each disc. The study was done in duplicates to check the reproducibility.
Disc diffusion assay to evaluate synergistic effects
A disc diffusion method was used to assay the various antibiotics for bactericidal activity against test strains on MHA plates. The standard antibiotics discs of erythromycin, novobiocin, lincomycin, penicillin G, vancomycin, and oleandomycin were used. To determine the combined effects, each standard paper disk was further impregnated with 30 μL of the freshly prepared silver nanoparticles solution in water at a final content of 15 μg/disc. A single colony of strains P. aeruginosa, E. coli, S. enterica, and V. parahaemolyticus were grown overnight in Muller-Hinton liquid medium on a rotary shaker (120 rpm) at 28 °C. The inocula were prepared by diluting the overnight cultures so that the final optical density is 0.5 at 620 nm. Hundred microliters of overnight log culture were applied to the MHA plates along with the standard (only antibiotics, control) and prepared disks containing silver nanoparticles. After incubation at 28 °C for 24 h, the zones of inhibition were measured. The assays were performed in triplicate.
Results and discussion
Screening and identification of strain
The screening results after incubation period showed the growth of bacterial strain THG-FG1.2 on NA plate supplemented with 1 mM of AgNO3, which suggest that the strain THG-FG1.2 was capable of tolerating silver salt. The 16S rRNA gene sequence of the strain determined in this study was a continuous stretch of 1425 bp. According to the EzTaxon-e server, strain THG-FG1.2 shared 97.5% similarity with Aeromonas schubertii. The 16S rRNA sequence of THG-FG1.2 has been submitted to NCBI with accession number KU523692.
Synthesis and characterization of nanoparticles
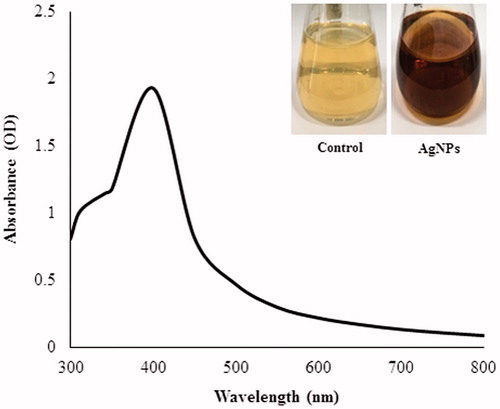
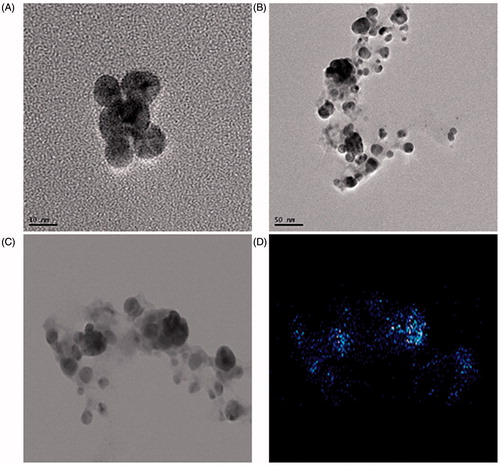
Nanoparticles synthesized by Aeromonas THG-FG1.2 were confirmed by visual observation with the appearance of color change in the reaction mixture. The color reaction was observed in which pale yellow supernatant and AgNO3 solution changed into dark brown color which indicates the synthesis of silver nanoparticles, as the silver nanoparticles cause surface plasmon resonance due to which brown color appears (Naqvi et al. Citation2013, Singh et al. Citation2015c). The absorption spectrum of this sample displayed in shows a well-defined plasmon band at 400 nm, characteristic of nanosized silver (Martınez-Castanon et al. Citation2008, Singh et al. Citation2015d, Wang et al. Citation2015a, Citation2015b). shows a representative TEM image of the silver nanoparticles that were synthesized by treating the AgNO3 solution with bacterial supernatant. The silver nanoparticles were spherical in shape with the size range of 8–16 nm.
Figure 2. TEM image of spherical shaped silver nanoparticles at 10 nm (A) and 20 nm (B). Elemental mapping results indicate distribution of silver elements, TEM micrograph of silver nanoparticles solution (C), and silver nanoparticles (D).
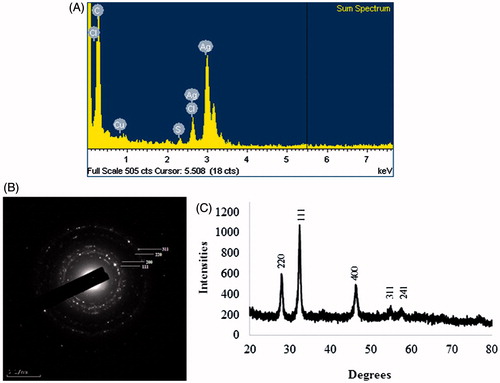
The results of elemental mapping of the biosynthesized silver nanoparticles showed the distribution of elements in the electron micrograph of purified silver nanoparticles. corresponds to the electron micrograph of silver nanoparticles and shows the distribution of elemental silver in the electron micrograph. The distribution of elemental silver was clearly visible in the elemental maps and was found to be the predominant element in the respective nanoproduct (Singh et al. Citation2015b, Wang et al. Citation2015a, Citation2015b). The silver nanoparticles display an optical absorption band peak at approximately 3 keV (), which is typical of the absorption of silver nanoparticles due to surface plasmon resonance (Singh et al. Citation2015e). The other metal ion groups also appeared in the EDX spectrum which correspond to the TEM grid utilized for study. The silver nanoparticles are crystalline, as can be seen from the SAED recorded from one of the nanoparticles in the aggregates (). shows the XRD pattern of nanoparticles, exhibited extreme peaks in the whole spectrum of 2θ value ranging from 20 to 80 and this pattern was analogous to the Braggs's reflection of silver nanocrystals (Gurunathan et al. Citation2014, Singh et al. Citation2015d). The obtained XRD results were similar with previous study which showed synthesis of silver nanoparticles by bacterial strains (Singh et al. Citation2015e, Vivek et al. Citation2011).
Antimicrobial and enhanced synergistic activity
To analyze the results, the zone of inhibition was measured around each disc, after the incubation period (Singh et al. Citation2015b). The results indicated that the extracellularly synthesized silver nanoparticles have antimicrobial activity. As shown in ; clear inhibition zones surrounded the discs impregnated with silver nanoparticles (50 μL, 500 ppm). The silver nanoparticles showed highest antimicrobial activity against C. albicans followed by P. aeruginosa, V. parahaemolyticus, S. aureus, C. tropicalis, B. cereus, B. subtulis, E. coli., and S. enterica. The results of zone of inhibition are interpreted in . The results were comparable and followed the previous studies based on antimicrobial activity of silver nanoparticles (Naqvi et al. Citation2013, Singh et al. Citation2015b,Citation2015d).
Figure 4. Zones of inhibition of 50 μL of silver nanoparticles against Candida albicans [KACC 30062] (A1), Candida tropicalis [KCTC 7909] (A2), Bacillus cereus [ATCC 14579] (B1), Bacillus subtulis [KACC 14741] (B2), Staphylococcus aureus [ATCC 6538] (B3), Salmonella enterica [ATCC 13076] (C1), Escherichia coli [ATCC 10798] (C2), Pseudomonas aeruginosa [ATCC 6538] (C3), and Vibrio parahaemolyticus [ATCC 33844] (C4). Note: AgNPs are silver nanoparticles solution (in water).
![Figure 4. Zones of inhibition of 50 μL of silver nanoparticles against Candida albicans [KACC 30062] (A1), Candida tropicalis [KCTC 7909] (A2), Bacillus cereus [ATCC 14579] (B1), Bacillus subtulis [KACC 14741] (B2), Staphylococcus aureus [ATCC 6538] (B3), Salmonella enterica [ATCC 13076] (C1), Escherichia coli [ATCC 10798] (C2), Pseudomonas aeruginosa [ATCC 6538] (C3), and Vibrio parahaemolyticus [ATCC 33844] (C4). Note: AgNPs are silver nanoparticles solution (in water).](/cms/asset/dbb46652-aa20-48b4-b1fd-422a033f423e/ianb_a_1163715_f0004_c.jpg)
Table 1. Antimicrobial activity of silver nanoparticles against pathogenic microorganism.
Additionally, the combination effect of silver nanoparticles with different antibiotics was also investigated against P. aeruginosa, E. coli, S. enterica, and V. parahaemolyticus using the disc diffusion method. The diameter of inhibition zones (in millimeters) around the different antibiotic discs with or without silver nanoparticles against test strains are shown in . The strains P. aeruginosa, E. coli, and S. enterica are completely resistant to the antibiotics, but showed a zone of inhibition when treated with silver nanoparticles. The other pathogenic strain, V. parahaemolyticus, showed sensitivity to standard antibiotics and enhanced zone of inhibition when the antibiotics discs were impregnated with silver nanoparticles. Silver nanoparticles displayed enhanced antibacterial activity with antibiotics (). The highest fold increases in area were observed for E15 and NV30 against S. enterica, for P 10 against P. aeruginosa and for MY15 against V. parahaemolyticus. Thus the biologically synthesized silver nanoparticles also showed a similar potent bactericidal activity against pathogens as reported previously (Chen et al. Citation2011, Elbeshehy et al. Citation2015, Saravanan et al. Citation2014, Singh et al. Citation2015b,Citation2015d, Wang et al. Citation2015b).
Figure 5. Zones of inhibition against Salmonella enterica; standard antibiotics; (A1), standard antibiotics + AgNPs 30 μL (500 ppm) (A2). Similarly, against Escherichia coli [ATCC 10798]; (B1–B2), against Psuedomonas aeruginosa [ATCC 6538]; (C1–C2), against Vibrio parahaemolyticus [ATCC 33844]; (D1–D2). Note: Erythromycin (E15) 15 μg/disc, Lincomycin (MY15) 15 μg/disc, Novobiocin (NB30) 30 μg/disc, Penicillin G (P10) 10 μg/disk, Vancomycin (VA30) 30 μg/disc, and Oleandomycin (OL15) 15 μg/disc.
![Figure 5. Zones of inhibition against Salmonella enterica; standard antibiotics; (A1), standard antibiotics + AgNPs 30 μL (500 ppm) (A2). Similarly, against Escherichia coli [ATCC 10798]; (B1–B2), against Psuedomonas aeruginosa [ATCC 6538]; (C1–C2), against Vibrio parahaemolyticus [ATCC 33844]; (D1–D2). Note: Erythromycin (E15) 15 μg/disc, Lincomycin (MY15) 15 μg/disc, Novobiocin (NB30) 30 μg/disc, Penicillin G (P10) 10 μg/disk, Vancomycin (VA30) 30 μg/disc, and Oleandomycin (OL15) 15 μg/disc.](/cms/asset/f8656349-12ef-4d5d-8775-c95c9cc36ac1/ianb_a_1163715_f0005_c.jpg)
Table 2. Zone of inhibition (mm) of different antibiotics against test strains (in absence and in presence of AgNPs at concentration of 15 μg/disc).
Conclusion
The study highlights the cost effective and environmental friendly biological synthesis of silver nanoparticles by the strain Aeromonas THG-FG1.2, isolated from soil. The formed silver nanoparticles were well characterized by UV–Vis spectra, transmission electron micrographs, elemental mapping, EDX, and XRD. The biosynthesized silver nanoparticles were crystalline, spherical, and stable with particle size of 8–15 nm. The silver nanoparticles revealed good antimicrobial activity against the selected pathogenic microorganisms. Additionally, they also displayed the enhanced bactericidal activity with different antibiotics. This biosynthesis approach appears to be a facile, cost-effective, non-toxic, ecofriendly alternative to the conventional physical and chemical methods. These silver nanoparticles may also be used in effluent treatment process for reducing the microbial load, wound healing, and other medical applications.
Funding information
This work was conducted under the industrial infrastructure program (No. N0000888) for fundamental technologies which is funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ankamwar B, Damle C, Ahmad A, Sastry M. 2005. Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol. 5:1665–1671.
- AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. 2009. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 3:279–290.
- Chen M, Yang Z, Wu H, Pan X, Xie X, Wu C. 2011. Antimicrobial activity and the mechanism of silver nanoparticle thermosensitive gel. Int J Nanomed. 6:2873–2877.
- Darroudi M, Ahmad MB, Abdullah AH, Ibrahim NA, Shameli K. 2010. Effect of accelerator in green synthesis of silver nanoparticles. Int J Mol Sci. 11:3898–3905.
- Elbeshehy EK, Elazzazy AM, Aggelis G. 2015. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against bean yellow mosaic virus and human pathogens. Front Microbiol. 6:453.
- Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M. 2009. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine. 5:382–386.
- Gurunathan S, Han JW, Kwon DN, Kim JH. 2014. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 9:373.
- Jo JH, Singh P, Kim YJ, Wang C, Mathiyalagan R, Jin CG, Yang DC. 2015. Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2015.1068792.
- Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, et al. 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 62:716–721.
- Lane DJ. 1991. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, Eds. Nucleic Acid Techniques in Bacterial Systematics. Chichester: Wiley, pp. 115–176.
- Marambio-Jones C, Hoek EM. 2010. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res. 12:1531–1551.
- Martinez-Castanon GA, Nino-Martinez N, Martinez-Gutierrez JR, Ruiz F. 2008. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res. 10:1343–1348.
- Nam JM, Thaxton CS, Mirkin CA. 2003. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 301:1884–1886.
- Naqvi SZ, Kiran U, Ali MI, Jamal A, Hameed A, Ahmed S, Ali N. 2013. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanomed. 8:3187–3195.
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. 2009. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 8:543–557.
- Nithya G, Shepangam NH, Balaji S. 2011. Biosynthesis of silver nanoparticle and its antibacterial activity. Arch Appl Sci Res. 3:377–380.
- Parak WJ, Gerion D, Pellegrino T, Zanchet D, Micheel C, Williams SC, et al. 2003. Biological applications of colloidal nanocrystals. Nanotechnology. 14:R15.
- Sadeghi B, Gholamhoseinpoor F. 2015. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc. 134:310–315.
- Sahoo PK, Kamal SK, Kumar TJ, Sreedhar B, Singh AK, Srivastava SK. 2009. Synthesis of silver nanoparticles using facile wet chemical route. Defence Sci J. 59:447–455.
- Saravanan M, Jacob V, Arockiaraj J, Prakash P. 2014. Extracellular biosynthesis, characterization and antibacterial activity of silver nanoparticles synthesized by Bacillus subtilis (NCIM-2266). J Bionanosci. 8:21–27.
- Schultz DA. 2003. Plasmon resonant particles for biological detection. Curr Opin Biotechnol. 14:13–22.
- Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. 2007. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 3:168–171.
- Sharma VK, Yngard RA, Lin Y. 2009. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 145:83–96.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2015a. Weissella oryzae DC6-facilitated green synthesis of silver nanoparticles and their antimicrobial potential. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2015.1064937.
- Singh P, Kim YJ, Singh H, Wang C, Mathiyalagan R, Yang DC. 2015b. Biosynthesis of anisotropic silver nanoparticles by Bhargavaea indica and their synergistic effect with antibiotics against pathogenic microorganisms. J Nanomater. 2015:234741.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, EL-Agamy Farh M, Yang DC. 2015c. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2015.1008514.
- Singh P, Kim YJ, Singh H, Wang C, Hwang KH, Farh Mel A, Yang DC. 2015d. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int J Nanomed. 10:2567–2577.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2015e. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2015.1011809.
- Song JY, Kim BS. 2009. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 32:79–84.
- Vivek M, Kumar PS, Steffi S, Sudha S. 2011. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J Med Biotechnol. 3:143–148.
- Wang C, Singh P, Kim YJ, Mathiyalagan R, Myagmarjav D, Wang D, Jin CG, Yang DC. 2015a. Characterization and antimicrobial application of biosynthesized gold and silver nanoparticles by using Microbacterium resistens. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2015.1089253.
- Wang C, Kim YJ, Singh P, Mathiyalagan R, Jin Y, Yang DC. 2015b. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2015.1011805.
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173:697–703.