Abstract
Objective: The aim of the present study was to develop and optimize topically applied nimesulide-loaded nanostructured lipid carriers. Materials and methods: Box–Behnken experimental design was applied for optimization of nanostructured lipid carriers. The independent variables were ratio of stearic acid: oleic acid (X1), poloxamer 188 concentration (X2) and lecithin concentration (X3) while particle size (Y1) and entrapment efficiency (Y2) were the chosen responses. Further, skin penetration study, in vitro release, confocal laser scanning microscopy and stability study were also performed. Results and discussion: The optimized nanostructured lipid carriers of nimesulide provide reasonable particle size, flux, and entrapment efficiency. Optimized formulation (F9) with mean particle size of 214.4 ± 11 nm showed 89.4 ± 3.40% entrapment efficiency and achieved mean flux 2.66 ± 0.09 μg/cm2/h. In vitro release study showed prolonged drug release from the optimized formulation following Higuchi release kinetics with R2 value of 0.984. Confocal laser scanning microscopy revealed an enhanced penetration of Rhodamine B-loaded nanostructured lipid carriers to the deeper layers of the skin. The stability study confirmed that the optimized formulation was considerably stable at refrigerator temperature as compared to room temperature. Conclusion: Our results concluded that nanostructured lipid carriers are an efficient carrier for topical delivery of nimesulide.
Introduction
In the beginning of the 1990s, solid lipid nanoparticles were developed as a drug delivery system. However, the application is limited owing to the restricted drug loading capacity and the expulsion of drug during storage caused by crystallization of lipid matrix (Luan et al. Citation2015). In order to overcome this shortage, a new colloidal delivery system named nanostructured lipid carriers (NLC) were developed on the basic of solid lipid nanoparticles.
NLC, as the second generation of lipid nanoparticles, consist of the mixture of solid lipid and liquid lipid. They not only possess the outstanding advantages of solid lipid nanoparticles, but also avoid the disadvantages such as drug leakage and low entrapment efficacy, which can be attributed to the incorporation of liquid lipid that breaks the ordered crystalline state of solid lipid and enlarges drug storage space (Iqbal et al. Citation2012, Pradhan et al. Citation2016). In addition, NLC possess a number of features advantageous for the topical route of application. They are well suited for use on damaged or inflamed skin because they are composed of physiological and biodegradable lipids of low systemic toxicity and also low cytotoxicity (Gonzalez-Mira et al. Citation2011, Jaiswal et al. Citation2016, Raj et al. Citation2015). Most of the used lipids have an approved status or are excipients used in commercially available topical cosmetic or pharmaceutical preparations. The small size of the lipid particles ensures close contact to the stratum corneum and can increase the amount of the drug penetrating into the skin. Because of their solid lipid matrix, controlled release from these carriers is possible. In recent years, NLC, as a mature novel pharmaceutical form have been widely applied into dermal drug delivery system (Muller et al. Citation2011, Patil et al. Citation2016, Tichota et al. Citation2014).
The present research explored the formulation and characterization of NLC system for the topical application of nimesulide.Nimesulide is a relatively cyclooxygenase-2 selective, non-steroidal anti-inflammatory drug. Its approved indications are the treatment of acute pain, the symptomatic treatment of osteoarthritis and primary dysmenorrhoea in adolescents and adults above 12 years age. Due to concerns about the risk of hepatotoxicity, nimesulide has been withdrawn from market in many countries (Bessone Citation2010, Ramjith and Mathew Citation2015). Although the use of nimesulide is banned for oral administration, due to its potential for inducing hepatotoxicity and thrombocytopenia, the use of nimesulide for topical delivery is prominent in the treatment of many inflammatory conditions including rheumatoid arthritis. Many studies have been done in recent years using nimesulide as model drug for topical delivery (Alves et al. Citation2007, Lenz et al. Citation2012, Shahzad et al. Citation2013, Vandana et al. Citation2014).
In view of characteristics of nimesulide including low molecular weight (308.31 Daltons), melting point (143–144.5 °C), and good solubility in lipophilic solvents (log P = 2.60, in octanol–water) (Khan et al. Citation2011). It seems that there is potential for investigating the nimesulide-loaded NLC system. In this study, NLC were used as a nimesulide topical therapeutic system. The system was developed, optimized and evaluated for the analysis of particle size, shape, entrapment efficiency, in vitro skin penetration, confocal laser microscopy, in vitro release, and stability study.
Materials and methods
Materials
Nimesulide was purchased from Denizen Pharmaceuticals Pvt. Ltd., Mumbai, India. Poloxamer 188 was purchased from Sigma Aldrich Chemicals Pvt. Limited, Mumbai, India. Isopropyl alcohol, methanol, and ethanol, were purchased from S D Fine Chemical Ltd, Mumbai, India. Lecithin soya, 30% was purchased from Himedia Laboratories, Pvt. Ltd., Mumbai, India. Oleic acid and stearic acid were supplied by Thomas Baker, Mumbai, India. Double-distilled water was used for all experiments.
Screening of lipids
On the basis of solubility studies of nimesulide in various solid lipids and oils, lipid phase was selected for its NLC formulation development.
Selection of liquid lipids
The saturation solubility of nimesulide in different liquid lipids was determined by adding excess amount of drug in 3 ml of oils in small vials. The vials were tightly closed and continuously stirred to reach equilibrium for 72 h at 25 °C in mechanical shaker (Metrex Scientific Instrument Ltd, New Delhi, India). Afterwards, oil and drug mixtures were centrifuged at 5000 rpm for 30 min (Beg et al. Citation2012, Dash et al. Citation2015). The supernatant was separated, dissolved in 10% methanol-phosphate buffer saline, pH 7.4 (PBS) and solubility was quantified by UV spectrophotometer at 396 nm (Singh et al. Citation2005).
Selection of solid lipids
The selection of solid lipid was based on the solubility of nimesulide to give a visibility clear solution in lipid when seen with the naked eye. Nimesulide (10 mg) and varying quantities of selected lipids were heated above the melting point of the lipid in a temperature controlled water bath in 15 ml glass vials. After melting the lipid in vials, the solubility of nimesulide was observed under normal light.
Partitioning behavior of nimesulide in various lipids
Nimesulide (10 mg) was dispersed in a mixture of melted lipid (1 g) and hot PBS (1 ml). The mixture was shaken for 24 h in a hot water bath at 65 ± 5 °C. After cooling, the aqueous phase was separated by centrifugation at 5000 rpm and the drug content was analyzed spectrophotometrically.
Miscibility of solid and liquid lipid
The solid and liquid lipid in different ratios (3:2, 7:3, 8:2, 9:1) with best solubilizing potential for nimesulide were subjected to miscibility studies. The lipid mixtures were agitated at 200 rpm for 1 h at 70 °C using magnetic stirrer, then samples were kept at room temperature for 24 h. The miscibility between two lipids was determined by visual observation. The samples which did not show phase separation were selected for further studies.
Formulation and optimization of nimesulide-loaded NLC
After screening and selection of constituents, nimesulide-loaded NLC were prepared by using melt emulsification ultrasound dispersion method (Patel et al. Citation2012, Thakkar et al. Citation2014) and optimized using 3-factors, 3-levels Box–Behnken design using Design Expert® (Version 7.1.6, Stat-Ease Inc., Minneapolis, MN). The independent variables selected were the ratio of stearic acid: oleic acid (X1), poloxamer 188 concentration (X2) and lecithin concentration (X3) with their low, medium and high levels used to prepare the 15 formulations. The dependent variables were particle size (Y1) and entrapment efficiency (Y2) with constraints applied to the formulation of NLC (). Precisely, oily phase was prepared by dissolving drug and lecithin in mixture stearic acid as solid lipid and oleic acid as liquid lipid heated at 75 °C. Meanwhile 10 ml of aqueous phase was prepared by dispersing surfactant (poloxamer 188) in distilled water and heated to same temperature. The hot aqueous phase was then added to the lipid phase at 75 °C under magnetic stirring at 600 rpm to form coarse emulsion. The coarse emulsion was sonicated using probe sonicator (Titanium probe, Ultrasonicator, Model-UP100H, Hielscher Ultrasonics GmbH, Berlin) at 37 °C for 4 min by maintaining output amplitude at 50%. The resultant nanoemulsion was cooled at room temperature to form nimesulide-NLC dispersion.
Table 1. Variables in Box–Behnken design to form nimesulide-loaded NLC formulations.
Particle size, polydispersity index and surface morphology of nimesulide-loaded NLC
Particle size and polydispersity index of developed formulations were determined by the dynamic light scattering method, using Zetasizer (HAS 3000; Malvern Instruments, Malvern, UK) at 25 ± 1 °C and at a scattering angle of 90°. Transmission electron microscopy (TEM) was conducted to characterize the surface morphology of nanoparticles. NLC particles were visualized by using a Philips, CM10 (Philips Research, Hamburg, Germany) electron microscope. Samples were dried on carbon-coated grid and negatively stained with aqueous solution of phosphotungstic acid. After drying the specimen was viewed under the microscope at 10–100 k-fold enlargements at an accelerating voltage of 100 kV (Madheswaran et al. Citation2014).
Determination of nimesulide entrapment efficiency
An aliquot of 2.0 ml of each drug-loaded sample was centrifuged at 12,500 rpm for 45 min to separate the lipid and aqueous phase. The supernatant was then diluted with methanol, filtered through 40 μm filter paper and the drug content was determined by the double beam UV-Visible spectrophotometer (Shimadzu, Japan) at 396 nm (Singh et al. Citation2005). The entrapment efficacy of NLC was calculated using the following equation:
where Wa stands for the mass of drug added to the formulation and Ws is the analyzed weight of the drug in supernatant (Doktorovova et al. Citation2010).
In vitro skin penetration study
In vitro skin penetration study was carried out to ascertain the extent of penetration of nimesulide across the rat skin using vertical Franz-type diffusion cell having area of diffusion 1 cm2 and 10 ml of receptor cell volume. The rat skin samples were mounted over the diffusion cells in such a way that stratum corneum side faced the donor compartment whereas the dermis faced the receiver compartment (Ahad et al. Citation2011a, Citation2011b, Citation2014). The PBS containing 10% polyethylene glycol 400 was used as the receptor phase (Babu et al. Citation2003). The receptor phase was maintained at 32 ± 1 °C by circulating jacket and was stirred at 500 rpm using a magnetic stirrer. 1 ml of nimesulide-loaded NLC formulation was placed in the donor compartment. Samples of 1 ml were withdrawn from the receptor compartment via the sampling port at different time intervals, i.e. 0, 1, 2, 3, 4, 5, 6, 7, and 24 h and analyzed for drug content by UV-Visible spectrophotometer at 396 nm (Singh et al. Citation2005). The receptor phase was immediately replenished with equal volume of fresh buffer.
Release kinetics
To study the release kinetics from optimized nimesulide-NLC dispersion formulation, data obtained from in vitro drug release studies were fitted in various kinetic models: zero-order, first-order, Higuchi's matrix (Higuchi Citation1963) and the Korsmeyer–Peppas model (Korsmeyer et al. Citation1983, Peppas Citation1985). To determine the mechanism of drug release, the data were fitted into the Korsmeyer–Peppas model and the release exponent (n) was calculated from the slope of the straight line. For the slab matrix, if the exponent is 0.5, then the diffusion mechanism is fickian; if 0.5 < n < 1.0, the mechanism is non-fickian, n = 1 to Case II (relaxational) transport, and n > 1 to super case II transport (Uprit et al. Citation2013).
Confocal laser scanning microscopy
Nimesulide-NLC optimized formulation loaded with Rhodamine B dye was applied homogeneously and non-occusively to the excised rat abdominal skin mounted on Franz diffusion cell for 18 h. The skin was washed with distilled water and placed on the slide with stratum corneum facing upward and observed under confocal microscope (Leica SP5C Spectral Confocal Laser Scanning Microscope 710, UK) with an argon laser beam with excitation at 488 nm and emission at 590 nm. The skin sample was sliced in section of 5–10 μm thickness through z-axis by laser confocal microscope.
Stability study
The optimized nimesulide NLC dispersion was subjected to stability study to evaluate any physical or chemical changes on storage. The formulation was kept at refrigerated condition (4 ± 2 °C) and at 25 ± 2 °C/60 ± 5% RH for 60 days in Borosil amber-colored glass container for stability study (Narala and Veerabrahma Citation2013). The sampling was done at intervals of 15, 30, 45 and 60 days after storage. Samples from each formula at each temperature were analyzed for drug retention efficiency. In addition, NLC dispersions were visually inspected for any physical instability (separation, aggregations, and so forth).
Result and discussion
Screening of lipids
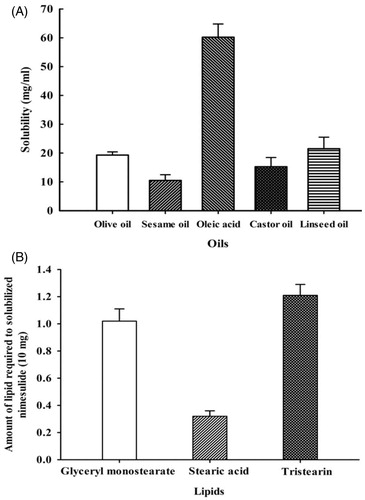
For the selection of oils and lipid, solubility of nimesulide in different oils and lipids were determined. As per obtained results (), nimesulide exhibits maximum solubility in oleic acid (60.2 ± 4.6 mg/ml), which was therefore selected as the oil phase for the development of NLC. For the selection of solid lipids, nimesulide had demonstrated the highest solubility in stearic acid (). The apparent partition coefficient of nimesulide in different solid lipids such as PBS/glyceryl monostearate, PBS/stearic acid, and PBS/tristearin was found to be 100.4 ± 7.61, 150.3 ± 3.31 and 79.9 ± 4.54 respectively; that showed the highest partitioning of nimesulide in stearic acid. In the present study, stearic acid was selected as the solid lipid for the formulation of NLC because stearic acid has more potential to solubilize the nimesulide as compared to the other lipids. Results showed that the drug has highest solubility in stearic acid and oleic acid. Both stearic acid and oleic acid were further subjected for miscibility study; the results of miscibility study have demonstrated that their mixture showed good miscibility in ratio of 9:1, 8:2, 7:3 and 6:4. It has been reported that incorporation of liquid oil into lipid nanoparticles perturbed the crystalline matrix resulting in enough space to accommodate drug molecules, and thus increased the entrapment efficiency (Thatipamula et al. Citation2011, Woo et al. Citation2014). In the present study 3 ratios of solid lipid:oil selected for further studies were 9:1, 8:2, and 7:3. It was reported (Woo et al. Citation2014) that nanoparticles with oleic acid amount higher than 30% has lower melting behavior and may be easily oxidized, especially when there is unexpected temperature fluctuation during storage and transportation.
Optimization of NLC and fitting the model to the data
The total 15 runs were generated and the responses so observed are shown in . All the responses observed for 15 formulations prepared were simultaneously fitted to first order, second order and quadratic models using Design Expert software (Minneapolis, MN). It is evident that all the three independent variables, namely the ratio of stearic acid: oleic acid (X1), poloxamer 188 concentration (X2), and lecithin concentration (X3) have interactive effects on the two responses, e.g., Y1; particle size (nm), Y2; entrapment efficiency (%).
Table 2. Observed response in the Box–Behnken design for nimesulide NLC formulations (mean ± SD).
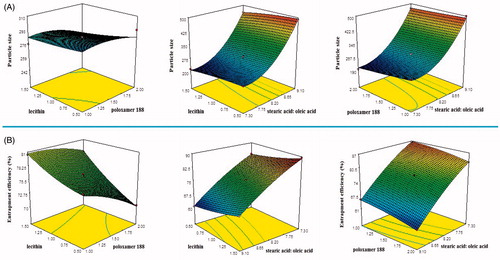
Three-dimensional plots were prepared and are shown in , for responses Y1 and Y2, respectively. These plots are known to study the interaction effects of the factors on the responses as well as are useful in studying the effects of two factors on the response at one time.
Response analysis through polynomial equations
Response 1 (Y1): effect of independent variables on NLC particle size
In the model fit for particle size of NLC, sequential P values suggested “quadratic model” for NLC particle size analyses. is the three-dimensional plot which shows the effect of independent variables on the particle size of NLC (Y1).
It was observed that, increase in surfactant concentration decreases particle size (). This decrease in size at higher surfactant concentrations might be due to reduction in interfacial tension between the aqueous and lipid phases, leading to the formation of emulsion droplets of smaller size (Liu et al. Citation2007). Higher surfactant concentrations effectively stabilized the particles by forming a steric barrier on the particle surface, and thereby protect smaller particles and prevent their coalescence into bigger ones (Rahman et al. Citation2010).
It is very evident from the model graph () that variation of liquid lipid concentration affects the particle size of NLC formulation. As ratio of solid lipid to liquid lipid decreased, the size of the particle increased. This decrease in particle size at higher liquid lipid concentration might be due to decrease in the viscosity of internal phase. Co-surfactant concentration also has the similar effect on the particle size of the NLC. Increased co-surfactant concentration led to decrease in NLC particle size (). Particle size analysis of prepared 15 formulations revealed a size range of 196.8 ± 18 to 499.8 ± 12 nm, which is primarily suitable for topical delivery (Desai et al. Citation2010; Nasr et al. Citation2008; Schramlova et al. Citation1997). Polydispersity index value of developed formulations ranged from 0.335 to 0.458 (), supporting the presence of homogeneity and narrow distribution of particle size.
Response 2 (Y2): effect of independent variables on entrapment efficiency
In case for entrapment efficiency, quadratic model was found to be significant and best fit for NLC entrapment efficiency analysis. is the three-dimensional plot which shows the effect of different independent variables on entrapment efficiency of nimesulide (Y2). In our study, it was revealed by the model graphs that increasing the amount of surfactant from 1 to 2% w/w resulted in a gradual decrease in the entrapment efficiency of NLC formulations (). This observed decrease in entrapment efficiency could be explained by partition phenomenon. High surfactant level in the external phase might increase the partition of drug from internal to external phase of the medium. This increased partition is due to the increased solubilization of the drug in the external aqueous phase so more drug can disperse and dissolve in it (Rahman et al. Citation2010). It has been observed that the entrapment efficiency of NLC formulation (F8) had increased from 69.01% to 85.013% (F9) with an increase in the percentage of oleic acid from 10 to 30%w/w (). It might be due to the incorporation of liquid lipids into solid lipids which have led to massive crystal order disturbance. Greater imperfections in the crystal lattice leave enough space to accommodate drug molecules, which ultimately improved drug entrapment efficiency. Higher entrapment in the formulation containing 30%w/w oleic acid indicates higher solubility of the drug in oleic acid, compared to stearic acid. Effect of the amount of lecithin (co-surfactant) on entrapment efficiency of drug in NLC was evaluated. reveals that the higher percentage entrapment efficiency was found when the amount of lecithin was increased from 0.5% to 1.5%w/w. It may be explained that by increasing the lecithin content reduces the possibility of drug loss to the external phase, and provides more space to incorporate the drug by forming multilayers around the particle, resulting in improved entrapment efficiency.
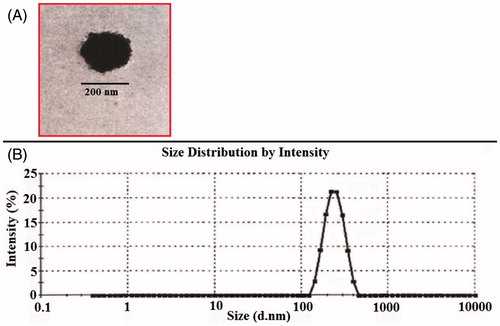
The optimum formulation of nimesulide-loaded NLC system was selected based on the criteria of attaining the maximum value of entrapment efficiency and minimizing the particle size by applying Point prediction method of the Design Expert software® (Minneapolis, MN). The formulation composition with stearic acid: oleic acid (7:3), poloxamer 188 (1.5%w/w), and lecithin (1.5%w/w) was found to fulfill requisites of an optimum formulation, i.e. F9. The optimized formulation has the entrapment efficiency of 89.4 ± 3.40% with particle size and flux across rat skin of 214.4 ± 11 nm and 2.66 ± 0.09 μg/cm2/h, respectively (). The electron micrographs of F9 are shown in . They show the outline and core of the well-identified spherical particle, displaying sealed NLC structure. Size distribution of optimized F9 NLC loaded with nimesulide is presented in . On the basis of the Box–Behnken design approach, optimized nimesulide NLC formulation was selected and further analyzed for in vitro skin penetration, drug release kinetic, confocal laser scanning microscopy and storage stability study.
In vitro skin penetration study
In vitro skin penetration study has been performed using Franz diffusion cell across rat skin. It was observed that the penetration of nimesulide enhanced on increasing the concentration of oleic acid in the NLC dispersion (). The NLC dispersion containing 30% w/w of oleic acid (F9) showed a flux of 2.66 μg/cm2/h, whereas the NLC dispersion containing 10% w/w of oleic acid (F10) produced a flux of 1.36 μg/cm2/h. This increase in flux on increasing the liquid lipid concentration may be due to adhering of liquid lipid to the lipid matrix, leading to decrease in the diffusion path length of the lipid matrix (Thatipamula et al. Citation2011). Moreover, it is observed that increasing the surfactant concentration also increased the percentage of nimesulide penetration across rat skin. The higher penetration noticed at higher surfactant concentration could be explained by the partitioning effects of the drug between the melted lipid phase and the aqueous surfactant phase during particle production. The higher the surfactant concentration, the greater is the solubility of the drug in the water phase, so the amount of drug in the outer shell increased and released in a relatively rapid way (Zur Muhlen and Mehnert Citation1998).
Drug release kinetics
The in vitro drug release model showing highest value of R2 was considered as best model for release of nimesulide from NLC formulation (F9). The highest value of the correlation coefficient (R2 = 0.984) was observed for the Higuchi matrix, followed by the first-order (R2 = 0.9654), Korsmeyer–Peppas (R2 = 0.9638) and zero order (R2 = 0.8314). Since the value of release exponent (n) for the proposed model was found to be 0.56, it implies that optimized NLC formulation follows Anomalous Diffusion (both diffusion and erosion) pattern (0.5 < n < l). The same release kinetics pattern for NLC for topical delivery of nitrendipine and piroxicam was described by previous researchers (Monica et al. Citation2014, Uner et al. Citation2014).
Confocal laser scanning microscopy
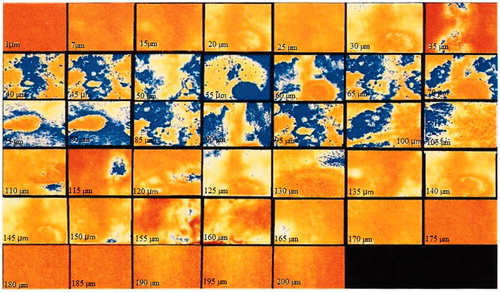
The confocal laser scanning microscopy study was conducted to evaluate the extent of penetration of the nimesulide-loaded NLC system. As observed by confocal laser scanning microscopy, NLC preparation traversed the skin thickness to extent of approximately 110 μm (. Much greater intensity of fluorescence was observed from NLC. The fluorescence in the horizontal section at 40–100 μm was very strong and showed that NLC was rich in the epidermis and upper dermis.
Stability study
Stability study indicated that there was no color fading, aggregation and phase separation was observed for optimized formulation (F9). The particle size and entrapment efficiency were measured to ensure that the formulation characteristics and drug retention capacity of the formulation remain unchanged. The difference in the particle size, and entrapment efficiency were found to be insignificant. The results exhibited that the drug retained above 83.98% in NLC dispersion for the period of 60 days at refrigerated condition. The stability study result indicated that the optimized nimesulide-loaded NLC dispersion was considerably stable at refrigerator temperature as compared to room temperature.
Conclusion
Nimesulide-loaded NLC were successfully prepared and optimized using a 3-factor, 3-level Box–Behnken design. The formulation optimizing study using statistical experimental design shows that optimum concentrations of stearic acid: oleic acid, poloxamer 188 and lecithin are required to provide the maximum value of flux and entrapment efficiency, minimizing the particle size. Further, nimesulide-loaded NLC could be incorporated into a gel formulation that would offer enhanced skin contact and ease of application without altering the release or skin penetration characteristics of the nanoparticles. Present results demonstrate that the NLC formulation is a potentially useful carrier for topical delivery of nimesulide.
Disclosure statement
All authors have approved the final manuscript, and the authors declare that they have no conflicts of interest to disclose.
References
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, Ali A. 2011a. Interactions between novel terpenes and main components of rat and human skin: mechanistic view for transdermal delivery of propranolol hydrochloride. Curr Drug Deliv. 8:213–224.
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, Ali A. 2011b. Role of novel terpenes in transcutaneous permeation of valsartan: effectiveness and mechanism of action. Drug Dev Ind Pharm. 37:583–596.
- Ahad A, Raish M, Al-Mohizea AM, Al-Jenoobi FI, Alam MA. 2014. Enhanced anti-inflammatory activity of carbopol loaded meloxicam nanoethosomes gel. Int J Biol Macromol. 67:99–104.
- Alves MP, Scarrone AL, Santos M, Pohlmann AR, Guterres SS. 2007. Human skin penetration and distribution of nimesulide from hydrophilic gels containing nanocarriers. Int J Pharm. 341:215–220.
- Babu RJ, Kanikkannan N, Kikwai L, Ortega C, Andega S, Ball K, Yim S, Singh M. 2003. The influence of various methods of cold storage of skin on the permeation of melatonin and nimesulide. J Control Release. 86:49–57.
- Beg S, Swain S, Singh HP, Patra CN, Rao ME. 2012. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS PharmSciTech. 13:1416–1427.
- Bessone F. 2010. Non-steroidal anti-inflammatory drugs: what is the actual risk of liver damage? World J Gastroenterol. 16:5651–5661.
- Dash RN, Mohammed H, Humaira T, Ramesh D. 2015. Design, optimization and evaluation of glipizide solid self-nanoemulsifying drug delivery for enhanced solubility and dissolution. Saudi Pharm J. 23:528–540.
- Desai P, Patlolla RR, Singh M. 2010. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol Membr Biol. 27:247–259.
- Doktorovova S, Araujo J, Garcia ML, Rakovsky E, Souto EB. 2010. Formulating fluticasone propionate in novel PEG-containing nanostructured lipid carriers (PEG-NLC). Colloids Surf B Biointerfaces. 75:538–542.
- Gonzalez-Mira E, Nikolic S, Garcia ML, Egea MA, Souto EB, Calpena AC. 2011. Potential use of nanostructured lipid carriers for topical delivery of flurbiprofen. J Pharm Sci. 100:242–251.
- Higuchi T. 1963. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 52:1145–1149.
- Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. 2012. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target. 20:813–830.
- Jaiswal P, Gidwani B, Vyas A. 2016. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif Cells Nanomed Biotechnol. 44:27–40.
- Khan SA, Ahmad M, Murtaza G, Aamir MN, Rasool F, Raees MA. 2011. Influence of process parameters on nimesulide-loaded poly(D,L-Lactide-Co-Glycolide) microcapsules. Latin Am J Pharm. 30:119–125.
- Korsmeyer RW, Gurny R, Doelker EM, Buri P, Peppas NA. 1983. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 15:25–35.
- Lenz QF, Guterres SS, Pohlmann A, Alves MP. 2012. Semi-solid topical formulations containing nimesulide-loaded nanocapsules showed in-vivo anti-inflammatory activity in chronic arthritis and fibrovascular tissue models. Inflamm Res. 61:305–310.
- Liu J, Gong T, Wang C, Zhong Z, Zhang Z. 2007. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm. 340:153–162.
- Luan J, Zheng F, Yang X, Yu A, Zhai G. 2015. Nanostructured lipid carriers for oral delivery of baicalin: In vitro and in vivo evaluation. Colloids and Surfaces A. 466:154–159.
- Madheswaran T, Baskaran R, Yong CS, Yoo BK. 2014. Enhanced topical delivery of finasteride using glyceryl monooleate-based liquid crystalline nanoparticles stabilized by cremophor surfactants. AAPS PharmSciTech. 15:44–51.
- Monica G, Jovita K, Sanjay T, Shubhini SA. 2014. Development of piroxicam loaded nanostructured lipid carriers for spondylitis treatment. Adv Sci Lett. 20:1066–1071.
- Muller RH, Shegokar R, Keck CM. 2011. 20 Years of lipid nanoparticles (SLN and NLC): present state of development and industrial applications. Curr Drug Discov Technol. 8:207–227.
- Narala A, Veerabrahma K. 2013. Preparation, characterization and evaluation of quetiapine fumarate solid lipid nanoparticles to improve the oral bioavailability. J Pharm. 2013:265741.
- Nasr M, Mansour S, Mortada ND, El Shamy AA. 2008. Lipospheres as carriers for topical delivery of aceclofenac: preparation, characterization and in vivo evaluation. AAPS PharmSciTech. 9:154–162.
- Patel D, Dasgupta S, Dey S, Ramani YR, Ray S, Mazumder B. 2012. Nanostructured lipid carriers (NLC)-based gel for the topical delivery of aceclofenac: preparation, characterization, and in vivo evaluation. Sci Pharm. 80:749–764.
- Patil GB, Patil ND, Deshmukh PK, Patil PO, Bari SB. 2016. Nanostructured lipid carriers as a potential vehicle for carvedilol delivery: application of factorial design approach. Artif Cells Nanomed Biotechnol. 44:12–19.
- Peppas NA. 1985. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 60:110–111.
- Pradhan M, Singh D, Singh MR. 2016. Influence of selected variables on fabrication of triamcinolone acetonide loaded solid lipid nanoparticles for topical treatment of dermal disorders. Artif Cells Nanomed Biotechnol. 44:392–400.
- Rahman Z, Zidan AS, Khan MA. 2010. Non-destructive methods of characterization of risperidone solid lipid nanoparticles. Eur J Pharm Biopharm. 76:127–137.
- Raj R, Mongia P, Ram A, Jain NK. 2015. Enhanced skin delivery of aceclofenac via hydrogel-based solid lipid nanoparticles. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2015.1036997.
- Ramjith US, Mathew T. 2015. A review on some of the banned drugs which are still available in india. Int J Curr Res Chem Pharma Sci. 2:76–83.
- Schramlova J, Blazek K, Bartackova M, Otova B, Mardesicova L, Zizkovsky V, Hulinska D. 1997. Electron microscopic demonstration of the penetration of liposomes through skin. Folia Biol (Praha). 43:165–169.
- Shahzad Y, Afreen U, Nisar Hussain Shah S, Hussain T. 2013. Applying response surface methodology to optimize nimesulide permeation from topical formulation. Pharm Dev Technol. 18:1391–1398.
- Singh B, Mehta G, Kumar R, Bhatia A, Ahuja N, Katare OP. 2005. Design, development and optimization of nimesulide-loaded liposomal systems for topical application. Curr Drug Deliv. 2:143–153.
- Thakkar HP, Desai JL, Parmar MP. 2014. Application of Box–Behnken design for optimization of formulation parameters for nanostructured lipid carriers of candesartan cilexetil. Asian J Pharm. 8:81–89.
- Thatipamula R, Palem C, Gannu R, Mudragada S, Yamsani M. 2011. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. Daru. 19:23–32.
- Tichota DM, Silva AC, Sousa Lobo JM, Amaral MH. 2014. Design, characterization, and clinical evaluation of argan oil nanostructured lipid carriers to improve skin hydration. Int J Nanomedicine. 9:3855–3864.
- Uner M, Karaman EF, Aydogmus Z. 2014. Solid lipid nanoparticles and nanostructured lipid carriers of loratadine for topical application: physicochemical stability and drug penetration through rat skin. Trop J Pharm Res. 13:653–660.
- Uprit S, Kumar Sahu R, Roy A, Pare A. 2013. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm J. 21:379–385.
- Vandana KR, Yalavarthi PR, Sundaresan CR, Sriramaneni RN, Vadlamudi HC. 2014. In-vitro assessment and pharmacodynamics of nimesulide incorporated Aloe vera transemulgel. Curr Drug Discov Technol. 11:162–167.
- Woo JO, Misran M, Lee PF, Tan LP. 2014. Development of a controlled release of salicylic acid loaded stearic acid-oleic acid nanoparticles in cream for topical delivery. Scientific World J. 2014:205703
- Zur Muhlen A, Mehnert W. 1998. Drug release and release mechanism of prednisolone loaded solid lipid nanoparticles. Pharmazie. 53:552–555.