Abstract
Objective: The aims of this study were to investigate the release of element from, and the biological response in vitro to, cobalt–chromium alloys and other base–metal alloys used for the fabrication of metal-ceramic restorations.
Material and methods: Eighteen different alloys were investigated. Nine cobalt–chromium alloys, three nickel–chromium alloys, two cobalt–chromium–iron alloys, one palladium–silver alloy, one high-noble gold alloy, titanium grade II and one type III copper–aluminium alloy. Pure copper served as positive control. The specimens were prepared according to the ISO standards for biological and corrosion testing. Passive leaching of elements was measured by using Inductively Coupled Plasma – Mass Spectrometry (ICP-MS) after incubation in cell culture media, MEM, for 3 days. Corrosion testing was carried out in 0.9% sodium chloride (NaCl) and 1% lactic acid for 7 days, and the element release was measured by Inductively Coupled Plasma – Optical Emission Spectroscopy (ICP-OES). The biological response from the extract solutions was measured though MTT cytotoxicity testing and the Hen's egg test-chorio-allantoic membrane (HET-CAM) technique for irritationt.
Results: The corrosion test showed similar element release from base-metal alloys compared to noble alloys such as gold. Apart from the high-copper alloy, all alloys expressed low element release in the immersion test, no cytotoxic effect in the MTT test, and were rated non-irritant in the HET-CAM test.
Conclusions: Minimal biological response was observed for all the alloys tested, with the exception of the high-copper alloy.
Introduction
The biocompatibility of dental materials used for fixed prosthodontic restorations is an ongoing discussion among dental practitioners. The materials chosen have to sustain the challenging environment of the oral cavity in the long-term and also meet the patient’s aesthetic demands. The types of alloys and the testing methods employed have shifted over the decades, but the goal remains constant; to find materials that combine function and aesthetics without harming the surrounding tissues or the patient.[Citation1] Cobalt–chromium alloys have recently gained a lot of interest in Sweden. These alloys have been used, mainly for removable partial dentures, for many decades,[Citation2] but have not been widely used for fixed prosthodontics. The advantage of these alloys compared to gold alloys is their low-cost, high-strength and high-elastic modulus that makes them useful in clinical cases where space for the restoration is limited, as very thin frameworks can be made. High-fusing porcelain can be used and, compared to titanium, the aesthetics are improved. The alloys also have good corrosion resistance due to protective chromium oxide layer. However, the amount of chromium should not exceed 30% as this would make the alloy brittle and reduce its castability.[Citation3] The disadvantages are that, unlike titanium, both cobalt and chromium are known allergens[Citation4] and therefore the increasing use of these metals for fixed restorations is possibly harmful. Dental technicians also experience increased dust-related health risk when grinding and polishing these alloys.[Citation5] The hardness of these alloys also makes the processing difficult and time-consuming.[Citation3]
In July 2008 the Swedish Ministry of Health and Social affairs developed a revised dental insurance system, protecting against high costs for patients in need of extensive dental treatment. It was decided not to reimburse for the gold alloys in prosthetic restorations, making prosthetic treatment using gold-based alloys very expensive for the patient. The cost of base-metal and titanium alloys for fixed prosthodontics is however reimbursed and these alloys therefore became the first choice for the restorations. Titanium properties have been documented both in vitro and in vivo and its advantages and disadvantages are well known.[Citation3] The clinical documentation of cobalt–chromium for fixed prosthetic restorations is scarce although the alloy is now frequently used. Eliasson et al. published a retrospective study in 2007 where no adverse reactions to the material were reported.[Citation6] Recently a number of case reports on allergic reactions to base metals have been published.[Citation7] However, in a 5-year retrospective study of cobalt–chromium-based fixed dental prostheses no adverse biological reactions were reported.[Citation8] Corrosion and biocompatibility are also of increasing concern as patients today are more aware of the potential disadvantages of metal restorations in the oral cavity. Altogether there is a need for more thorough evaluation of the alloys in use. Corrosion is the major reason for the release of elements and is increased in conditions of low pH which are often experienced in the oral cavity. Previous studies have also shown that the leakage of metal elements is higher from base metal alloys than from noble alloys and titanium.[Citation9] In order to evaluate the biocompatibility of dental alloys, in vitro studies can be performed which attempt to imitate the clinical conditions as far as possible in order to be able to anticipate possible negative effects. The aims of this study were to investigate the release of elements from, and the biological response in vitro to cobalt–chromium alloys and other base metal alloys used for the fabrication of metal-ceramic restorations such as single crowns and fixed partial dentures.
Material and methods
Samples of commercially available dental alloys used for metal-ceramic applications were received from the manufacturers (). Pure copper served as positive control. All specimens but six were cylindrical in shape when received from the manufacturers. The titanium, IPS d.SIGN 30 and copper samples were disc shaped, and the U-gold, Albabond A and NPG alloys were supplied as plates. The specimens were kept in their original shapes during the experiments as the surfaces were suitable for grinding and measuring in all cases.
Table 1. Trade name, manufacturer, batch number and composition of the investigated alloys given by the manufacturers.
Preparation of elution extracts
Minimal essential medium
The specimens were sandblasted with 110 μm alumina oxide, rinsed in water and wet ground with 1200 SiC paper (Struers A/S, Ballerup, Denmark) and rinsed again. Fresh abrasive paper was used for each alloy. The specimens were then ultrasonically cleaned in 70% ethanol for 5 minutes and dried in water and oil-free air. The specimens were incubated in cell culture medium
Minimal essential medium (MEM), without FBS, is used as the extraction medium. The volume of media used was calculated from the surface area of each specimen, 1.25 cm2/ml according to ISO 10993-12.[Citation8] MEM without specimens served as negative control. The vials were sealed and placed in an agitated water bath at 37 °C for 24 hours. The specimens were removed from the extraction media on the second day. The media were sterilized and filtered in Syringe filters 0.22 (TPP. Switzerland) and 950 μl was added to 50 μl of FBS (Fetal Bovine Serum, Sigma, St Louis, USA).
Lactic acid
For five of the alloys (Wirobond C, Albabond A, Wirobond LFC, Heraenium S and NPG) an additional MTT assay was performed using lactic acid as extraction media. The alloys were chosen to represent the various groups of metals included in the study: Co–Cr, Ag–Pd, Co–Cr–Fe, Ni–Cr and a high-copper alloy. The specimens were heat-treated, sandblasted, polished and ultrasonically cleaned before being incubated in a solution of lactic acid (pH 2.3) for 7 days, all according to the procedure described above. After the final day, the specimens were removed and the media neutralized with MEM and the procedure continued according to the MTT manual.[Citation10] In the following discussion, this test will be referred to as the corrosion MTT.
Characterization of elution extracts
In order to determine the element leakage from the dental alloys a method for quantitative determination was developed.[Citation9] Eighteen specimens in 2–6 parallel (altogether 81 samples) were sandblasted, polished and cleaned before incubation in minimum essential cell culture medium (MEM) at 37 °C for 72 hours as described above. Teflon cleaned in HNO3 was used as control. The solutions were then analyzed with ICP-MS. An inter-laboratory comparison was made between five different laboratories to evaluate the methods.[Citation9]
Preparations of corrosion extracts
Two samples of each specimen were sandblasted in 110 μm alumina oxide, Al2O3. Heat treatment of the specimens was performed according to the manufacturer's instructions, between 790 and 980 °C, and bench cooled (Jelenko Flagship VPC, Sekisui Chemical Co., Japan). The specimens were polished, wet ground with 320 and 1200 grit abrasive paper (Struers, Denmark), ultrasonically cleaned in 70% ethanol for 5 minutes and dried in water and oil free air. According to the ISO standard 10271:2001 [Citation11] borosilicate glass containers were used for incubation of the specimens in a solution of lactic acid (C3H6O3) and NaCl. The volume of media used was calculated from the surface area of each specimen (1.0 ml/cm2). The corrosion test was performed twice and the pH for the solutions was prepared at 2.32 and 2.33 according to ISO standard 22674:2006.[Citation12] The incubations were performed at 37 °C in CO2 for 7 days. Pure copper served as positive control.
Characterization of corrosion extracts
On day seven, the specimens were removed and the extraction media together with a blank control were sent for the analysis of metal elements (Sheffield Analytical Services, Sheffield, UK). Each solution was analyzed for metallic elements by ICP-OES.
Cell culture and MTT tests
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) a yellow tetrazole, is reduced to purple formazan in living cells to determine the level of mitochondrial activity when exposed to extracts of the test material. The method that was described by Edmondson [Citation13] is easy and reproducible, and has been performed in a number of studies to estimate cytotoxicity for various alloys.[Citation14,Citation15] Mouse fibroblasts L-929 (American Type Culture Collection CCL1) were cultured in minimum essential medium with Earle's salts (MEM) containing 2 mmol L−1 L-Glutamine (both PAA Laboratories GmbH, Pasching, Germany), supplemented with penicillin (100 U/ml)/streptomycin (100 μg/ml), (Lonza, Verviers, Belgium) and 5% FBS (Fetal Bovine Serum. Sigma, St Louis, MO). The cell culture was adjusted to 7.5 × 104 cells/ml and 200 μl of the cell suspension was seeded into wells of a 96 well plate and incubated for 24 hours at 37 °C in an air atmosphere containing 5% CO2 and 95% humidity. The cell culture medium in the wells was replaced by 100 μl of the elution extracts or corrosion extracts of five alloys (Wirobond C, Albabond A, Wirobond LFC, Heraenium S and NPG) after adjusting the pH by adding 10 M NaOH. Six parallel wells were used for each sample and the plates were incubated for 24 hours at 37 °C in 5% CO2 and 95% humidity. On the third day, the extract media was removed and 100 μl/well of MTT (Sigma, St Louis, USA) dissolved in phosphate buffered saline (Lonza, Verviers, Belgium) to a concentration of 0.5 mg/ml was added to each well. The plates were incubated for 1 h at 37 °C in 5% CO2 and 95% humidity. The media were removed and 100 μl DMSO (Dimethyl sulfoxide, Sigma, St Louis, MO) added to each well. The plates were kept at room temperature under agitation for 20 minutes and the spectrophotometric absorbance was measured at 540 nm using a Multiscan EX microplate reader (Labsystems, Helsinki, Finland). The mean value of the negative control was set at 100% and the absorbance values for the samples were calculated in relation to the control value. The MTT tests were performed three times for each alloy according to the method described by Edmondson el al.[Citation13]
The HET-CAM procedure
The Hen's egg test-chorio-allantoic membrane (HET-CAM) technique was performed to measure the irritation potential of the alloys.[Citation16] Extracts were prepared as described under the Section “Characterization of elution extracts". On the testing day, the specimens were removed and the pH of the extraction media was adjusted to 5.2–6.0 by adding 0.1 M NaOH. This was done in order to avoid potential negative effects on the chorio-allantoic membrane caused by the low pH of the test solutions. The solutions were tested immediately after preparation. The HET-CAM procedure has been found to be an acceptable alternative to in vivo tests and was performed according to Kalweit et al.[Citation16] with minor modifications. Fertilized eggs were purchased (Samvirkekylling, Våler, Norway) and placed in a rotating incubator in a humidified atmosphere at 37 °C until testing on day 9. The shell above the air cell of the eggs and the inner membrane were removed using forceps and the chorioallantoic membrane (CAM) was assessed. 300 μl of the test solution was applied directly onto the CAM which was then examined for 5 minutes by using a photo macroscope with illumination (M400, Wild, Heerbrugg, Switzerland). Each solution was tested three times and the experiment was repeated once. The irritation score of the CAM was measured by recording the haemorrhage, coagulation and vascular lysis for a period of up to 5 minutes, according to Kalweit et al.[Citation16] The results were transformed into an irritation score between 0 and 21 and the test solutions could be classified as non-irritant if they scored 0–0.9, slightly irritant if they scored 1–4.9, moderately irritant if they scored 5–8.9 and strongly irritant if they scored 9–21.
Results
Corrosion and elution tests
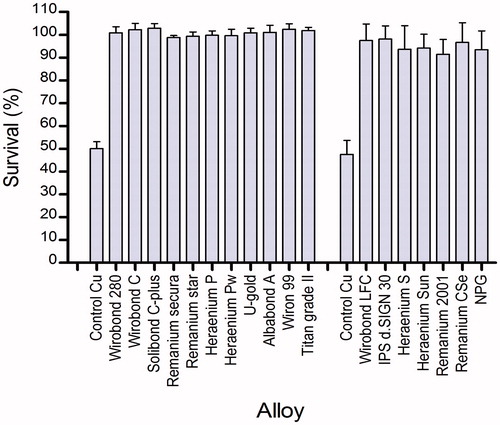
The results from the corrosion tests are presented in . The results show that all except two of the samples gave yields well below the limit of 200 μg/cm2 per 7 days suggested by the ISO 22674:2006.[Citation12] The NPG alloy and the copper control (tested twice) expressed results well above the limit, with values of 329 μg/cm2 per 7 days and 348 μg/cm2 per 7 days, respectively. The quantitative analysis with ICP-MS showed element leakage corresponding with the manufacturer's declaration of the compositions, and higher leakage of elements could be seen from the base-metal alloys compared to the noble alloys such as gold (). The copper control was not included in this test. The inter-laboratory comparison showed similar results between all five laboratories.[Citation9] In the results from the leakage studies in corrosion solution are compared with results from the MEM-extract test. Higher ion release could be seen from the copper-based alloy NPG in the corrosion test compared to elution test. A tendency towards higher ion release from the alloys no 2, 3, 14, 15 and 16 was also noticed ().
Table 2. The results from corrosion and immersion tests.
MTT (Cytotoxicity) assay and corrosion MTT
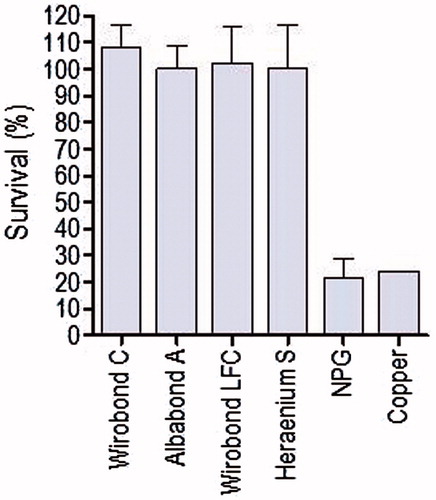
The elution extracts of tested alloys expressed no cytotoxic effects in the MTT assay. The greater standard deviation in the second group is explained by a higher density of cells as the amount of cells is not always constant (). A grading of cytotoxic effects was suggested by Sletten and Dahl[Citation17] where extracts were rated as severely, moderately or slightly cytotoxic when the activity relative to a control was less than 30%, between 30% and 60%, or greater than 60% respectively. As seen from the mean value from all tests, none of the alloys could be rated cytotoxic except for pure copper which served as control. Pure copper had the lowest value for cell viability, 50.1% and 47.6% and was rated moderately cytotoxic (). A slightly different result could be seen when lactic acid was used as the extraction medium for the MTT assay, the corrosion MTT. A selection of five alloys was used for incubation in lactic acid. This time both the copper alloy NPG and pure copper were rated severely cytotoxic ().
HET-CAM tests
The results from the HET-CAM tests showed that all test extracts except one were classified as non-irritant material with no evidence of hemorrhage, coagulation or vascular lysis. The class III alloy NPG showed a delayed effect on the CAM with bleeding and was classified as slightly irritant. The copper specimen showed a reaction on the CAM within 60 s and was classified as moderately irritant. The positive control of 0.1M NaOH had an immediate reaction on the CAM with bleeding followed by coagulation and vascular lysis and was classified as strongly irritant, with an irritation score of 12 according to Kalweit et al.[Citation16]
Discussion
The aims of this study were to investigate the release of elements from, and the biological response in vitro to, cobalt–chromium and other base–metal alloys used for the fabrication of metal ceramic restorations. However, to carry out such tests in clinical conditions would be problematic as the oral environment is subject to constant changes that are difficult to imitate artificially. In vitro studies are easier to standardize and to control compared to in vivo studies but many in vitro studies attempt to imitate clinical conditions and the composition of the extraction media in such studies are therefore subject to variations.[Citation18] Extraction media such as MEM,[Citation9,Citation14,Citation15] artificial saliva,[Citation19,Citation20] lactic acid[Citation19] or a combination of saline solution and lactic acid [Citation21,Citation22] are used among others when extracting testing material. MEM is used for extraction in toxicity testing on cell lines, whereas the lactic acid will be toxic for the cells due to its low pH. When it comes to corrosion testing, a solution of lactic acid and saline solution with a pH of 2.3 are suggested by the ISO standard.[Citation11] This is an attempt to imitate the clinical situations where acidic food and beverages, low oxygen conditions in narrow interproximal spaces and the presence of dental plaque create an acidic environment that facilitates a corrosive reaction. Corrosion is an oxidation–reduction process affecting a material in a chemical or electrochemical process.[Citation23] Dry corrosion occurs when a metal surface reacts with atmospheric oxygen to create a thin layer of oxide on the metal surface. Apart from gold and few other exceptions, most metals form a thin surface oxide which is chemically stable and which may, or may not, form a barrier to further oxidation of the underlying metal. In the moist environment of the oral cavity where dental restorations are functioning, electrochemical or galvanic corrosion is much more common.[Citation24] Electrochemical corrosion takes place when a metal is placed into a solution (or electrolyte) and a reaction between the metal and the solution occurs. This type of corrosion can take place in almost any solution, but is especially pronounced in acidic environments and is also accelerated by the presence of chloride ions which can be present in the oral cavity. Other accelerating factors include the presences of different alloys which can form battery like cells across the liquid which connects them, and local fluctuations in surface chemistry or electrolyte chemistry (e.g. the lack of oxygen inside cracks and crevices). Metal ions are released during such corrosion processes. The released ions could eventually saturate the solution but in the oral cavity they are continuously washed away by food, saliva and other fluids, which is why the corrosion process continues.[Citation23] In our study the alloys were incubated in either MEM cell culture medium (pH 7) for passive leaching, or underwent corrosion testing in lactic acid and NaCl (pH 2.3) followed by the analysis of the solutions by ICP-MS or ICP-OE, respectively. The results are presented in where it can be seen that slightly higher amounts of released elements are detected in the acidic corrosion solution for some of the alloys. In general, the release of ions was low and in line with the studies by Beck et al.[Citation24] and Syverud et al.[Citation21] where a corrosion test was performed in lactic acid and NaCl (pH 2.3) and compared to the results from immersion in NaCl at pH 7. It could be seen that the release was pH dependant, since a lower concentration of ions was detected in the solution of only NaCl. Previous studies have also demonstrated a higher release of elements when the pH value is decreased.[Citation4,Citation19] The high ion release seen from pure copper and the copper-alloy NPG has been demonstrated in previous studies after immersion in various solutions.[Citation14,Citation25,Citation26] The process of corrosion is however complex and depends not only on the extraction media but also the type and composition of the alloy and factors such as surface characteristics, chemical content, presence of multiple phases and solubility. The presence of pits and impurities on the surface might enhance corrosion as mentioned earlier. The initial preheating and polishing of the specimens before the immersion test is part of the regular technical procedure when producing a metal-ceramic restoration. The heat-treatment is crucial for the porcelain–metal binding properties, but the altered surface is also responsible for an increased element release as shown by Schmalz, which is why polishing of the surface to remove these elements is recommended.[Citation26] This procedure was also in line with the study by Syverud et al. where palladium alloys with or without copper were preoxidized and ground/polished in two steps.[Citation21] It could be seen that more metal ions were released from the surface when remnants of the oxide layer remained on the surface. In another study by Qui[Citation20] where Co–Cr and Ni–Cr alloys were compared before and after simulated porcelain firing, an alteration of surface properties could be seen and in general the release of metal ions increased after the firing process. However, opposing results have also been demonstrated in a study by Ardlin et al.[Citation22], where some of the alloys showed higher element release without preoxidization. Another study on Pd-based alloys showed both reduced and increased corrosion after heat treatment depending on the type of alloy.[Citation27]
In order to evaluate the biological responses in our study a cell culture test, MTT and an irritation test, HET-CAM were performed. In the MTT test and corrosion MTT ( and ), the cells were exposed to the extract media for 24 hours according to the ISO standard.[Citation10] A longer time of exposure is not recommended as this might increase the risk of microbial contamination or cell overgrowth. The extraction time, on the other hand, can sometimes be extended to 72 hours[Citation28] or longer[Citation14], which is an attempt to imitate the even longer exposure of the alloy in the oral cavity. This was also done in the corrosion MTT where the alloys were exposed for 7 days compared to 24 hours in the MTT test. The extraction media were lactic acid and MEMy. In the corrosion MTT, the high-copper alloy was rated severely cytotoxic as was pure copper ( and ). In the MTT study by Sjögren et al.[Citation14], the high-copper alloy was found to be as cytotoxic as pure copper when the specimens were incubated in MEM for 5 days, which indicates that the time of exposure is important. The HET-CAM showed similar results as the study by Ardlin[Citation22] when testing cobalt–chromium alloys with no evidence of haemorrhage, coagulation or vascular lysis on the CAM. As in our study the alloys Wirobond C, Wirobond LFC and U-gold were tested but only the 1 mmol−1 solution of Cu2+ and the positive control were rated slight irritant and strongly irritant respectively, which is in line with our findings. However, in a study by Syverud et al.[Citation21], a salt solution of Cu2+ and Pd2+ had the highest irritation score. Although our in vitro tests show no signs of cytotoxicity or membrane damage from cobalt–chromium alloys, a recent study by Hjalmarsson et al.[Citation29] demonstrated improved viability of both epithelial cells and fibroblast on titanium compared to cobalt–chromium alloy. Altogether, the situation which exists in clinical conditions remains to be examined.
To conclude, in our study base-metal alloys, apart from the high-copper alloy (NPG), performed comparable to the high-gold alloy (U-gold) and the silver–palladium-based alloy (Albabond A) in regard to corrosion and elution of elements.
Acknowledgements
Authors thank the staff at NIOM for valuable help during this work, especially to Inger-Sofie Dragland, Ketil Kvam, Morten Syverud and Anne Wesman for skilful assistance with the immersions tests and the HET-CAM experiments.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Polyzois GL, Dahl JE, Hensten-Pettersen A. Biological testing of dental materials: development of national and international standards. J Biomater Appl. 1995;9:255–362
- Stenberg T. Release of cobalt from cobalt chromium alloy restorations in the oral cavity of man. Scand J Dent Res. 1982;90:472–479
- Van Noort R. Casting alloys for metallic restorations. In: Van Noort R. Introduction to Dental Materials. Edinburgh, New York. Mosby Elsevier; 2007. p 227–236
- Wataha JC. Biocompatibility of dental casting alloys: a review. J Prosthet Dent. 2000;83:223–234
- Seldén A, Persson B, Bornberger-Dankvardt SI, Winström LE, Bodin LS. Exposure to cobalt–chromium dust and lung disorders in dental technicians. Thorax. 1995;50:769–772
- Eliasson A, Arnelund CF, Johansson A. A clinical evaluation of cobalt–chromium metal-ceramic fixed partial dentures and crowns: a three-to-seven-year retrospective study. J Prosthet Dent. 2007;98:6–16
- Levi L, Barak S, Katz J. Allergic reactions associated with metal alloys in porcelain-fused-to-metal fixed prosthodontic devices – a systematic review. Quintessence Int. 2012;43:871–877
- Svanborg P, Längström L, Lundh RM, Bjerkstig G, Örtorp A. A 5-year retrospective study of cobalt–chromium-based fixed dental prostheses. Int J Prosthodont. 2013; 26: 343–349
- Kalfoss T. Determination of element leakage from dental alloys into cell culture medium using inductively coupled plasma mass spectrometry (ICP-MS) [MSc thesis]. Ås: Norwegian University of Life Sciences; 2010
- International Organization for Standardization. Biological evaluation of medical devices: part 12. Sample preparation and reference materials. ISO 10993-12:2009. Geneva; 2009
- International Organization for Standardization. Dental metallic materials – corrosion test methods. ISO 10271:2001. Geneva; 2001
- International Organization for Standardization. Dentistry – Metallic materials for fixed and removable restorations and appliances. ISO 22674:2006. Geneva;2006
- Edmondson JM, Armstrong LS, Martinez AO. A rapid and simple MTT based spectrophotometric assay for determining drug sensitivity in monolayer cultures. J Tissue Cult Meth. 1988;11:15–17
- Sjögren G, Sletten G, Dahl JE. Cytotoxicity of dental alloys, metals, and ceramics assessed by Millipore filter, agar overlay and MTT tests. J Prosthetic Dent. 2000;84:229–236
- Wataha JC, Malcolm CT, Hanks CT. Correlation between cytotoxicity and the elements released by dental casting alloys. Int J Prosthodont. 1995;8:9–14
- Kalweit S, Besoke R, Gerner I, Spielmann H. A national validation project of alternative methods to the Draize rabbit eye test. Toxicol In Vitro. 1990;4:702–706
- Sletten G, Dahl JE. Cytotoxic effects of extracts of compomers. Acta Odontol Scand. 1999;57:316–322
- Geurtsen W. Biocompatibility of dental casting alloys. Crit Rev Oral Biol Med. 2002;13:71–84
- Elshahawy WM, Watanbe I, Koike M. Elemental ion release from four different fixed prosthodontic materials. Dent Mater. 2009;25:976–981
- Qiu J, Yu W-Q, Xhang F-Q, Smales RJ, Zhang Y-L, Lu C-H. Corrosion behaviour and surface analysis of a Co-Cr and two Ni-Cr dental alloys before and after simulated porcelain firing. Eur J Oral Sci. 2011;119:93–101
- Syverud M, Dahl JE, Herö H, Morisbak E. Corrosion and biocompatibility testing of palladium alloy castings. Dent Mater. 2001;17:7–13
- Ardlin BI, Dahl JE, Tibballs JE. Static immersion and irritation tests of dental metal-ceramic alloys. Eur J Oral Sci 2005;113:83–89
- Anusavice KJ, Brantley WA. Physical properties of dental materials. In: Anusavice K, ed. Phillips' science of dental materials. St. Louis, Missouri, US. Saunders; 2003. p.41–71
- Beck KA, Sarantopoulos DM, Kawashima I, Berzins DW. Elemental release from CoCr and NiCr alloys containing palladium. J Prosthodont. 2012;21:88–93
- Chaturvedi TP. An overview of the corrosion aspect of dental implants: titanium and its alloys. Indian J Dent Res. 2009;20:91–98
- Schmalz G, Langer H, Schweikl H. Cytotoxicity of dental alloys extracts and corresponding metal salt solutions. J Dent Res. 1998;77:1772–1778
- Berzins DW, Kawashima I, Graves R, Sarkar NK. Heat treatment effects on electrochemical corrosion parameters of high-Pd alloys. J Mater Sci Mater Med. 2008;19:335–341
- Nelson SK, Wataha JC, Neme AML, Cibirka RM, Lockwood PE. Cytotoxicity of dental.casting alloys pretreated with biological solutions. J Prosthet Dent. 1999;81:591–596
- Hjalmarsson L, Smedberg J-I, Aronsson G, Wennerberg A. Cellular responses to cobalt-chromium and CP titanium – an in vitro comparison of frameworks for implant-retained oral prostheses. Swed Dent J. 2011;35:17–18