Abstract
Dextran sulfate sodium (DS) was allowed to interact ionically with ropinirole hydrochloride (ROPI HCl, an anti-Parkinsonian agent) to synthesize self-assembled ROPI-DS nanoplex. The preliminary objective behind ROPI-DS complexation was to enhance the partitioning of ROPI HCl and thereby its encapsulation into nanocarriers and to improve the nasal membrane permeability. Molecular interactions were computed using in silico molecular modeling. Nanoplex were characterized for physicochemical and partitioning behavior. Optimized ROPI-DS nanoplex was further characterized by spectroscopic and thermal analysis, diffraction studies, morphological and histopathological analysis. In summary, ROPI-DS nanoplex represents a promising nanocarrier material for intranasal administration.
Introduction
Dextran sulfate sodium (DS) is one among such biopolymers which has widely been studied for pharmaceutical and biomedical application because of its biodegradability, biocompatibility, and excellent safety profile (Schatz et al. Citation2004). The anionic trait of DS, due to the presence of sulfate groups, corroborates strong electrostatic interactions with di-cationic functional groups of ropinirole hydrochloride (ROPI HCl) and hence could be self-assembled into ROPI-DS nanocomplex (nanoplex).
ROPI HCl is a non-ergot dopamine D2- and D3-receptor agonist, generally used alone or as an adjunct to levodopa in the management of Parkinson’s disease (PD). Chemically, it is 4-[2-(dipropylamino)-ethyl]-1,3-dihydro-2H-indol-2-one (Mahaki et al. Citation2013). ROPI HCl is a highly hydrophilic, low molecular weight anti-Parkinsonian drug with a plasma half-life of 5–6 h. It suffers an oral bioavailability only ∼50% owing to its extensive hepatic first-pass metabolism. Its hydrophilic nature may also hamper its permeation across the biological membranes (Pardeshi et al. Citation2013a). Intranasal administration generally bypasses the transport of drug across the BBB and also avoid hepatic first-pass metabolism, thereby aid to overcome the above-mentioned shortcomings (Kulkarni et al. Citation2015, Pardeshi and Belgamwar Citation2013, Patel et al. Citation2012a, Patel et al. Citation2012b). Intranasal administration of ROPI HCl has been investigated by our group (Pardeshi et al. Citation2013a, Pardeshi et al. Citation2013b). However, we observed low encapsulation of ROPI HCl into lipid-based nanocarriers (Pardeshi et al. Citation2013a, Pardeshi et al. Citation2013b) and this accounts for the evolution of new strategies to enhance its partitioning behavior and thus, the encapsulation inside the nanocarriers.
Electrostatic interactions can result in complexation between cationic drugs and anionic polymers. It has previously been utilized for improving drug encapsulation efficiency and controlling the drug release from polymeric nanocarriers (Ding et al. Citation2011, Ehtezazi et al. Citation2000, Ranjan et al. Citation2010). Previously, Janes et al. (Citation2001) reported the complexation of doxorubicin (DOX) with DS to increase the encapsulation of DOX into chitosan (CHT) nanoparticles. Cheow and Hadinoto (Citation2012) investigated the ionic complexation between hydrophilic ciprofloxacin hydrochloride (CIP) and DS for increasing the dissolution rate and saturation solubility of CIP. The self-assembly is a green and cost-effective material-synthesis technique. It does not require the use of sophisticated instrument or organic solvents for the fabrication of biomaterial particles (Cheow and Hadinoto Citation2012).
The specific objectives of the present study are to enhance the partition behavior of hydrophilic ROPI HCl, which would increase its encapsulation efficiency into nanocarriers and also to enhance the permeation characteristics after intranasal administration. The authors strongly believe that the well-addressed capacity of DS to form self-assembled nanoplex with ROPI HCl at various molar ratios grant to such kind of nanoparticulate carriers an exceptional attention for the development of robust nanocarrier material.
The molecular level interactions of self-assembled ROPI-DS nanoplex were computed using in silico molecular modeling analysis. The nanoplex particles were analyzed for complexation efficiency (CE), partition coefficient, and drug release studies in vitro. Fabricated nanoplex were further applied to the excised sheep nasal mucosal membrane to investigate their nasal toxicity and nasal membrane permeability ex vivo.
Materials and methods
Materials
ROPI HCl was received as a gift sample from Wockhardt Pharmaceuticals Pvt. Ltd. (Aurangabad, India). DS, sodium salt (CAS 9011-18-1, Mw ∼5000) and dialysis membrane (Mw cut-off 12–14 kDa) were purchased from Hi-Media Laboratories (Mumbai, India). CHT (CAS 9012-76-4, 75–85% deacetylation) was supplied by Central Institute of Fisheries Technology (Cochin, India). Water used throughout the study was bi-distilled and filtered through 0.22 μ nylon filter immediately before use. All other chemicals and reagents were purchased from SD Fine-Chem (Mumbai, India) and used as received.
Synthesis of self-assembled ROPI-DS nanoplex
The ionic ROPI-DS nanoplex were synthesized according to a protocol previously reported by (Yousefpour et al. Citation2011). Briefly, a 10 mM solution each of ROPI HCl and DS were prepared in bidistilled water. Aliquots of ROPI HCl solution were added to a DS solution under constant magnetic stirring at moderate speed and room temperature (RT) for 2 h to obtain ROPI-DS molar ratios of 0.1:1, 0.5:1, 1:1, 1.5:1, 2:1, 3:1, 4:1, 6:1, 8:1, and 10:1. The cloudy solution immediately appears as soon as the ROPI-DS complexes self-assembled into nanoparticles. The nanoplex suspension is allowed to equilibrate for 3 h at ambient conditions. The obtained nanoplex were collected after three cycles of centrifugation and washing with bidistilled water and lyophilized in a freeze dryer (VirTis Benchtop, SP Scientifics, Gardiner, NY) employing standard lyophilization protocol, after preliminary physicochemical characterization and nanoplex optimization. The physical mixture (PM) of an equimolar ratio (1:1) of ROPI and DS was prepared by thoroughly mixing ROPI and DS for 10 min in a mortar until a homogeneous mixture was obtained, further passed through 40# mesh (nominal pore size: 0.0165 inches) and stored in a desiccator (Jena et al. Citation2014).
In silico molecular modeling analysis
The aim of in silico molecular modeling analysis was to confirm the binding of ROPI HCl to the DS, to examine the molecular interactions between binding molecules and to predict the most favorable structure of ROPI-DS complex. In silico molecular modeling analysis gives an insight about the binding sites in ligand (ROPI HCl) and substrate (DS), thereby enable ROPI-DS complexation. The molecular docking tool, GLIDE (Schrodinger Inc., NY) was used to study the ligand (ROPI HCl) docking into the pocket of substrate (DS). The low-energy conformation of the ROPI HCl was selected and was docked into the DS pocket using docking mode (Evans et al. Citation2007, Halgren et al. Citation2004, Zhong et al. Citation2009).
Characterization of self-assembled ROPI-DS nanoplex
Physicochemical characterization
Nanoplex production yield was determined as the total weight of ROPI-DS nanoplex produced after freeze drying (practical weight) with respect to the total weight of ROPI HCl and DS added initially (theoretical weight) (EquationEq. 1(1) ).
(1)
Nanoplex size and zeta potential measurements were accomplished by dynamic light scattering (DLS) using a Zetasizer (Nano ZS90, Malvern Instruments Ltd., Malvern, UK) at 25 °C and at a scattering angle of 90°. All the samples were diluted with bidistilled water (1:10) to get optimum 100–200 kilo-counts s−1 (kcps) for measurements.
CE of ROPI-DS nanoplex was estimated by ultracentrifugation of nanoplex at 20,000 RPM for 30 min. The concentration of remaining ROPI HCl in the supernatant was measured spectrophotometrically (1700, Shimadzu®, Tokyo, Japan) at 250 nm and percent CE was determined as the difference between the amount of ROPI added and the amount of non-complexed ROPI in the supernatant after ultracentrifugation (EquationEq. 2(2) ; Cheow and Hadinoto Citation2011, Yousefpour et al. Citation2011).
(2)
Partition coefficient (logP) of bulk ROPI and ROPI-DS nanoplex was determined using a procedure reported by Zafar et al. (Citation2014) employing octanol-water solvent system. A predefined amount of ROPI or ROPI-DS nanoplex was added to octanol-water solvent mixture in a separating funnel. The funnel was subjected to equilibrium shaking in a shaking incubator for 24 h at RT. After 24 h, aliquots of water and octanol phase were withdrawn, sufficiently diluted and analyzed for ROPI concentration spectrophotometrically (1700, Shimadzu®, Tokyo, Japan) at 250 nm. The logP was calculated using the following EquationEquation (3)(3) :
(3)
where, logP is the partition coefficient, Co represents nanoplex concentration in n-octanol, and Cw represents nanoplex concentration in water.
The criteria of attaining a minimum particle size, optimum zeta potential, maximum CE was set to decide the optimum sample of ROPI-DS nanoplex.
Analysis of ROPI-DS interactions
To understand the interactions involved in ROPI-DS nanoplex formation, complexation was studied in the presence of ethanol (hydrogen bond disrupting agent) and NaCl (electrostatic shielding agent). To the optimized ROPI-DS nanoplex suspension, ethanol was added at varying concentrations (0–80% v/v), the absorbance was measured at 250 nm and compared with the absorbance of pure ROPI solution in ethanol-water cosolvent system. The influence of varying ionic strengths on the absorbance of ROPI-DS nanoplex was also studied in the presence of 0–0.5 M NaCl (Yousefpour et al. Citation2011).
In addition, the absorbance of ROPI-DS nanoplex was investigated in presence of CHT, a cationic polysaccharide. CHT was added to the optimized ROPI-DS nanoplex suspension at CHT/DS (w/w) ratio of 0.25–6 and the resulting absorbance was measured at 250 nm. Dissociation of ROPI from ROPI-DS nanoplex was studied further to characterize the nature of interactions involved in ROPI HCl and DS complexation. Briefly, ROPI-DS nanoplex equivalent to 100 μg of ROPI was incubated in the presence of 100 mM phosphate buffer saline (PBS) or bidistilled water. The solution was vortexed and kept aside for 6 h at RT. The solution was then subjected to centrifugation and the resulting supernatant was analyzed for ROPI concentration spectrophotometrically at 250 nm (Yousefpour et al. Citation2011).
Solid-state characterization of ROPI-DS nanoplex particles
FTIR spectra of ROPI, DS, ROPI-DS PM, and ROPI-DS nanoplex were registered in solid state as potassium bromide (KBr) dispersion on an FTIR spectrometer-430 (Shimadzu 8400S, Tokyo, Japan) in a scan range of 4000–500 cm−1 and resolution of 4 cm−1.
The thermal profiles of ROPI, DS, ROPI-DS PM, and ROPI-DS nanoplex were recorded on a Differential Scanning Calorimeter (DSC 1, Mettler Toledo®, Zurich, Switzerland) at a heating rate of 10 °C min−1 from 30–300 °C under a nitrogen purge of 50 mL min−1.
The thermogravimetric (TGA/DTG) analyses of ROPI, DS, ROPI-DS PM, and ROPI-DS nanoplex were performed on a Thermogravimetric Analyzer (NETZSCH, STA 409 PG/4/G Luxx, New York) at a heating rate of 10 °C min−1 from 40–700 °C under nitrogen purge of 50 mL min−1.
The X-ray diffractograms of ROPI, DS, ROPI-DS PM, and ROPI-DS nanoplex were recorded on an X-ray Diffractometer (Brucker AXS D8 advance®, Karlsruhe, Germany). Diffractograms were scanned in a scattering range from 3–80° (2θ) with a resolution of 0.02° and scanning speed of 2.0° min−1, applying an accelerating voltage of 40 kV and a current intensity of 35 mA.
Dispersions of bulk ROPI and ROPI-DS nanoplex were visualized by High-resolution Transmission Electron Microscope (HR-TEM, JEOL, JEM-2100, Peabody, MA). Samples were carbon-coated on copper grids and stained with phosphotungstic acid (2% w/v), air-dried and viewed under HR-TEM. Photomicrographs were captured at 200 kV at different magnifications.
In vitro drug diffusion and diffusion kinetics
Modified Franz’s diffusion cell (12 mL capacity; 3.14 cm2 diffusion area) was used to study the diffusion of ROPI in vitro from optimized ROPI-DS nanoplex particles across the dialysis membrane (Mw cut-off 12–14 kDa). The ROPI-DS nanoplex, equivalent to 1 mg of ROPI, was spread over the membrane into the donor compartment previously soaked with 3 mL of simulated nasal electrolyte fluid (SNEF). Diffusion medium (PBS, pH 6.8, 35 ± 2 °C) was filled in receptor compartment and maintained under constant stirring. 300 μL of the medium was periodically withdrawn from a receptor compartment at pre-determined time points, withdrawn samples were replaced with same quantity of fresh PBS, and assayed spectrophotometrically (1700, Shimadzu®, Tokyo, Japan) at 250 nm.
To study kinetics of drug release from ROPI-DS nanoplex, data obtained from in vitro drug release studies were plotted in various kinetic models viz. zero order (see EquationEq. 4(4) as cumulative percentage of drug released versus time, first order (see EquationEq. 5
(5) as log cumulative percentage of drug remaining versus time and Higuchi’s model (see EquationEq. 6
(6) as cumulative percentage of drug released versus square root of time).
(a) Zero order
(4)
where, K0 is zero-order rate constant (concentration/time) and t is time (min). A graph of concentration versus time would yield a straight line with slope equal to K0.
(b) First order
(5)
where, C0 is initial concentration of drug, K is first-order rate constant and t is time (min).
(c) Higuchi
(6)
where, Qt is amount of drug released in time t, K is kinetic constant and t is time (min).
Mechanism of drug release from ROPI-DS nanoplex was evaluated by subjecting the data obtained from in vitro drug diffusion studies to Korsemeyer–Peppas model (see EquationEq. 7(7) as log cumulative percentage drug released versus log time). Release exponent (n) and kinetic constant (K) were calculated from slope of straight line.
(7)
where, Mt represents amount of drug released at time t, M∞ is total amount of drug released after an infinite time, K is diffusional (kinetic) constant of drug-polymer complex system and n is release exponent that determines mechanism of drug release from ROPI-DS nanoplex. If n = 0.5 then, drug release mechanism is Fickian diffusion, if n < 0.5, mechanism is quasi-Fickian diffusion, if n = 0.5–1.0 then, it is non-Fickian or anomalous diffusion, if n = 1.0, mechanism is non-Fickian case II diffusion, and if n > 1.0 then, the mechanism is non-Fickian super case II diffusion (Pardeshi et al. Citation2013a, Pardeshi et al. Citation2013b).
Ex vivo permeation studies
The use of biological membranes is very crucial to predict the drug permeation characteristics. For this reason, the excised sheep nasal mucosa, covering the ventral nasal conchae, was chosen for present permeation experiment. The tissue is also easy to handle and there are more chances of getting intact, unstrained pieces of nasal mucosa. In addition, an ethical advantage is that the mucosal tissue can be easily available from slaughterhouse and the sacrificing of animal can be avoided only for a single piece of tissue (Tas et al. Citation2006).
Ex vivo drug permeation studies were performed on lyophilized ROPI-DS nanoplex using modified Franz’s diffusion cell (receptor capacity: 12.0 mL; permeation area: 3.14 cm2) across an excised sheep nasal mucosa (thickness: ∼0.20 mm) as a model permeation membrane (barrier). Permeation medium (PBS, pH 6.8, 35 ± 2 °C) was filled in receptor compartment and maintained under constant stirring. The excised sheep nasal mucosa, obtained from local butchery, was mounted onto the diffusion chamber with mucosal and serosal surfaces keeping toward the donor and receptor compartments, respectively. Optimized nanoplex formulations (ROPI-DS 1.5:1), equivalent to 1 mg of ROPI HCl, were spread over the mucosal membrane in donor compartment, previously soaked with 3.5 mL of SNEF. 300 μL of medium from receptor compartment was withdrawn at pre-determined time points and the sampled volume were replaced using the same volume of fresh PBS, and assayed using UV-visible spectrophotometer (1700, Shimadzu®, Tokyo, Japan) at 250 nm (Pund et al. Citation2013).
The cumulative amount of ROPI permeated (μg) per unit surface area (cm2) of the nasal mucosa was plotted as a function of time (h) and the slope of the linear portion of the curve was considered as a steady-state flux (Jss) (EquationEq. 8(8) ). The apparent permeability coefficient (Papp) and the steady-state diffusion coefficient (D) were calculated using EquationEquations 9
(9) and Equation10
(10) , respectively (Pund et al. Citation2013).
(8)
(9)
(10)
where, ΔQt/Δt is the cumulative amount of drug permeated per unit of mucosal surface area (μg cm−2) and t is time (h). Cd is the initial concentration of ROPI in the donor compartment (mg) and S is the effective surface area of nasal mucosa. K is the partition coefficient of ROPI HCl and L is the diffusion path length.
Ex vivo nasal toxicity evaluation
The nasal toxicity studies were conducted in order to ensure the biocompatibility of ROPI-DS nanoplex ex vivo on the excised sheep nasal mucosa. The sheep nasal mucosa (conchae), obtained from local butchery (Shirpur, India) within 60 min of sacrificing, was cleaned with saline solution. After application of predefined amount of ROPI-DS nanoplex, mucosal tissues were fixed in 10% formalin solution, routinely processed and implanted in paraffin. Paraffin sections (5–6 μm) were stained with hematoxylin–eosin (HE) and observed under digital light microscope (Besto BE-12, Chandigarh, India) equipped with a C-mount 1/3″ CMOS 1.3MP camera imaging accessory (Moticam® 1000, Motic Instruments Inc., Toronto, Canada) and computer-controlled image analysis software (Motic Images Plus ver. 2.0 ML). The mucosa incubated with PBS (pH 6.8) directly after isolation at local butchery was used as a control (Joshi et al. Citation2012, Pardeshi et al. Citation2012). To determine the nasal toxicity, the toxicity indicators such as epithelial necrosis or sloughing of epithelial cells, and fibrosis were used.
Results and discussion
Mechanism of nanoplex formation
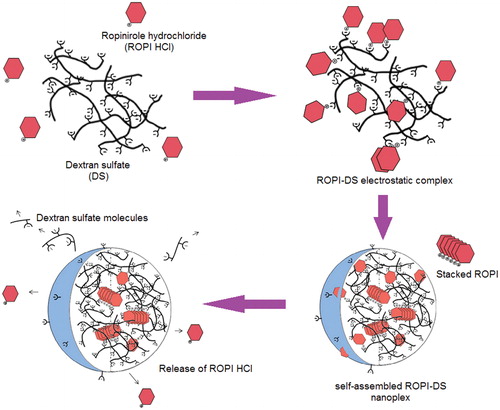
The nanoplex are expected to be self-assemble in water to form spherical nanoparticles, most probably with the hydrophobic ROPI-coupled DS units forming the inner particle core while the anionic sulfate groups of DS remaining near the surface of the nanoplex. A model illustrating the structure of the ROPI-DS nanoplex and release of ROPI HCl from the nanoplex is shown in Scheme 1 (Lanz-Landazuri et al. Citation2014).
Scheme 1. Schematic model illustrating the mechanism of ROPI-DS nanoplex formation and release of ROPI HCl from the nanoplex. Reproduced from Lanz-Landazuri et al. (Citation2014) with kind permission of the copyright holder, Elsevier, Amsterdam.
In silico molecular modeling analysis
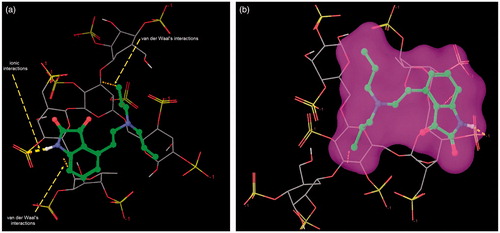
The representative docking conformations of ROPI HCl and DS complexation are presented in . Docking interactions indicate that -NH of ROPI HCl is involved in ionic interaction with the sulfate groups of DS. On the other hand, indole ring and isopropyl side chain of ROPI HCl is showing van der Waals type of interactions with DS monomer. In silico molecular modeling analysis gives an insight about the ROPI-DS complexation which could be useful for further complex optimization through physicochemical and solid-state characterization.
Physicochemical characterization of ROPI-DS nanoplex
The production yield of self-assembled ROPI-DS nanoplex was obtained in the range of ∼90–95% validating ionic complexation as a cost-effective and organic solvent-free materials-synthesis process. The average particle diameter was obtained in the nanoscale dimensions for all the prepared complexes with uniform particle size distribution. The zeta potential values appeared to be negative for all fabricated ROPI-DS nanoplex samples, which may be ascribed to the excess of anionic sulfate groups of DS preferentially located near to the surface of the nanoplex ().
Table 1. Composition and physicochemical characterization of ROPI-DS nanoplex.
The CE was found to be significantly increased as the concentration of ROPI HCl increased during complexation and reached a plateau at a molar ratio 1.5:1 (97.20 ± 3.6), beyond which CE declined slightly with increase in ROPI-DS ratio (). Complete charge neutralization of ROPI HCl at 1.5:1 molar ratio could be the reason behind high CE. No further increments in CE were observed after saturation.
The apparent partition coefficient (logP) was determined in octanol-water system to ensure the increased lipophilicity of ROPI-DS nanoplex over ROPI HCl. The logP values of ROPI HCl and ROPI-DS nanoplex (1.5:1) were estimated to be 2.477 ± 0.35 and 6.84 ± 0.82, respectively. The increased logP values of ROPI-DS nanoplex over ROPI HCl indicated that the fabricated nanoplex was more lipophilic compared to bulk ROPI HCl, which may be credited to the charge neutralization of the individual ionic components in the nanoplex (Zafar et al. Citation2014).
Analysis of ROPI-DS interactions
The role played by hydrogen bonding and electrostatic interactions in ROPI-DS nanoplex formation was studied by investigating the influence of ethanol (hydrogen bond disrupting agent) and NaCl (electrostatic shielding agent) on the corresponding nanoplex absorbance. Data () revealed that the absorbance was increased upon addition of either of the above agents beyond 50% ethanol and >0.25 M NaCl, which was equal to that of absorbance of bulk ROPI HCl. This indicated that the hydrogen bonding and electrostatic interactions involved in ROPI-DS nanoplex formation were inhibited by the addition of ethanol or NaCl (Yousefpour et al. Citation2011).
Figure 2. Effect of (a) ethanol concentration, (b) NaCl concentration, and (c) CHT concentration on the absorbance of ROPI-DS nanoplex (ROPI-DS 1.5:1), and (d) dissociation efficiency of ROPI from ROPI-DS nanoplex (ROPI-DS 1.5:1) in different media. Data expressed as mean ± SD (n = 3).
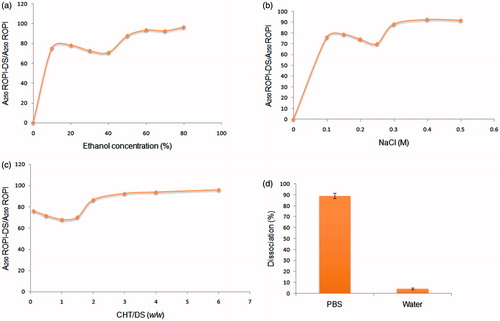
demonstrated the influence of cationic polysaccharide (CHT) on the ROPI-DS interactions. It was seen that the addition of CHT up to CHT/DS (w/w) 1.5 resulted in a decreased ROPI-DS absorbance. However, the higher CHT/DS (w/w) ratio significantly increased the ROPI-DS absorbance till the ROPI-DS absorbance reached up to that of bulk ROPI. The alterations in charge density of ROPI-DS nanoplex due to CHT might be the reason behind increased nanoplex absorbance at higher CHT/DS (w/w) ratios.
Approximately 90% of ROPI was dissociated from ROPI-DS nanoplex in PBS (a strong ionic medium) whereas negligible dissociation was observed in water (). These findings validated the involvement of ionic interactions in ROPI-DS nanoplex formation.
Solid-state characterization of ROPI-DS nanoplex particles
The ionic ROPI-DS nanoplex formation in the solid state could be well ensured by infrared spectroscopy. The FTIR spectra registered from the ROPI-DS nanoplex (1.5:1) and from its components, both individually and in physically mixed form, are compared in Supplementary Figure S1a–d. In addition, the relative vibrations and characteristic peak assignments are listed in . The three characteristic peaks assigned to sulfate group of DS in the FTIR region are at 983 cm−1 [ν(SOO-) symmetrical], and 1231 cm−1 [ν(SOO-) asymmetrical]. The disappearance or altered intensity of above-mentioned peaks for a sulfate group of DS confirms the presence of ionic interactions during nanoplex formation. The shifts recorded for the peaks at 1157 cm−1 to 1193 cm−1 due to aliphatic ν(C–N) and from 3421 cm−1 to 3400 cm−1 due to ν(N–H) from ROPI HCl to ROPI-DS nanoplex supports the involvement of -NH groups of ROPI HCl in coupling with the sulfate groups of DS, most probably via ionic interactions. The peaks at 2880, 1722, 1389, 1613, 1456, and 1280 cm−1 registered in the FTIR spectrum of ROPI HCl were almost identical compared to those registered for ROPI-DS nanoplex indicating that the native secondary structure conformation of ROPI HCl was retained in the nanoplex.
Table 2. Relevant vibrations of the chemical bonds and the characteristic absorption peaks (cm−1) in FTIR spectra of ROPI, DS, ROPI-DS PM, and ROPI-DS nanoplex (1.5:1).
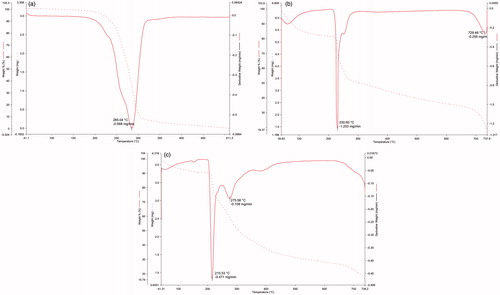
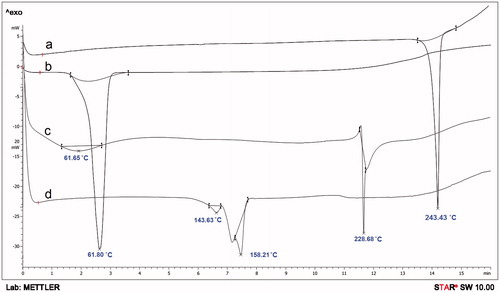
The DSC thermal profiles of parent materials (ROPI HCl and DS), their physical mixture (ROPI-DS PM), and ROPI-DS nanoplex (1.5:1) are illustrated in . The sharp endotherm at 243.43 °C indicates melting of ROPI HCl (curve a). A sharp endothermic peak at 61.80 °C signifies the glass transition of DS (curve b). Results show that ROPI HCl exists in crystalline form while the DS is in an amorphous form. In the ROPI-DS PM, the melting peak of ROPI shifted downside to 228.68 °C while the glass transition of DS shifted upside to 61.65 °C due to physical binding of ROPI HCl with DS in solid state (curve c). The endothermic peak of ROPI becomes slightly broader; while the crystalline nature of ROPI still exists in the PM. Lyophilized ROPI-DS nanoplex sample do not exhibit an endothermic melting peak of crystalline ROPI HCl indicating conversion of ROPI HCl into an amorphous state owing to its complexation with DS and entry of ROPI molecules between the DS branches (Yuan et al. Citation2009). A small exothermic peak is noticed at 143.63 °C in the nanoplex thermogram (curve d) that could be attributed to the interaction of very low residual amounts of free ROPI with the remaining sulfate groups of DS due to increased mobility of both the materials at high temperatures during DSC analysis (Li et al. Citation2006).
Figure 3. Overlaid DSC thermograms of (a) ROPI HCl, (b) DS, (c) ROPI-DS PM, and (d) ROPI-DS nanoplex (1.5:1).
The TGA and DTA thermograms of native materials (ROPI HCl, DS), and ROPI-DS nanoplex (1.5:1) are presented in . The crystalline ROPI HCl exhibited a sharp endothermic peak at 285.04 °C indicating its thermal decomposition with a mass loss in the range of c.a. 4–94% (). The thermal decomposition of amorphous DS occurred at 230.60 °C with a mass loss ranging from 18–67% (). The ROI-DS nanoplex formation is evidenced from the decomposition temperatures of nanoplex at 215 and 275 °C, indicating the decomposition of individual components of the nanoplex. The mass loss occurred in the range of 10–80% (). Results revealed that the ROPI-DS nanoplex (1.5:1) are thermally less stable as compared to parent materials viz. ROPI HCl or DS.
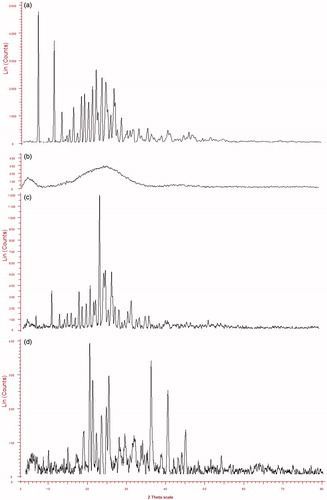
The X-ray diffractograms of native materials (ROPI HCl, DS), ROPI-DS PM and ROPI-DS nanoplex (1.5:1) are overlaid in . The strong diffraction patterns appearing at (2θ) 7.37°, 11.42°, 22.21°, 23.66°, 24.70°, and 26.79° confirms the crystalline state of ROPI HCl (diffractogram a). The absence of any sharp diffraction peak indicates that the DS is an amorphous material (diffractogram b). The ROPI-DS physical mixture (diffractogram c) shows that the diffraction peaks exhibited by native ROPI HCl (7.37° and 11.42°) are well reduced in intensity (7.25°, 11.25°) indicating the partial conversion of ROPI HCl from crystalline to amorphous state, may be due to trituration during preparation of PM. Some new diffraction peaks were observed with the overall high intensity of the diffraction patterns indicating that it was a mixture of crystalline ROPI HCl and amorphous DS. The diffractogram of lyophilized ROPI-DS nanoplex (1.5:1) (diffractogram d) displays broad and slightly diffused diffraction peaks suggesting that the ROPI-DS nanoplex exists in an amorphous state. The complexation of ROPI HCl with DS disrupts the hydrogen bonds among the amine group of ROPI and more possibly the random distribution of residual ROPI HCl within the chains of DS leads to a disappearance of diffraction peaks in the XRD spectrum of ROPI-DS nanoplex which were previously exhibited by bulk ROPI (Cheow and Hadinoto Citation2012, Li et al. Citation2006). The appearance of new diffraction peaks at (2θ) 35.83°, 40.11°, and 44.62° in the XRD spectrum of ROPI-DS nanoplex, which were not observed in the spectrum of bulk ROPI, indicates the formation of new crystalline domains in the nanoplex sample that may be attributed to the electrostatic interactions between di-cationic ROPI HCl and anionic DS leading to nanoplex formation.
Figure 5. Overlaid XRD diffractograms (a) ROPI HCl, (b) DS, (c) ROPI-DS PM, and (d) ROPI-DS nanoplex (1.5:1).
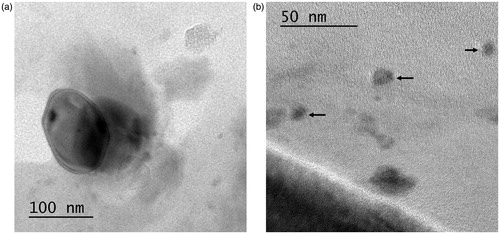
The HR-TEM images () clearly indicated that the ROPI-DS nanoplex (1.5:1) are spherical to somewhat elliptical-shaped particles. The nanoplex particles are well dispersed and free from any aggregation. All nanoplex particles have uniform sizes between 50–150 nm at ROPI/DS molar ratio of 1.5:1. The nanoplex particle size observed in HR-TEM images are smaller compared to that obtained by DLS due to the swelling of DS in an aqueous environment (Li et al. Citation2006).
In vitro drug diffusion studies
The in vitro diffusion profiles of ROPI-DS nanoplex (1.5:1) are presented in while the inset shows the magnified view of comparative release profiles of bulk ROPI HCl and ROPI-DS nanoplex at initial 240 min in PBS (pH 6.8) across dialysis membrane. The release pattern followed by nanoplex sample (1.5:1) was found to be controlled release. This might be attributed to the strong ionic coupling between ROPI HCl and DS, including hydrophobic interactions, which may explain the retention of ROPI HCl by the DS molecules (Lanz-Landazuri et al. Citation2014). The fitting of release data to kinetic models demonstrated that the best fit model was Higuchi’s model since the Higuchi’s kinetic plot showed the highest regression coefficient value (R2 = 0.982) compared to zero-order (R2 = 0.929) and first-order (R2 = 0.970) models (Supplementary Figure S2). Peppa’s exponential model was utilized to investigate the mechanism of drug diffusion from ROPI-DS nanoplex. The corresponding Korsemeyer–Peppas plot showed a linearity regression coefficient (R2), release exponent (n), and kinetic constant (K) values of 0.972, 0.389, and 0.575, respectively (). The value of the release exponent (n) suggested the quasi-Fickian diffusion mechanism of drug release from the ROPI-DS nanoplex sample (1.5:1) that signifies the diffusion of stacked ROPI HCl from the self-assembled ROPI-DS nanoplex particles (Scheme 1) (Chalikwar et al. Citation2013, Lanz-Landazuri et al. Citation2014).
Figure 7. (a) In vitro diffusion profile of ROPI-DS nanoplex at 24 h through the dialysis membrane mounted onto a vertical Franz’s diffusion cell. Inset represents the comparative diffusion profiles in vitro of bulk ROPI HCl and ROPI-DS nanoplex at initial 240 min (error bar indicates mean ± SD, n = 3). (b) Ex vivo permeation profiles of ROPI HCl at 24 h from ROPI-DS nanoplex through the excised sheep nasal mucosa mounted onto a vertical Franz’s diffusion cell. Inset represents the comparative permeation profiles ex vivo of bulk ROPI HCl and ROPI-DS nanoplex at initial 240 min (error bar indicates mean ± SD, n = 3).
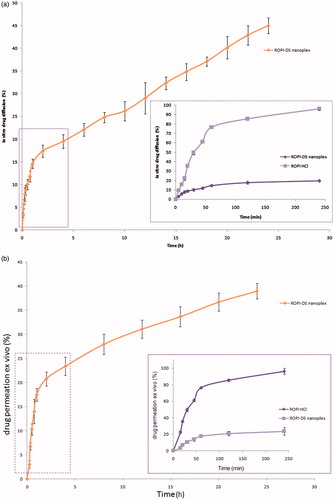
Table 3. In vitro and ex vivo release characteristics for ROPI-DS nanoplex (1.5:1).
Ex vivo drug permeation studies
The ex vivo permeation profiles of ROPI-DS nanoplex (1.5:1) are presented in while the inset view shows the comparative permeation profiles of bulk ROPI HCl and ROPI-DS nanoplex at initial 240 min in PBS (pH 6.8) across excised sheep nasal mucosa. Controlled delivery of ROPI HCl was observed from the self-assembled nanoplex formulation at 24 h. The total amount of ROPI recovered from the permeation experiment was 91.23% of the initial amount applied (1 mg) that includes the amount recovered from the mucosal surface (52.3 ± 2.1%) and from the receptor compartment (38.93 ± 1.6%). The permeability parameters calculated (using EquationEqs. 8–10) for ROPI-DS nanoplex through the excised sheep nasal mucosa are listed in . The steady-state flux (Jss), apparent permeability coefficient (Papp), and the steady-state diffusion coefficient (D) of ROPI-DS nanoplex through the nasal mucosa were calculated to be 254.6 ± 3.2 μg cm−2 h−1, 0.05093 ± 0.06 cm−2 h−1, and 0.1542 ± 0.06 cm−2 h−1, respectively. These parameters suggested the significantly high permeability of ROPI-DS nanoplex across the excised sheep nasal mucosa. This high permeability of nanoplex may be attributed to the paracellular transport of ROPI HCl (small polar molecule with Mw 296.8 Da, pKa value of 9.5, and logP value of 2.47), that takes place between the adjacent epithelial cells through hydrophilic pores and the tight junctions between the cells (Kumar et al. Citation2013, Mudry et al. Citation2007, Pardeshi and Belgamwar Citation2016).
Ex vivo nasal toxicity evaluation
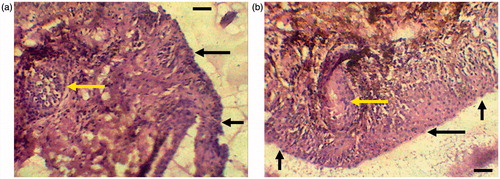
shows the histopathological specimens of excised sheep nasal mucosa stained with HE. Tissue examinations revealed the appearance of ciliated respiratory epithelium and goblet cells in the control mucosa (). The epithelial cells were seen as intact layers in the specimen treated with ROPI-DS nanoplex (1.5:1) (). No alterations were observed in basal membrane, and in the superficial part of the submucosa. Goblet cells and mucus layer were also appeared normal. No detrimental signs of damage such as appearance of epithelial necrosis or sloughing of epithelial cells were detected on the integrity of the nasal mucosa (Karasulu et al. Citation2008, Kaur et al. Citation2015, Kulkarni et al. Citation2016a,Citationb). Our observations revealed that the mucosal surface retains a good morphology after treatment, suggesting that the ROPI-DS nanoplex formulation was biocompatible ex vivo and could be used safely without irritation or damage to the nasal epithelium.
Figure 8. Optical light photomicrographs of histological sections of excised sheep nasal mucosa (scale bar: 10 μm, magnification: 400×), (a) untreated control nasal mucosa, (b) nasal mucosa treated with ROPI-DS nanoplex (1.5:1) (sample treatment includes application of 100 μL suspension of ROPI-DS nanoplex formulations for 2 h; Black arrow indicates mucus layer and yellow arrow indicates goblet cells).
Conclusions
In aqueous media, anionic DS had shown ionic interactions with di-cationic ROPI HCl producing self-assembled nanoplex. The virtues of the ROPI-DS nanoplex are evidenced by the (i) enhanced lipophilicity, (ii) enhanced permeability, and (iii) controlled drug release as compared to those addressed by the bulk ROPI HCl, validating electrostatic complexation as a rapid, aqueous-based, and energy-efficient process. Nasal administration could have further implications in reduction of dose and the dose-related adverse effects. The potential of the nanoplex formulation as a novel nanocarrier material must be raised with regard to brain pharmacokinetics and neurotoxicity studies in order to transmit it to the clinical studies, so after it could extend to the pharmaceutical market.
Funding information
The authors are grateful to University Grants Commission (UGC, New Delhi, India) for providing financial assistance to C.V. Pardeshi under the title “Development of Novel Nanocarriers for Nose to Brain Drug Delivery” (Grant number: 47-832/13-WRO).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Chalikwar SS, Mene BS, Pardeshi CV, Belgamwar VS, Surana SJ. 2013. Self-assembled, chitosan grafted PLGA nanoparticles for intranasal delivery: design, development and ex vivo characterization. Polym Plast Technol Eng. 52:368–380.
- Cheow WS, Hadinoto K. 2011. Factors affecting drug encapsulation and stability of lipid-polymer hybrid nanoparticles. Colloids Surf B Biointerfaces. 85:214–220.
- Cheow WS, Hadinoto K. 2012. Self-assembled amorphous drug-polyelectrolyte nanoparticle complex with enhanced dissolution rate and saturation solubility. J Colloid Interface Sci. 367:518–526.
- Ding D, Li K, Zhu Z, Pu KY, Hu Y, Jiang X, Liu B. 2011. Conjugated polyelectrolyte-cisplatin complex nanoparticles for simultaneous in vivo imaging and drug tracking. Nanoscale. 3:1997–2002.
- Ehtezazi T, Govender T, Stolnik S. 2000. Hydrogen bonding and electrostatic interaction contributions to the interaction of a cationic drug with polyaspartic acid. Pharm Res. 17:871–877.
- Evans DA, Doman TN, Thorner DA, Bodkin MJ. 2007. 3D QSAR methods: phase and catalyst compared. J Chem Inf Model. 47:1248–1257.
- Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL. 2004. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. 47:1750–1759.
- Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MJ. 2001. Chitosan nanoparticles as delivery systems for doxorubicin. J Control Release. 73:255–267.
- Jena SK, Singh C, Dora CP, Suresh S. 2014. Development of tamoxifen-phospholipid complex: novel approach for improving solubility and bioavailability. Int J Pharm. 473:1–9.
- Joshi AS, Patel HS, Belgamwar VS, Agrawal A, Tekade AR. 2012. Solid lipid nanoparticles of Ondansetron HCl for intranasal delivery: development, optimization and evaluation. J Mater Sci Mater Med. 23:2163–2175.
- Karasulu E, Yavasoglu A, Evrensanal Z, Uyanikgil Y, Karasulu HY. 2008. Permeation studies and histological examination of sheep nasal mucosa following administration of different nasal formulations with or without absorption enhancers. Drug Deliv. 15:219–225.
- Kaur P, Garg T, Rath G, Goyal AK. 2015. In situ nasal gel drug delivery: a novel approach for brain targeting through the mucosal membrane. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi:10.3109/21691401.2015.1012260.
- Kulkarni AD, Bari DB, Surana SJ, Pardeshi CV. 2016a. In vitro, ex vivo and in vivo performance of chitosan-based spray-dried nasal mucoadhesive microspheres of diltiazem hydrochloride. J Drug Deliv Sci Tech. 31:108–117.
- Kulkarni AD, Patel HM, Surana SJ, Belgamwar VS, Pardeshi CV. 2016b. Brain-blood ratio: implications in brain drug delivery. Expert Opin Drug Deliv. 13:85–92.
- Kulkarni AD, Vanjari YH, Sancheti KH, Belgamwar VS, Surana SJ, Pardeshi CV. 2015. Nanotechnology-mediated nose to brain drug delivery for Parkinson’s disease: a mini review. J Drug Target. 23:775–788.
- Kumar M, Pandey RV, Patra KC, Jain SK, Soni ML, Dangi JS, Madan J. 2013. Evaluation of neuropeptide loaded trimethyl chitosan nanoparticles for nose to brain delivery. Int J Biol Macromol. 61:189–195.
- Lanz-Landazuri A, de Ilarduya AM, Garcia-Alvarez M, Munoz-Guerra S. 2014. Poly(b,L-malic acid)/doxorubicin ionic complex: a pH-dependent delivery system. React Funct Polym. 81:45–53.
- Li Y, Taulier N, Rauth AM, Wu XY. 2006. Screening of lipid carriers and characterization of drug-polymer-lipid interactions for the rational design of polymer-lipid hybrid nanoparticles (PLN). Pharm Res. 23:1877–1887.
- Mahaki H, Yazdi M, Chamani J, Saberi MR. 2013. Interaction between ropinirole hydrochloride and aspirin with human serum albumin as binary and ternary systems by multi-spectroscopic, molecular modeling and zeta potential. J Lumin. 134:758–771.
- Mudry B, Carrupt P, Guy RH, Delgado-Charro MB. 2007. Quantitative structure-permeation relationship for iontophoretic transport across the skin. J Control Release. 122:165–172.
- Pardeshi CV, Belgamwar VS. 2013. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: an excellent platform for brain targeting. Expert Opin Drug Deliv. 10:957–972.
- Pardeshi CV, Belgamwar VS. 2016. Controlled synthesis of N,N,N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int J Biol Macromol. 82:933–944.
- Pardeshi CV, Belgamwar VS, Tekade AR, Surana SJ. 2013a. Novel surface modified polymer-lipid hybrid nanoparticles as intranasal carriers for ropinirole hydrochloride: in vitro, ex vivo and in vivo pharmacodynamic evaluation. J Mater Sci Mater Med. 24:2101–2115.
- Pardeshi CV, Rajput PV, Belgamwar VS, Tekade AR. 2012. Formulation, optimization and evaluation of spray-dried mucoadhesive microspheres as intranasal carriers for Valsartan. J Microencapsul. 29:103–114.
- Pardeshi CV, Rajput PV, Belgamwar VS, Tekade AR, Surana SJ. 2013b. Novel surface modified solid lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: application of factorial design approach. Drug Deliv. 20:47–56.
- Patel S, Patel B, Patel Z, Pardeshi CV. 2012a. Nanocarriers as novel nose to brain drug delivery platforms. Indian J Novel Drug Deliv. 4:243–251.
- Patel S, Patel B, Patel Z, Pardeshi CV. 2012b. Nose to brain targeted drug delivery bypassing the blood-brain barrier: an overview. Drug Invent Today. 4:610–615.
- Pund S, Rasve G, Borade G. 2013. Ex vivo permeation characteristics of venlafaxine through sheep nasal mucosa. Eur J Pharm Sci. 48:195–201.
- Ranjan A, Pothayee N, Seleem M, Jain N, Sriranganathan N, Riffle J, Kasimanickam R. 2010. Drug delivery using novel nanoplexes against a Salmonella mouse infection model. J Nanopart Res. 12:905–914.
- Schatz C, Domard A, Viton C, Pichot C, Delair T. 2004. Versatile and efficient formation of colloids of biopolymer-based polyelectrolyte complexes. Biomacromolecules. 5:1882–1892.
- Tas C, Ozkan CK, Savaser A, Ozkan Y, Tasdemir U, Altunay H. 2006. Nasal absorption of metoclopramide from different Carbopol 981 based formulations: in vitro, ex vivo and in vivo evaluation. Eur J Pharm Biopharm. 64:246–254.
- Yousefpour P, Atyabi F, Farahani EV, Sakhtianchi R, Dinarvand R. 2011. Polyanionic carbohydrate doxorubicin–dextran nanocomplex as a delivery system for anticancer drugs: in vitro analysis and evaluations. Int J Nanomed. 6:1487–1496.
- Yuan H, Jiang SP, Du YZ, Miao J, Zhang XG, Hu FQ. 2009. Strategic approaches for improving entrapment of hydrophilic peptide drugs by lipid nanoparticles. Colloids Surf B Biointerfaces. 70:248–253.
- Zafar S, Negi LM, Verma AK, Kumar V, Tyagi A, Singh P, Iqbal Z, Talegaonkar S. 2014. Sterically stabilized polymeric nanoparticles with a combinatorial approach for multi drug resistant cancer: in vitro and in vivo investigations. Int J Pharm. 477:454–468.
- Zhong H, Tran LM, Jenna L. 2009. Induced-fit docking studies of the active and inactive states of protein tyrosine kinases. J Mol Graph Model. 28:336–346.