Abstract
We herein report a severe case of pyoderma gangrenosum occurring in a burn patient and discuss the possibility of occult complications of this pathological state in daily treatment.
Introduction
Pyoderma gangrenosum (PG) is a non-infectious neutrophilic dermatosis.[Citation1,Citation2] This condition is usually discussed in the dermatological field and is rarely encountered by burn surgeons. Typical PG represents with rapidly progressive, painful ulcers with an irregular, violet-colored and undermined border. The standard treatment is oral and topical corticosteroids and occasionally immunosuppressants.[Citation3,Citation4] However, obtaining a diagnosis of PG is not simple and largely depends on the ability to rule out other ulcer-forming diseases and/or the response of the lesion to diagnostic treatment.[Citation1,Citation2] Therefore, the disease is frequently diagnosed incorrectly, thus resulting in a delay in the proper diagnosis.[Citation5]
Subsequent to the very early descriptions,[Citation6,Citation7] it has been shown that PG is often associated with inflammatory bowel diseases, such as ulcerative colitis and Crohn’s disease, as well as rheumatoid arthritis and hematological disorders.[Citation1,Citation2] In addition to these systemic disorders, it is widely accepted that surgical wounds are associated with PG.[Citation8,Citation9] Colorectal stoma construction and breast surgery are the two major background factors for PG.[Citation10,Citation11] However, burn wounds have not been recognized to be an etiology of PG, and an association with PG in patients with burn wounds has been infrequently reported.[Citation12,Citation13] In this report, we describe a rare case of widespread PG occurring on grafted skin following burn treatment.
Case report
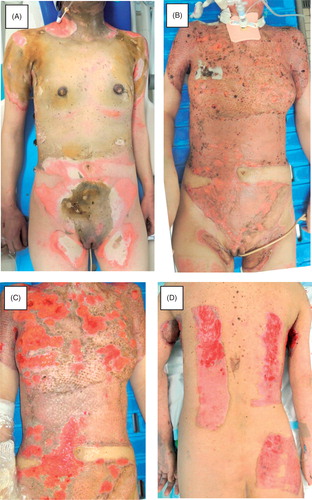
A 51-year-old female presented with a 35% third-degree burn and 4% deep dermal burn as a result of a fire in the beginning of February (). The patient did not have past history of inflammatory bowel diseases, hematological or rheumatic disorders. The patient was immediately admitted to the burn unit. She received debridement followed by split-thickness skin grafts, an artificial dermal graft, and autologous cultured epidermal sheet grafts five times during the next two months, during which most of the injured tissue was covered ().
Figure 1. Clinical course of the patient during the first admission. A: Initial clinical appearance. The anterior surface of the trunk exhibits a deep burn. B: Appearance after the first series of debridement and skin grafting. C and D: Multiple ulcers in the grafted skin and donor sites.
Approximately 80 days after the burn injury, multiple ulcers appeared on the trunk and right temporal area, and the lesions rapidly increased in both size and number (). The ulcers were located in regions once covered with split-thickness skin grafts together with autologous cultured epidermal sheet grafts. Finally, the patient received a sixth round of debridement and skin grafts on the 139th day. At that time, the white blood cell (WBC) count was 12,290/μl, with 73.5% neutrophils, and the CRP level was 10.32 mg/dl just prior to the operation. She was transferred to another hospital on the 158th day; however, multiple ulcers rapidly recurred in the grafted skin and donor sites. Therefore, she was readmitted to our hospital 17 days after the first discharge.
Upon an examination, many punched-out ulcers were scattered along the trunk and donor sites on the back and thighs (), and some had fused to form irregularly shaped lesions (). The lesions first presented as pustules and then became covered by crust, with ulcers finally appearing under the crust (, –). A bacterial culture examination revealed methicillin-resistant Staphylococcus aureus in the ulcers, then, multidrug-resistant Pseudomonas aeruginosa followed. Biopsy specimens demonstrated the findings of an ulcer with heavy neutrophil infiltration devoid of any evident vasculitis (). Blood sampling and immunofluorescent analyses of frozen biopsy specimens showed no autoantibodies against the basement membrane area. Based on these clinical and laboratory findings, a diagnosis of PG was made; no underlying diseases were identified according to internal examinations. Considering from clinical appearance, the onset of PG was thought to have been around the 80th day after the burn.
Figure 2. Clinical course after the second admission. A and B: Initial appearance on the second admission. Multiple recurrent ulcers are scattered in the grafted skin (A) and donor sites (B). C: Close-up view of the recurrent ulcers. D: Progression of the lesions. The lesions started as pustules (1), which then transformed to form a crust (2). When the crust was removed, a fresh ulcer appeared (3). E: Recent appearance of the patient after treatment with oral corticosteroids. The ulcers were restricted to limited areas; no ulcers are seen in this field.
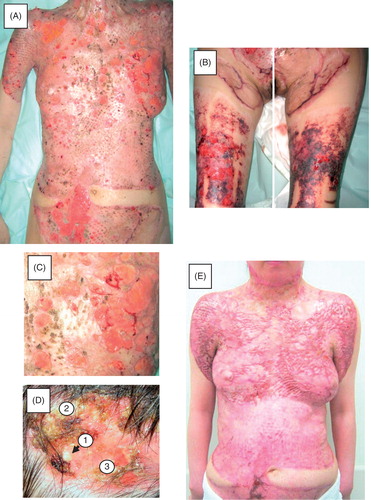
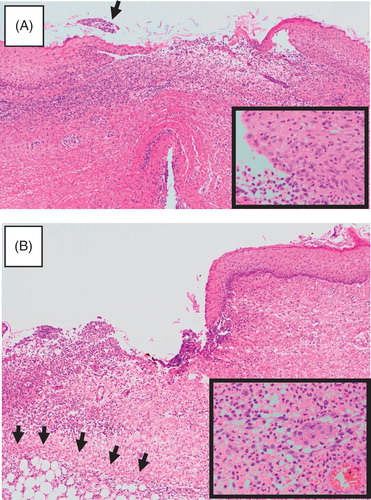
Figure 3. Histological findings of a recurrent ulcer. A: Early lesion. A pustule was biopsied, although it broke during handling. Heavy infiltration is seen in the upper dermis. A crust is already noted (arrow). Inlet: A close-up view of the epidermis at the periphery of the pustule, showing many neutrophils. B: Advanced lesion. The periphery of an ulcer was biopsied. Note the sharply demarcated border and heavy infiltration that extends to a part of the fat layer (arrows). Fibrosis is already seen on the right side. Inlet: A close-up view of the infiltration, showing neutrophils to predominate.
First, we applied a topical corticosteroid, difluprednate, to some of the ulcers, and they became dry. Then, a daily dose of 50 mg of prednisolone was administered, and the amount of exudate greatly decreased and the size of the ulcers began to decrease. The dose of prednisolone was tapered by 5 mg every a few weeks, and most of the ulcers healed by 46 days after the commencement of the oral corticosteroid therapy. The patient is currently under follow-up at our outpatient clinic, with residual small-sized ulcers restricted to limited areas ().
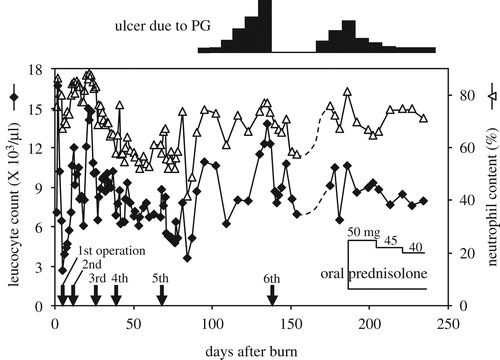
The clinical course of this case is summarized in . After the fourth operation, both the WBC counts and neutrophil contents became stable at around 7000/μl and 50%, respectively. After approximately the 80th day, the neutrophil content showed steady elevation during the presence of the PG ulcers, while WBC counts did not. The elevation of the neutrophil content reflected the appearance of the ulcers better than the WBC counts: it decreased once after the sixth operation but subsequently increased, coinciding with the second episode of ulcers. After the initiation of oral prednisolone, the WBC count and the neutrophil content became stable.
Discussion
Typical ulcers in PG are characterized by a punched-out appearance with a violet-colored edge[Citation1]; however, it is quite common for PG-associating ulcers to exhibit a non-specific appearance, and the diagnosis of PG must be based on the exclusion of other ulcer-forming diseases. For this reason, obtaining a diagnosis of PG remains difficult if the burn surgeon does not consider the possibility of the disease. In general, the ulcers in PG are believed to be aggravated by surgery.[Citation14] In the current case, we fortunately obtained a fair result after the operation for the first episode of PG; however, recurrence of the ulcers occurred in the right upper chest, anterior chest, left breast, and the right groin after re-debridement and re-grafting (compare and ). If we had made a diagnosis of PG earlier, reoperation would have been considered after achieving control of the ulcers with oral corticosteroids. This observation indicates that it is important to obtain a proper diagnosis of PG.
Pyoderma gangrenosum is characterized by the presence of excessive neutrophilic infiltration and is classified as a neutrophilic dermatosis.[Citation2] Abnormal activation of neutrophils is a key event in the neutrophilic dermatosis,[Citation15] and a systemic as well as local over- or sustained expression of interleukin-8 (IL-8) is thought to be a candidate cause of the disease.[Citation16] However, because the overproduction of IL-8 is not always constitutive, the contribution of interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) may be attributed to the actions as upstream players of IL-8 abnormalities.[Citation17]
Pyoderma gangrenosum has been described to be associated with leukocytosis[Citation1,Citation3]; however, the relationship between the leukocytosis and ulcer progression is not shown. This time, we could observe WBC trend in association with the onset and progression of the PG ulcers. In the current case, elevation of WBC count roughly coincided with episodes of skin ulcers; moreover, we found that the neutrophil content showed a better correlation with the presence of the ulcers than the WBC count alone. On the other hand, this parameter remained elevated thereafter, most likely because of the large amount of oral corticosteroids.[Citation18] Because we could not find previous reports that mentioned relationships between the WBC counts/neotrophil content and the ulcers, it is not clear if the observed correlation between these parameters is a general finding in widespread PG patients or restricted only to our case. This point remains to be solved by collecting relevant clinical data in the future.
It is widely known that PG occurs after local injuries.[Citation10] Among various types of injuries, breast surgery is the most known cause of PG,[Citation10,Citation19] whereas the onset of PG due to burn injuries has rarely been reported.[Citation12,Citation13] Interestingly, the serum IL-8 levels are similar in burn patients and subjects who undergo breast operation, rising one day after injury in both conditions and staying at a high level for some time.[Citation20,Citation21] These findings indicate that these pathological states have a similar background.
Skin surgeons sometimes encounter cases of small ulcers or even pustules in burn scars, grafted skin or donor sites, and the topical corticosteroids occasionally bring successful results. Among the skin grafts, sometimes we see one or a few small superficial ulcers on split-thickness skin grafts, but we have not seen the ulcers in region covered with artificial dermis. Although the precise reason is not clear, we suppose that because the split-thickness skin graft is very commonly used and is usually wide, such troubles are noticed in this type of the skin graft. Concerning autologous cultured epidermal sheet grafts, although they were used together with split-thickness skin grafts, the present case would be the first case that we see the ulcers. Based on our experience, we imagine that milder cases of PG occur more frequently than we expect. It is possible that these ulcers and pustules indicate milder PG; however, because skin biopsies have been hardly done and diagnostic findings are not sufficient in these cases, it would be speculative. In order to establish the possible entity of PG, accumulation of clinical and pathological information is required.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cox NH, Jorizzo JL, Bourke JF. Vasculitis and neutrophilic vascular reactions. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s textbook of dermatology. Vol. 3, 8th edn. Oxford: Blackwell Science; 2010. p. 64–73.
- Prat L, Bouaziz JD, Wallach D, et al. Neutrophilic dermatoses as systemic diseases. Clin Dermatol. 2014;32:376–388.
- Brooklyn T, Dunnill G, Probert C. Diagnosis and treatment of pyoderma gangrenosum. Brit Med J. 2006;333:181–184.
- Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525–531.
- Napoli B, D’Arpa N, Conte F. Pyoderma gangrenosum and full-thickness burns: is there a problem of differential diagnosis? Ann Burns Fire Disasters. 2006;19:71–73.
- Brocq L. Nouvelle contribution a l'étude du phagédénisme géométrique. Ann Dermatol Syphiligr. 1916;6:1–39.
- Brunsting LA, Goeckerman WH, O’Leary PA. Pyoderma gangrenosum: clinical and experimental observation in five cases occurring in adults. Arch Dermatol. 1930;22:655–680.
- Ouazzani A, Berthe JV, de Fontaine S. Post-surgical pyoderma gangrenosum: a clinical entity. Acta Chir Belg. 2007;107:424–428.
- Baldea A, Gamelli RL. Postoperative pyoderma gangrenosum after elective abdominoplasty: a case report and review of the literature. J Burn Care Res. 2010;31:959–963.
- MacKenzie D, Moiemen N, Frame JD. Pyoderma gangrenosum following breast reconstruction. Br Plast Surg. 2000;53:441–443.
- Poritz LS, Lebo MA, Bobb AD, et al. Management of peristomal pyoderma gangrenosum. J Am Coll Surg. 2008;206:311–315.
- West CC, Morritt AN, Ralston DR. Pyoderma gangrenosum in burns: a report of 3 cases and review of the literature. Burns. 2010;36:56–59.
- Kalu PU, Greg Williams. Scalding as an unusual cause of pyoderma gangrenosum. Burns. 2007;33:105–108.
- Barańska-Rybak W, Kakol M, Naesstrom M, et al. A retrospective study of 12 cases of pyoderma gangrenosum: why we should avoid surgical intervention and what therapy to apply. Am Surg. 2011;77:1644–1649.
- Alavi A, Sajic D, Cerci FB, et al. Neutrophilic dermatoses: an update. Am J Clin Dermatol. 2014;15:413–423.
- Schinkel C, Zimmer S, Kremer JP, et al. Comparative analysis of transcription and protein release of the inflammatory cytokines interleukin-1 beta (IL-1 beta) and interleukin-8 (IL-8) following major burn and mechanical trauma. Shock. 1995;4:241–246.
- Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217.
- Schimmer BP, Funder JW. ACTH, adrenal steroids, and pharmacology of the adrenal cortex. In: Brunton LL, Chabner BA, Knollman BC, editors. Goodman and Gilman's The pharmacological basis of therapeutics. New York: McGraw Hill Medical; 2011. p. 1209–1236.
- Liaqat M, Elsensohn AN, Hansen CD, et al. Acute postoperative pyoderma gangrenosum case and review of literature identifying chest wall predominance and no recurrence following skin grafts. J Am Acad Dermatol. 2014;71:e145–e146.
- Kim HS, Kim JH, Yim H, Kim D. Changes in the levels of interleukins 6, 8, and 10, tumor necrosis factor alpha, and granulocyte-colony stimulating factor in Korean burn patients: relation to burn size and postburn time. Ann Lab Med. 2012; 32:339–344.
- Schmidt A, Bengtsson A, Tylman M, Blomqvist L. Pro-inflammatory cytokines in elective flap surgery. J Surg Res. 2007;137:117–121.