Abstract
A suspicious breast mass was intra-operatively found in a female patient previously operated for breast augmentation with the Poly Implant Prothèse (PIP) implants. The neoplasm was verified as xanthoma and an asymptomatic microrupture of PIP was also detected. This report discusses possible association of breast xanthoma with PIP implant rupture.
Keywords:
Introduction
Poly Implant Prothèse (PIP) company is known to manufacture breast implants using unapproved industrial grade silicone and their products have been banned after numerous studies reporting an increased risk of complications such as implant rupture and gel leakage.[Citation1–4] Over 400,000 PIP implants have been utilized for breast augmentation worldwide, and approximately 10,000 have been sold in Ukraine.
Overall, various complications have been reported in patients with ruptured breast implants or after free biomaterial injections, which also include, but not limited to neoplasms as breast carcinoma, mesenchymal tumors, and anaplastic large cell lymphoma (ALCL).[Citation5–10] However, little is known about possible biological effect of industrial grade silicone in individuals with ruptured PIP implants.[Citation11]
Xanthoma is a benign lesion that is frequently diagnosed in skin, kidney or gallbladder, but rare in breast.[Citation12,Citation13] It is histopathologically characterized by foamy histiocytes and lymphoplasmatic cellular infiltrate. Xanthoma is rarely diagnosed in patients with breast implants and is mainly described in case reports.[Citation12,Citation14]
We report a clinical case of a patient previously operated on for breast augmentation, who opted for implant replacement and was diagnosed with a unilateral breast xanthoma associated with PIP implant rupture.
Case report
A 30-year-old female was seen by the senior plastic surgeon (VK) in January 2015 after bilateral aesthetic breast augmentation in 2007. The patient was concerned about her PIP breast implants and knowing possible risks of the devices opted for their removal and replacement with other authorized breast implants.
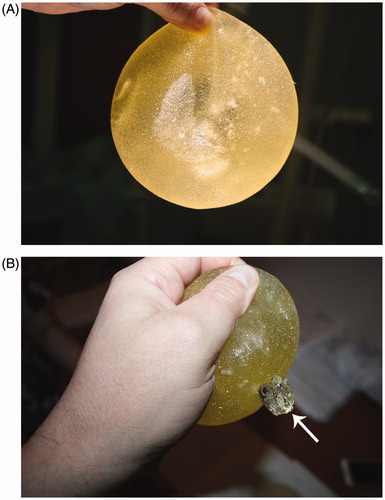
Preoperative clinical assessment revealed subglandular breast implants with no signs of capsular contracture or other implant-related concerns. The inframammary scars healed well and were inconspicuous. The woman was otherwise healthy; non-obese (body mass index 20 kg/m2) and non-smoker. The patient was scheduled for the desired procedure under general anesthesia. Both implants were removed via previous inframammary incisions. We started with the right side where no noteworthy changes have been seen, i.e. no paraprosthetic fluid, implant was intact with no irregularities, and the capsule looked normal. During the removal of the left implant, however, we received about 50 ml of milky seroma fluid. Inspection of the left device revealed no signs of macroscopic rupture. Further, some visible air bubbles within the implant and small “fatty” smears on the gloves were discovered, indicating possible subtle ruptures of the implant’s shell. To test this hypothesis, both explanted devices were subjected to manual compression. This maneuver resulted in rupture of the left implant, whereas the right one remained intact ().
Figure 1. Illustration of the ruptured PIP implant. (A) A yellow implant without signs of rupture and with visible air bubbles inside indicating possible microruptures of the implant shell. (B) Ruptured PIP device with leakage of the silicone gel after manual compression
Further revision of the left implant pocket revealed a yellowish well-delimited mass 4 × 5 cm in the central lower part of the breast over the fascia of the pectoral major muscle. A malignant neoplasm was suspected and an excisional biopsy with histopathological and immunohistochemical (IHC) analyses were performed. We did not proceed with concurrent breast implantation and prioritized rapid tumor verification, evaluation and treatment.
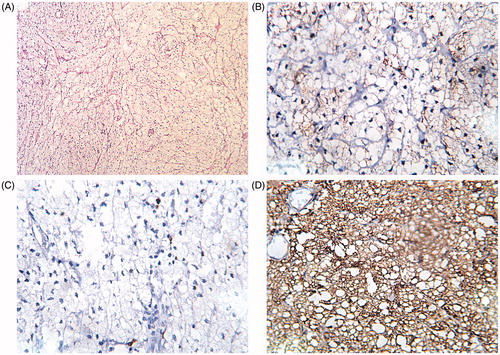
The histopathology of the specimen revealed xanthoma with no evidence of malignant neoplasm (). IHC was performed to discriminate the case from soft tissue sarcomas by using antibodies against proteins CD68 (clone KP1) and S100 (clone 4C4.9); proliferation was evaluated by analyses of Ki-67 (clone MIB-1) expression. Analyses of IHC for histiocytic marker CD68 revealed a strong positive expression in 100% of xanthoma cells, a weak staining of S100 in <10% of xanthoma cells (considered as negative). Several positively stained cells with MIB-1 indicated the absence of sarcoma and low proliferation, which supported the histopathological diagnosis of benign lesion, i.e. xanthoma ().
Figure 2. Illustration of histopathological and immunohistochemical (IHC) findings. (A) Histopathological features of H&E stained xanthoma tissue showing foamy histiocytes with multinucleate giant cells (magnification ×200). (B) Negative expression of protein S100 by IHC in xanthoma (magnification ×400). (C) Illustration of several xanthoma cells with positive nuclear expression of MIB-1 indicating low proliferation (magnification ×400). (D) Strong cytoplasmatic expression of CD68 in xanthoma (magnification ×400)
Both breasts were evaluated by ultrasonography, showing some signs of remaining xanthoma tissue but no evidence of other pathological findings postoperatively (). Considering the clinical findings, and to perform a secondary reconstruction with the new breast implants creating new submuscular pockets ().
Figure 3. Ultrasonography of the breast with the ruptured PIP implant after implant removal. The remnant xanthoma tissue is visualized as 1 mm hypoechogenic mass (marked by white crosses)
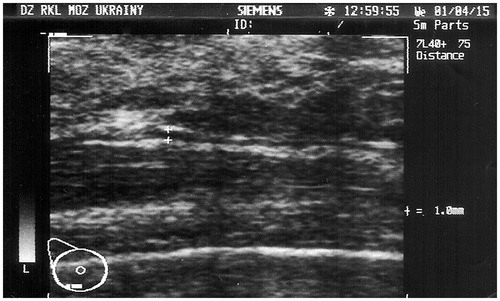
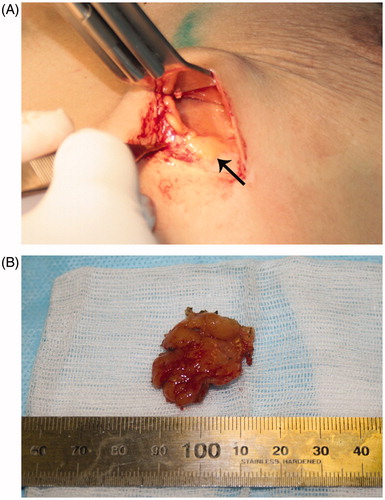
Figure 4. Surgical findings. (A) Remnant xanthoma tissue was identified as 2 × 2 cm yellowish neoplasm in the lower part of the left breast. (B) Surgically removed specimen of xanthoma.
Blood biochemistry was performed to evaluate the post-operative status of certain compounds relating to lipid methabolism. The analyses of clinical biochemistry revealed that majority of studied parameters were within the normal ranges: triglycerides 0.74 mmol/L (normal range: <1.7), cholesterol 4.24 mmol/L (normal range: <5), high-density lipoprotein cholesterol 2.04 mmol/L (normal range: >1.2), low-density lipoprotein cholesterol 1.9 mmol/L (normal range: <3), very low-density lipoprotein cholesterol 0.34 mmol/L (normal range: 0.26–1.04), apolipoprotein A1 1.33 g/L (normal range: 0.76–2.1), apolipoprotein B 0.58 g/L (normal range: 0.46–1.42), lipoprotein (a) 9.59 mg/dL (normal range: <30).
Considering the findings and patient’s desire to reconstruct the breast, we proceeded with the excision of the remained xanthoma tissue and the delayed secondary breast augmentation was scheduled (). Thus, a total capsulectomy was performed and new implants (450 ml, round shaped, Polytech Health & Aesthetics, Germany) were placed in the submuscular position. The 6-months follow up revealed good results with no post-operative complications clinically or on ultrasound.
As part of the routine assessment, health-related quality of life (HRQoL) was evaluated before surgery, after implant removal and 6 months after secondary breast augmentation. An EQ-5D-5L instrument with a visual analogue scale (VAS) was used to assess self-rated overall health status (http://www.euroqol.org/EuroQol Group, Netherlands) according to the previously published protocol.[Citation15] The patient reported no problems related to physical activity, but a problem in the anxiety/depression domain was detected after implants removal; this returned to normal after the breast implantation. Self-rated overall health status revealed a decrease from 50 VAS units preoperatively to 30 units after implants removal. However, 90 VAS units were reported 6 months after the placement of the new implants.
Discussion
In this study, we reported an unusual case of a ruptured PIP implant associated with breast xanthoma. This neoplasm is a rare lesion of the breast and an uncommon complication of PIP implant rupture. To the best of our knowledge, xanthoma is not included in the list of clinical findings in the current opinion of the Scientific Committee on Emerging and Newly Identified Health Risks of European Commission (SCENIHR, February, 2014).[Citation3] According to SCENIHR and other studies, commonly reported signs of the PIP implant rupture include breast pain, breast and axillary lymphadenopathy, and palpable breast mass associating with migration of silicone gel.[Citation3,Citation16] In the current case, we did not observe any of these signs, which might indicate a different clinical course for patients with xanthoma and “micro” ruptured PIP, also supported by the findings from other studies.[Citation11,Citation12]
Another differential diagnosis to consider in patients with a breast mass following breast augmentation is anaplastic large cell lymphoma (ALCL). Although ALCL is a rare breast malignancy, it is reported as an implant-associated malignant neoplasm developing due to a chronic periprosthetic inflammation.[(Citation7] However, no clear association between PIP implants and ALCL has been yet confirmed according to SCENIHR or other investigations.[Citation1,Citation4,Citation11] It is also worth to mention, that before the surgery we were unaware of any seroma in the breast and did not perform any fluid aspiration and cytological evaluation. However, an excisional biopsy of the tumor with histhopatological assessment had been performed with following immunohistochemistry, which was considered sufficient to describe the morphological patterns of the tumor and rule out ALCL.
Apart from ALCL, various mesenchymal tumors (i.e. fibromatosis, lipomatous tumors or sarcomas) are known to be associated with breast silicone implants.[Citation10] These neoplasms are infrequent and differential diagnosis could be established using histopathological analyses along with IHC staining for S100 protein. Although malignant mesenchymal tumors were not reported among patients with ruptured PIP implants, we performed IHC analyses of S100 expression in our patient, which is in agreement with other studies.[Citation10,Citation11]
Previously, xanthogranulomatous inflammatory processes in breast were demonstrated in patients with implant rupture, breast cancer patient, or individuals without history of breast lesions. [Citation12,Citation14,Citation16] Similarly to Hernanz et al. we did not observe any specific symptoms of xanthoma, unlike Hussain et al. who reported an abnormal mass in a breast cancer patient, possibly indicating different mechanisms of xanthoma pathogenesis.[Citation12,Citation14,Citation16,Citation17]
An association between xanthoma and foreign body reaction is a rare observation and was previously demonstrated in several case reports. According to the data from these studies xanthoma was associated with: oophoritis as a reaction to talcum powder, with cystitis secondary to sutures, as well as with cholecystitis as a reaction to gallstones.[Citation13,Citation18,Citation19] Literature search revealed no evidence of association between breast xanthoma and foreign body reaction.
In our study, the microrupture of the PIP device was suspected only after removal of the left implant. The risk of rupture is known and it was previously shown in a study on elastomeric properties of PIP implants that detected a random reduction of thickness and defects of microscopical structures of the shell.[Citation20] Moreover, the hypothesis of microscopical structural defects is supported by the presence of the yellow color of the ruptured implant in our case. This may arise due to absorption of the fat-soluble compounds due to a high permeability of the PIP shell.[Citation1] Similarly to other studies we observed some fluid effusion during implant removal as a result of synovial metaplasia associating with implant rupture.[Citation1,Citation4,Citation21,Citation22]
Finally, we also identified a negative impact of implants removal and neoplasm identification on HRQoL; levels of anxiety and depression increased postoperatively, but improved 6 months after the secondary breast augmentation and tumor related reassurance.
To summarize, we report a case of breast xanthoma following breast augmentation associated with an asymptomatic microrupture of the PIP implant. Current study supports findings of other papers investigating larger cohorts of patients with PIP implants; it also raises awareness of possible risks among individuals without clinical symptoms of implant rupture and leakage of the silicone gel. In asymptomatic patients who would like to keep their PIP implants we recommend more intensive clinical surveillance with the breast ultrasound screening.
Disclosure statement
Authors have nothing to disclose.
References
- Oulharj S, Pauchot J, Tropet Y. PIP breast implant removal: a study of 828 cases. J Plast Reconstr Aesthet Surg. 2014;67:302–307.
- Moschetta M, Telegrafo M, Cornacchia I, et al. PIP breast implants: rupture rate and correlation with breast cancer. G Chir. 2014;35:274–278.
- Epstein M, Emri I, Hartemann P, et al. The safety of Poly Implant Prothèse (PIP) silicone breast implants update of the opinion of february 2012 May, 2014 ed: Luxembourg: Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) of European Commission; 2014.
- Chummun S, McLean NR. Poly implant prothèse (PIP) breast implants: our experience. Surgeon. 2013;11:241–245.
- Unukovych D, Khrapach V, Wickman M, et al. Polyacrylamide gel injections for breast augmentation: management of complications in 106 patients, a multicenter study. World J Surg. 2012;36:695–701.
- Pinchuk V, Tymofii O, Tkach O, et al. Implant ruptures after augmentation mammoplasty. Aesthetic Plast Surg. 2013;37:60–67.
- Kellogg BC, Hiro ME, Payne WG. Implant-associated anaplastic large cell lymphoma: beyond breast prostheses. Ann Plast Surg. 2014;73:461–464.
- Nakahori R, Takahashi R, Akashi M, et al. Breast carcinoma originating from a silicone granuloma: a case report. World J Surg Oncol. 2015;13:72.
- Santanelli di Pompeo F, Laporta R, Sorotos M, et al. Breast implant-associated anaplastic large cell lymphoma: proposal for a monitoring protocol. Plast Reconstr Surg. 2015;136:144e–151e.
- Balzer BL, Weiss SW. Do biomaterials cause implant-associated mesenchymal tumors of the breast? Analysis of 8 new cases and review of the literature. Hum Pathol. 2009;40:1564–1570.
- Dieterich M, Stubert J, Stachs A, et al. Ruptured poly-implant protheses breast implant after aesthetic breast augmentation: diagnosis, case management, and histologic evaluation. Aesthetic Plast Surg. 2013;37:91–94.
- Hernanz F, Baeza S, Serna E, et al. Xanthogranulomatous capsulitis mimicking a polypoid neoplasm disease: an unusual presentation of ruptured Poly Implant Prothèse (PIP) breast implant. Eur J Plas Surg. 2013;36:797–800.
- Yabanoglu H, Aydogan C, Karakayali F, et al. Diagnosis and treatment of xanthogranulomatous cholecystitis. Eur Rev Med Pharmacol Sci. 2014;18:1170–1175.
- Hussain T, Elahi B, Long E, et al. Xanthogranulomatous inflammation involving latissimus dorsi donor site and implant breast reconstruction: case report and literature review. World J Surg Oncol. 2012;10:166.
- Tsymbaliuk I, Unukovych D, Shvets N, et al. Cardiovascular complications secondary to Graves' disease: a prospective study from Ukraine. PLoS One. 2015;10:e0122388.
- Hwang SH, Son EJ, Oh KK, et al. Bilateral xanthogranuloma of the breast: radiologic findings and pathologic correlation. J Ultrasound Med. 2007;26:535–537.
- Janssen D, Fölster-Holst R, Harms D, et al. Clonality in juvenile xanthogranuloma. Am J Surg Pathol. 2007;31:812–813.
- Chouairy CJ, Hajal EA, Nehme YA. Xanthogranulomatous oophoritis secondary to talcum powder. Case report and review of the literature. J Med Liban. 2012;60(3):169–72.
- Chung MK, Seol MY, Cho WY, et al. Xanthogranulomatous cystitis associated with suture material. J Urol. 1998;159:981–982.
- Beretta G, Panseri S, Manzo A, et al. Analytical investigations on elastomeric shells of new Poly Implant Prothèse (PIP) breast and from sixteen cases of surgical explantation. J Pharm Biomed Anal. 2014;98:144–152.
- Krishnanandan S, Abbassian A, Sharma AK, et al. Capsular synovial metaplasia mimicking silicone leak of a breast prosthesis: a case report. J Med Case Rep. 2008;2:277.
- Kolios L, Hirche C, Spiethoff A, et al. Complications of Poly Implant Prothèse breast implants: the current discussion. Expert Rev Med Devices. 2013;10:167–170.
