Abstract
Objective: Gram-positive cariogenic bacteria are etiological agents in dental caries; therefore, strategies to inhibit these bacteria to reduce the incident of this disease have intensified. In this study, we investigated antibacterial activities of titanates and gold-titanates against Lactobacillus casei (Lc) and Streptococcus mutans (Sm).
Materials and methods: Monosodium titanate (MST), nanomonosodium titanate (nMST) and amorphous peroxo-titanate (APT), which are inorganic compounds with high-binding affinity for specific metal ions, were used. Total bacterial proteins were measured to represent bacterial cell mass after 24 h incubation with gold-titanates. We further examined the effect of nMST-Au(III) concentrations (10,200,400 mg/L) on Lc and Sm cell viability over time via Live/Dead fluorescent staining and colony forming units (CFUs). Transmission electron microscopy (TEM) was used to determine specific locations on the bacterial cells affected by the nMST-Au(III).
Results: We found all gold-titanates and APT alone reduced bacterial protein for Lc (p value <0.001) while only MST-Au(III) and nMST-Au(III) affected Sm growth (p value <0.001). Overall, nMST-Au(III) showed the most effectiveness against both Lc and Sm at 400 mg/L. The Live/Dead staining showed all concentrations of nMST-Au(III) affected Lc growth but only 200 and 400 mg/L nMST-Au(III) interrupted Sm growth. The growth curves based on CFUs/mL showed all nMST-Au(III) concentrations affected growth of both Lc and Sm. TEM images showed nMST-Au(III) attached to Lc and Sm cell wall and were internalized into both cells.
Conclusions: nMST-Au(III) demonstrated potential antimicrobial activity against Gram-positive cariogenic bacteria. These results support further development of nMST-Au(III) as a potential novel material to prevent dental caries.
Introduction
The caries process consists of both remineralization and demineralization. There are many factors that can shift the balance of these processes. Sugar and bacteria are the dominant factors shifting the balance toward net mineral loss, which leads to initiation and progression of dental caries.[Citation1] Streptococcus mutans (Sm) are the major pathogens of human dental caries since they are frequently isolated from cavitated caries lesions and are highly acidogenic and aciduric. Also, Sm can produce surface antigens I/II and water-insoluble glucans that promote bacterial adhesion to the tooth surface and to other bacteria, leading to biofilm formation.[Citation2–4] Lactobacillus casei (Lc) have been detected in dental biofilms covering white-spot lesions and have also been determined to be cariogenic bacteria because of their capacity to produce acids as well as their ability to grow and survive in an acidic environment.[Citation5]
Many recent studies have shown that nanoparticles (NPs) have various properties that can be exploited in many biomedical applications, including in dental application. Silver, gold, platinum and palladium as NPs have been used as antimicrobial agents.[Citation6–9] Additionally, oral applications of nanoparticles have recently been considered.[Citation10,Citation11] The potential of NPs to control oral biofilm formation is related with their biocidal and anti-adhesive capabilities. NPs have been incorporated into dental materials to improve antimicrobial activity. For example, silver nanoparticles (AgNPs) were incorporated to orthodontic adhesive and reduced Sm growth.[Citation12] Moreover, the incorporation of AgNPs into bonding agent showed antimicrobial activity but did not affect physical properties of the material.[Citation13]
Monosodium titanate (MST), nanomonosodium titanate (nMST) and amorphous peroxotitanate (APT) are inorganic ion exchangers that can bind various metal ions.[Citation14,Citation15] In the previous studies, MST and APT have been used as sorbents/ion exchangers for the removal of radionuclides from nuclear wastes.[Citation14,Citation16] Sodium titanate and peroxotitanate have also been studied for their capacity to deliver therapeutic ions to target cells or to inhibit bacterial growth.[Citation17–20] Another previous study from our group documented the antibacterial activity of MST and APT with metal ions on periodontal pathogens.[Citation20] They found gold (Au)-titanates most often inhibited bacterial growth compared with palladium (Pd) or platinum (Pt)-titanates, and no antibacterial effect of titanates without metal ions was observed. Therefore, metal ions play an important role as antimicrobial agents, whereas the titanates themselves could work as stabilizers and release the ions to target cells. Gram-positive oral bacteria are important etiological factors in caries formation, but antimicrobial efficacy of gold-titanates against these bacteria is still unknown.
Our hypothesis is that NPs of gold-titanates [nMST-Au(III)] have antimicrobial activity against Gram-positive cariogenic bacteria, Lc and Sm. In our study, we developed a method to examine the effect of titanates and gold-titanates at various concentrations against Lc and Sm by first using bacteria protein as an indicator of total bacteria cell mass. We expanded our study to determine the antibacterial activity (based on direct fluorescent cell counts of Live/Dead cells) of nMST-Au(III) against Lc and Sm over time and investigate the interaction between nMST-Au(III) and bacteria by using transmission electron microscopy (TEM).
Materials and methods
Bacterial strains and growth conditions
Lactobacillus casei (ATCC® 15008™, VA, USA) was cultured aerobically at 37 °C in 55 g/L DifcoTM Lactobacilli MRS broth (BD#2388130, Difco Laboratories, Becton Dickinson, Franklin Lakes, NJ).
Streptococcus mutans UA 159 (ATCC® 700610™) was cultured aerobically at 37 °C in 37 g/L BactoTM Brain Heart Infusion (BHI) broth (BD#237500, Difco Laboratories).
All bacteria were grown overnight before inoculation into experimental cultures. After overnight culture, each bacterial species was added to new medium combined with different titanates, gold-titanates, sterile water or erythromycin as described in experimental design below.
Titanates and gold-titanates preparation
Micro-sized and nano-sized of monosodium titanate (MST and nMST) and amorphous peroxotitanate (APT) were prepared using a sol–gel or hydrothermal synthetic techniques as described in previous studies.[Citation14,Citation15,Citation21] HAuCl4i•3H2O was combined with a suspension of MST, nMST or APT for Au ion exchanger experiment to get MST-Au(III), nMST-Au(III) and APT-Au(III). Titanate and gold-titanate materials were prepared and sent to University of Washington from Dr. David Hobbs group (Savannah River National Laboratory, Aiken, SC). Once received, titanates and gold-titanates solution were prepared in sterile water. Each titanate was kept in 15 mL polyethylene tube covered by aluminum foil to avoid light exposure. The final concentrations of stock titanates and gold-titanates were 4000, 2000, 1000, 500, 250 and 100 mg/L.
Bacterial protein assay
Exposure of titanates and gold-titanates to bacterial samples
Three types each of titanates and gold-titanates used in this experiment were MST, APT, nMST, MST-Au(III), APT-Au(III) and nMST-Au(III). Total 108 CFUs of Lc or Sm were prepared in 900 µL volume MRS or BHI medium. In each bacterial suspension, 100 µL of stock titanate or gold-titanate solution was added to constitute a final concentration of 400, 200, 100, 50, 25 or 10 mg/L in a total 1 mL sample. Hundred microliters of sterile water and 50 mg/mL erythromycin were used as negative and positive controls, respectively.
Bacterial protein extraction and protein assay
After 24 h exposure, all cultures were collected for protein assays. B-PER® Bacterial Protein Extraction Reagent (Pierce®, Rockford, IL) was used to extract the bacterial protein. Bacterial protein concentration was measured on the bacterial extract using a BCA protein assay kit (Pierce®) as per manufacturer’s instructions. Bovine serum albumin was used to calibrate the BCA assay. BCA samples were measured at 560 nm by microplate reader (Magellan™, TECAN, San Jose, CA).
Live/Dead (L/D) direct cell fluorescent staining assay
nMST-Au(III) was further divided into low, medium and high concentrations (10, 200 and 400 mg/L) in order to test its effects on bacterial growth over time. Untreated bacteria were used as controls.
Lc and Sm were grown aerobically as pure species in batch suspended growth cultures at 37 °C overnight. Bacterial cultures (108 CFUs in 50 mL) were exposed to 400, 200 or 10 mg/L of nMST-Au(III) at time = 0 of batch suspended culture. Untreated control cultures for both species were used to establish their normal growth rates. Triplicate samples of cell suspension were collected every 2 h from each culture for 16 h. The LIVE/DEAD® BacLight™ Bacterial Viability kit (Molecular Probes®, Eugene, OR) was used to quantify bacterial cell viability. The staining kit is composed of two dyes. Syto9, green fluorescence nucleic acid stain, labels all bacteria DNA. Syto9 has fluorescence excitation/emission at 480/500 nm. Propidium iodide (PI), red fluorescence nucleic acid stain, can only penetrate bacteria with damaged membranes (i.e. “dead” bacteria), causing a reduction in the Syto9 stain fluorescence intensity. The fluorescence excitation/emission for PI is 490/635 nm. Each stained sample was filtered through a 0.2 µm Whatman® Cyclopore® polycarbonate and polyester membranes (Sigma-Aldrich®, St. Louis, MO), and mounted onto standard glass microscope slide for counting. The live and dead cell images were taken on the same field by fluorescence microscope at 100 × magnification. Five image sets per sample were taken and counted with ImageJ™ image analysis software (National Institutes of Health (NIH), Bethesda, MD).
Colony forming units
Separate Lc and Sm culture samples were diluted serially with phosphate-buffered saline (PBS). Species-specific agar plates, MRS and BHI agars, were used to determine Lc and Sm colony forming units (CFUs), respectively. All agar plates were incubated aerobically at 37 °C for 24 h. Bacterial colonies were counted and CFUs per volume were determined after appropriate dilution factor correction and used as another indicator of viable bacterial cells.
Transmission electron microscopy
Lc and Sm were grown aerobically at 37 °C overnight with MRS and BHI media, respectively. Bacterial cultures (108 CFUs/mL) were treated with 400 mg/L nMST-Au(III) or sterile water as a control. After 16 h exposure, bacterial cells were removed from suspension by centrifugation. Bacterial pellets were fixed in 2.5% glutaraldehyde solution for 3 h and washed with PBS and then stained with 0.1% osmic acid for 30 min at room temperature. The bacterial pellets were serially dehydrated in ethanol mixture (v/v, in water) up to 100% ethanol, embedded in epoxy resin and thin-sectioned. Each sample was deposited on TEM copper grids with a lacy carbon film (Electron Microscopy Sciences, Hatfield, PA), stained again with Reynold’s lead citrate for 10 min, and washed with sterile water.
Samples of nMST-Au(III) for TEM imaging were prepared by slow evaporation of nMST-Au(III) suspension on TEM copper grids with a lacy carbon film (Electron Microscopy Sciences) at room temperature. All bacteria and gold-titanate samples were examined with TEM (JEM-1230, JEOL, Tokyo, Japan).
Statistical analysis
In all experiments, the 95% confidence intervals and p values indicate whether the difference is statistically significant. In the preliminary bacterial culture studies, each bacterium was analyzed separately. After 24 h exposure, all the samples exposed to titanates were assessed whether they differed in average protein concentrations versus the bacterial controls, using Wilcoxon rank-sum tests.
Regarding the expanded bacterial studies, using L/D cell counts and the CFUs in each time point, all three-concentration groups were tested to determine whether the average bacterial cell numbers differed from the control using one-way analysis of variance and Tukey’s multiple comparisons test.
Results
Effect of gold-titanates and titanates on total bacterial protein
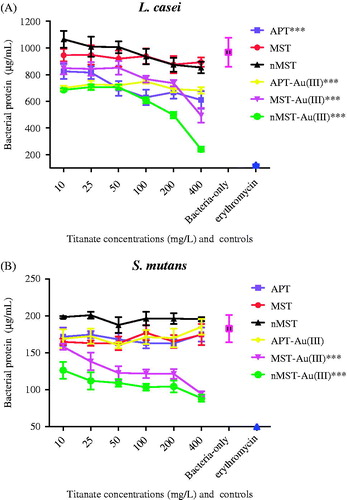
MST and nMST had no effect at any concentration on bacterial protein concentrations recovered from Lc at 24 h (). All other gold-titanates and APT decreased Lc bacterial protein concentrations at 24 h when compared with bacteria-only control (p value <0.001). At 200 and 400 mg/L nMST-Au(III), Lc protein concentrations were decreased by 29% and 70%, respectively, when compared with concentration of 10 mg/L. However, when testing with Sm (), only MST-Au(III) and nMST-Au(III) NPs showed any significant reduction in Sm bacterial proteins (p value <0.001). Overall, nMST-Au(III) at 400 mg/L was the most effective at reducing bacterial protein levels in both Lc and Sm.
Figure 1. The effect of three different titanates and gold-titanates on total bacterial proteins (A) L. casei (Lc) and (B) S. mutans (Sm) after 24 h incubation. Sterile water (H2O) and erythromycin were used as negative and positive controls, respectively. In each group (labeled as X-axis), the means ± the standard deviation (SD) of three independent experiments with each experiment set up in triplicate were expressed as bacterial protein concentration after treatment with various concentrations of titanates or gold titanates compared with controls. Each titanate or gold-titanate group is represented by different colors. ***p value <0.001 compared with negative control (bacteria-only).
Antibacterial effect of nMST-Au(III)
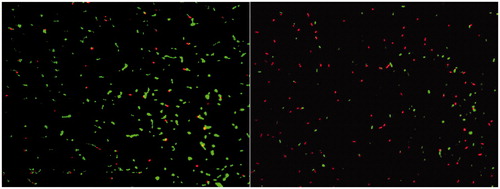
In , the images of untreated and treated with 400 mg/L nMST-Au(III) live and dead Sm cells are shown as in green and red cells, respectively. After using ImageJ™ image analysis software, live and dead cell images were merged.
Figure 2. Image of live (green) and dead (red) cells of untreated Sm (left) and treated Sm with 400 mg/L nMST-Au(III) (right) after stained with L/D staining kit at 14 h, taken under fluorescence microscope (100×), and processed by using ImageJ™ image analysis program.
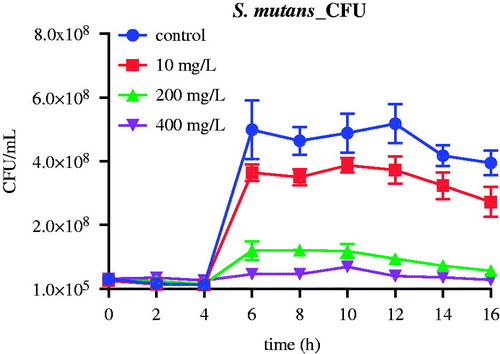
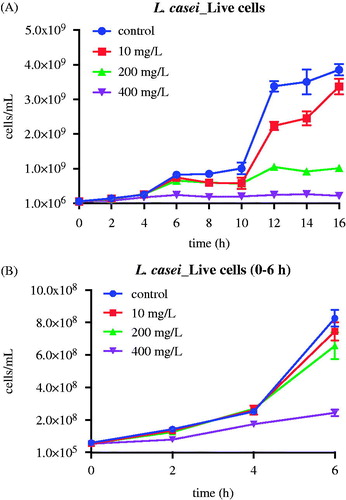
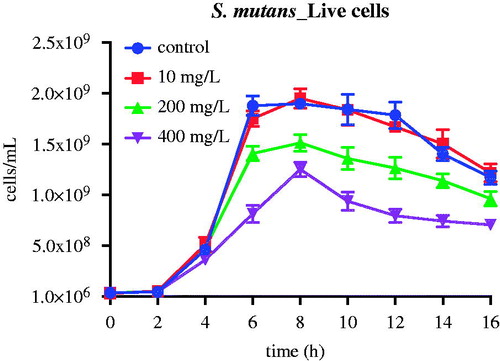
Batch suspended cultures of Lc and Sm were repeated at varying concentrations of nMST-Au(III). Samples were collected every 2 h for 16 h to determine viable cell counts, either by L/D staining or CFU counts. In Lc group (), we found that the high concentration (400 mg/L) of nMST-Au(III) significantly decreased Lc cell numbers at as early as 2 h (p value <0.01). The medium concentration (200 mg/L) of nMST-Au(III) also significantly reduced Lc growth rate by 6 h, ultimately reducing the numbers of cells at 16 h by a factor of 4× (p value <0.0001). The low concentration (10 mg/L) of nMST-Au(III) had only a marginal effect on the growth rate and extent of Lc (p value <0.05). According to this study, high concentration (400 mg/L) showed the best antibacterial activity against Lc and Sm growth compared with medium (200 mg/L) and low (10 mg/L) concentrations. Lc appeared more sensitive to nMST-Au(III) than Sm. Bacterial species may have varied defense mechanisms responding to external stimuli.
Figure 3. The effect of 10, 200 and 400 mg/L (low, medium and high concentrations) nMST-Au(III) on Lc growth curves at 37 °C over time (A) all time points (0–16 h) and (B) 0–6 h. All concentrations showed antibacterial activity on Lc growth. High concentration worked as antibacterial agent to highly inhibit Lc at as early as 2 h.
Unlike Lc, only high and medium concentrations nMST-Au(III) had any effect on Sm growth (). At the low concentration of nMST-Au(III), no significant difference of bacterial cell numbers compared with the control was found (p value >0.05). The medium concentration of nMST-Au(III) appeared to have no effect on Sm growth rate for up to 6 h and only marginally affected the maximum cell numbers attained at 16 h (p value <0.05). The high concentration of nMST-Au(III) only reduced the maximum cell extent at 16 h by a factor of 2× (p value <0.001).
Figure 4. The effect of 10, 200 and 400 mg/L (low, medium and high concentrations) nMST-Au(III) on Sm growth curves at 37 °C over time. Only medium and high concentrations were able to affect Sm growth. High concentration had no effect on Sm cell numbers for up to 6 h.
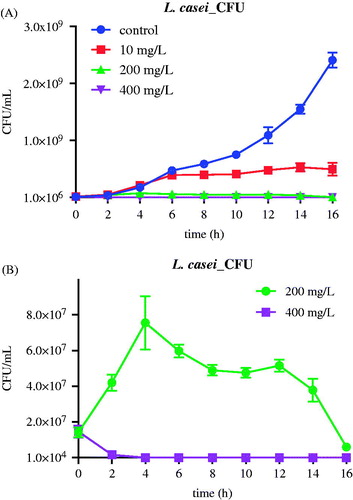
Based on CFU cell counts, bacterial growth curves of Lc exposed to the various concentrations of nMST-Au(III) exhibited the same levels of reduction as seen above (). Conversely, in Sm CFU study, both 200 and 400 mg/L group similarly affected exponential phase at 4–6 h, which did not correspond with L/D staining study ().
Figure 5. The effect of nMST-Au(III) on CFUs of Lc after 16 h. (A) Untreated control and all three concentrations compared (B) only high and medium concentrations compared. All concentrations of nMST-Au(III) showed inhibitory effect on Lc growth. High concentration was the most effective concentration against Lc growth and affected lag phase.
Transmission electron microscopy
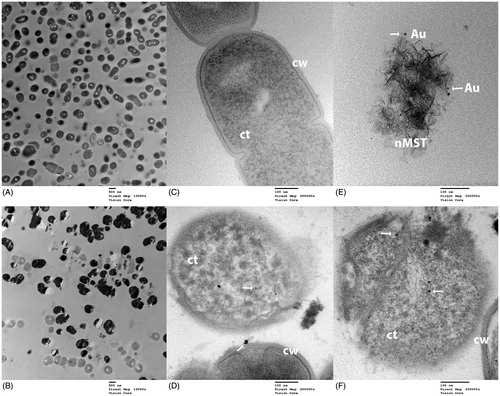
TEM images of an interaction between nMST-Au(III) and cariogenic bacteria Lc and Sm are shown in and , respectively. TEM images of both Lc and Sm revealed many dead bacterial cells in nMST-Au(III)-treated samples, while relatively few dead bacteria were found in untreated controls. Regarding the treated groups, NPs were observed on bacterial cell wall and internalized into both Lc and Sm. Partial rupture of Sm cell wall showed leakage of bacterial organelles with these particles included ().
Figure 7. TEM images of L. casei (Lc) and nMST-Au(III) (A) and (C) untreated Lc images taken at magnification 12,000× and 120,000×, respectively. Bacterial cell wall (cw) and cytoplasm (ct) were marked. (B) and (D) Lc with nMST-Au(III) images taken at magnification 12,000× and 120,000×, respectively: arrows point to the nanoparticles (NPs) attached to bacterial cell wall (cw) and internalized into bacterial cytoplasm (ct) (E) arrows point to gold (Au) NPs in nMST compared in size, shape and density with (F) arrows pointing to NPs in Lc with nMST-Au(III). Image taken at magnification 250,000×.
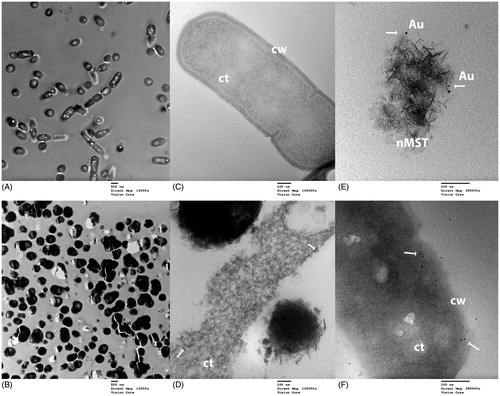
Figure 8. TEM images of S. mutans (Sm) and nMST-Au(III) (A) and (C) untreated Sm images taken at magnification 12,000× and 120,000×, respectively. Bacterial cell wall (cw) and cytoplasm (ct) were marked. (B) and (D) Sm with nMST-Au(III) images taken at magnification 12,000× and 120,000×, respectively: arrows point to the nanoparticles (NPs) attached to bacterial cell wall (cw) and internalized into bacterial cytoplasm (ct) (E) arrows point to gold (Au) NPs in nMST compared in size, shape and density with (F) arrows pointing to NPs in partial rupture of Sm cell with nMST-Au(III). Image taken at magnification 250,000×.
Discussion
Titanates can bind with several metal ions, and in the present study, we focused on gold-titanates to assess their antibacterial activity against Gram-positive cariogenic bacteria. Gold nanoparticles (AuNPs) were discovered in 1857 and the antimicrobial activity of AuNPs has been studied in both Gram-positive and Gram-negative bacteria such as Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa.[Citation22–25] AuNPs have been recommended for use in different biomedical applications such as labeling and imaging, clinical diagnostics, and therapeutics, since AuNPs present more biocompatibility and less toxicity than other nanoparticles.[Citation25] Kovvuru et al. [Citation26] found AgNPs-induced irreversible DNA damage and alteration of genome in mice after a short-term oral exposure, which may cause cancer. Thus, toxicity of NPs to mammalian cells is an important factor to consider in potential applications of NPs.
We initially investigated the effect of various types and concentrations of titanates and gold-titanates against Gram-positive cariogenic bacteria. Since determining cell suspended concentration by OD reading was compromised due to the colloidal nature of the added titanates,[Citation20,Citation27,Citation28] we determined the total bacteria cell mass based on bacterial protein. This method eliminated the OD interference issue as well as interference with direct bacterial mass (dry weight) since bacterial protein was extracted and the bacterial pellets with any titanates were discarded before the analysis.
Based on the bacterial protein assay, nMST-Au(III) showed the best effectiveness among titanates and gold-titanates to decrease bacterial protein concentrations. This may be because of differences among titanates that are carriers to release gold ions to bacterial environment. Nanosize-sodium titanate [nMST-Au(III)] has a smaller size but similar morphology to micron-sized titanates [MST-Au(III) or APT-Au(III]. Consequently, this increases particle surface area, which may help improve its antibacterial activity. In a previous study, AuNPs with various surface modification and composition showed differential antimicrobial activities against E. coli.[Citation24] With the same shape and type of AuNPs, a citrate-capping agent increased AuNPs aggregation and reduced the surface area of NPs that interacted with bacterial cells, leading to decreased inhibitory effect. Therefore, increasing the surface area of NPs generates further biophysical interaction between NPs and bacteria. Hernández-Sierra et al. [Citation29] investigated antibacterial activity of silver, zinc oxide and gold NPs against Sm and found that AuNPs had an effect on Sm only at the initial concentration of 197 μg/mL. At lower concentration, AgNPs showed better antibacterial activity than the other NPs. According to their electron microscopy images, the average size of AgNPs (25 nm) was smaller than AuNPs (80 nm) and zinc oxide NPs (125 nm). This study supports that smaller sized NPs have better interaction with bacterial cells. Similar to our materials, nMST-Au(III) showed better effect on bacterial growth by reducing bacterial protein levels in both Lc and Sm, when compared with micro-sized MST-Au(III) and APT-Au(III). According to our TEM images ( and ), average size of AuNPs is less than 10 nm. Thus, smaller sized NPs could improve antibacterial activity.
However, the protein level would represent either live or dead cells in bacterial suspension so L/D staining was used to determine the number of live and dead cells as a function of time in a batch growth culture exposed to different concentrations of nMST-Au(III). Overall CFU results corresponded with L/D assay. Unlike L/D staining, low concentration (10 mg/L) of nMST-Au(III) showed antibacterial effect on Sm exponential phase in CFU assay while no significant difference in activity was seen between low concentration and untreated control on Sm cell numbers in L/D staining. Most likely the residual gold-titanate particles on agar may have affected bacterial growth since titanates cannot be separated from the bacterial suspension before adding to agar plates.
Our TEM images also showed the treated Lc and Sm groups had many more dead cells, compared with a few dead cells in untreated groups. These images demonstrated nMST-Au(III) can cause bacterial cell death. TEM images of nMST-Au(III) in suspension were also taken to compare AuNPs on nMST ( and ) and black particles that were observed in treated groups ( and ). We found that AuNPs on nMST have similar size, shape and density to particles that were inside and attached to bacterial cell wall. These would suggest the particles that were internalized and attached to bacterial cell wall are AuNPs from nMST-Au(III) compound. The TEM images confirm AuNPs were both attached to and internalized within Lc and Sm. However, no nMST itself was closely attached or internalized into bacterial cells, which would support the notion that titanate itself does not have antibacterial activity. The function of titanate as a carrier and gold ion-exchanger to target bacterial cells could improve antibacterial activity of AuNPs. Lokina et al. [Citation30] reported that synthesizing AuNPs with a reducing and stabilizing agent showed a high degree of antimicrobial activity against several organisms such as Candida albicans and S. aureus. The reducing and stabilizing agent promoted the reduction of gold ions, which became AuNPs against bacterial cells.
Zhao et al. [Citation22] studied antibacterial effect of AuNPs on Gram-negative bacteria, with amino-substituted pyrimidine-capped AuNPs being tested with P. aeruginosa and E. coli. These pyrimidine-capped AuNPs were able to disrupt the bacterial cell membranes, leading to leakage of cytoplasmic components. The NPs were also internalized into bacterial cells, resulting in an interaction with bacterial DNA. Conversely, another study found AuNPs could only be absorbed onto Salmonella typhimurium cell wall but unable to penetrate into the bacterial cells. These AuNPs did not show a toxic effect on S. typhimurium.[Citation31] Antibacterial activity of AuNPs may require NPs to interact or destroy intracellular structures.
Corresponding to Zhao et al. [Citation22] TEM findings, AuNPs-induced morphological changes of cell membrane and leakage of nucleic acids on P. aeruginosa. Interaction between AuNPs and subcellular structures such as DNA and ribosome were determined. Cui et al. [Citation32] reported that cell endocytosis of small AuNPs cause cytotoxicity of both human cervical carcinoma (HeLa) cells and E. coli. They also mentioned the relationship between the size of AuNPs and NPs toxicity. Small NPs could aggregate inside the cells leading to cell toxicity. In contrast, large NPs did not enter cells but attached onto cell surfaces, which may enhance cell growth. According to these studies, two possible antibacterial activities of nMST-Au(III) mechanisms could be proposed. Once bacteria are exposed to nMST-Au(III), Au3+ ions could be released from nMST into the bacterial suspension and interact with bacteria by electrostatic attraction between negative charges of bacterial cell wall/membrane and positive charges of ions, and also enter the cells via ion transport. When Au3+ ions are internalized into the bacteria, they could be reduced to neutral gold atoms as observed in TEM images. Another explanation is that released Au3+ ions would be reduced to AuNPs outside the cells by bacteria themselves or surrounding environmental conditions. AuNPs are also able to attach to and be engulfed by bacteria as mentioned in previous studies.[Citation22,Citation31,Citation32]
Based on our data, we suggest that antibacterial activity of AuNPs depends on several factors: type, size, as well as bacteria species. Since we tested only one strain of Sm and Lc, the effect may be different in other strains of cariogenic bacteria. Also, microorganisms in the oral cavity are likely to be in biofilms adhered to tooth surfaces. According to previous studies, compare with bacteria in planktonic culture, bacterial biofilms have dramatically higher resistance to antimicrobial agents, including NPs and antibiotics.[Citation10,Citation33] Therefore, studies the effect of AuNPs on cariogenic bacterial biofilm would be noteworthy to explore. Moreover, further studies on the mechanism of nanoparticle action would help us better understand the function of gold-titanate NPs affecting on these cariogenic bacteria.
Conclusions
Gold-titanate NPs showed the most effectiveness and could be a potential antimicrobial agent against Gram-positive cariogenic bacteria, Sm and Lc. The innovative incorporation of gold-titanate NPs into dental materials such as restorative or endodontic material is an exciting possibility. These would improve antimicrobial capacity of materials and help to decrease secondary caries risk.
Acknowledgements
We would like to thank Dr David Hobbs (Savannah River National Laboratory) for providing titanate materials, Dr Charles Spiekerman for statistical advice, and Mr Edward Parker for TEM support.
Declaration of interest
This study is supported by the National Institute of Dental and Craniofacial Research Grant No. RO1DE021373-01 (NIH) and the Royal Thai Government. Authors W.O.C. and D.C.N.C. jointly hold a US patent on the titanate complex reported in this manuscript.
References
- Hara AT, Zero DT. The caries environment: saliva, pellicle, diet, and hard tissue ultrastructure. Dent Clin North Am. 2010;54(3):455–467
- Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65(10):1028–1037
- Matsui R, Cvitkovitch D. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol. 2010;5(3):403–417
- Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2010;90(3):294–303
- Badet C, Thebaud NB. Ecology of lactobacilli in the oral cavity: a review of literature. Open Microbiol J. 2008;2:38–48
- Amarnath K, Kumar J, Reddy T, Mahesh V, Ayyappan SR, Nellore J. Synthesis and characterization of chitosan and grape polyphenols stabilized palladium nanoparticles and their antibacterial activity. Colloids Surf B Biointerfaces. 2012;92:254–261
- Lopes I, Ribeiro R, Antunes FE, Rocha-Santos TAP, Rasteiro MG, Soares AM, Gonçalves F, Pereira R.Toxicity and genotoxicity of organic and inorganic nanoparticles to the bacteria Vibrio fischeri and Salmonella typhimurium. Ecotoxicology. 2012;21(3):637–648
- Ma S, Izutani N, Imazato S, Chen J, Kiba W, Yoshikawa R, Takeda K, Kitagawa H, Ebisu S. Assessment of bactericidal effects of quaternary ammonium-based antibacterial monomers in combination with colloidal platinum nanoparticles. Dent Mater J. 2012;31(1):150–156
- Mohanty S, Mishra S, Jena P, Jacob B, Sarkar B, Sonawane A. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomedicine. 2012;8(6):916–924
- Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89(11):1175–1186
- Mohamed Hamouda I. Current perspectives of nanoparticles in medical and dental biomaterials. J Biomed Res. 2012;26(3):143–151
- Ahn SJ, Lee SJ, Kook JK, Lim BS. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009;25:206–213
- Moszner N, Salz U. Recent developments of new components for dental adhesives and composites. Macromol Mater Eng. 2007;292:245–271
- Hobbs DT, Barnes MJ, Pulmano RL, Marshall KM, Edwards TB, Bronikowski MG, Fink SD. Strontium and actinide separations from high level nuclear waste solutions using monosodium titanate 1. Simulant testing. Separ Sci Technol. 2005;40(15):3093–3111
- Hobbs DT. The properties and uses of sodium titanates and peroxotitanates. J S C Acad Sci. 2011;9(1):20–24
- Nyman M, Hobbs DT. A family of peroxo-titanate materials tailored for optimal strontium and actinide sorption. Chem Mater. 2006;18:6425–6435
- Davis RR, Hobbs DT, Khashaba R, Sehkar P, Seta FN, Messer RLW, Lewis JB, Wataha JC. Titanate particles as agents to deliver gold compounds to fibroblasts and monocytes. J Biomed Mater Res A. 2009;93A(3):864–869
- Wataha JC, Hobbs DT, Lockwood PE, Davis RR, Elvington MC, Lewis JB, Messer RLW. Peroxotitanates for biodelivery of metals. J Biomed Mater Res B Appl Biomater. 2009;91B(2):489–496
- Wataha JC, Hobbs DT, Wong JJ, Dogan S, Zhang H, Chung KH, Elvington MC. Titanates deliver metal ions to human monocytes. J Mater Sci Mater Med. 2010;21(4):1289–1295
- Chung WO, Wataha JC, Hobbs DT, An J, Wong JJ, Park CH, Dogan S. Elvington MC. Rutherford RB. Peroxotitanate- and monosodium metal-titanate compounds as inhibitors of bacterial growth. J Biomed Mater Res A. 2011;97A(3):348–354
- Elvington MC, Tosten M, Taylor-Pashow KML, Hobbs DT. Synthesis and characterization of nanosize sodium titanates. J Nanopart Res. 2012;14:1114
- Zhao Y, Tian Y, Cui Y, Liu W, Ma W, Jiang X. Small molecule-capped gold nanoparticles as potent antibacterial agents that target Gram-negative bacteria. J Am Chem Soc. 2010;132(35):12349–12356
- Brown AN, Smith K, Samuels TA, Lu J, Obare SO, Scott ME. Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol. 2012;78(8):2768–2774
- Zhou Y, Kong Y, Kandu S, Cirillo JD, Liang H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guerin. J Nanobiotechnology. 2012;10–19
- Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38(6):1759
- Kovvuru P, Mancilla PE, Shirode AB, Murray TM, Begley TJ, Reliene R. Oral ingestion of silver nanoparticles induces genomic instability and DNA damage in multiple tissues. Nanotoxicology. 2015;9(2):162–71
- Davis RR, Lockwood PE, Hobbs DT, Messer RL, Price RJ, Lewis JB, et al. In vitro biological effects of sodium titanate materials. J Biomed Mater Res B Appl Biomater. 2007;83(2):505–511
- Drury JL, Jang Y, Taylor-Pashow KM, Elvington M, Hobbs DT, Wataha JC. In vitro biological response of micro- and nano-sized monosodium titanates and titanate-metal compounds. J Biomed Mater Res B Appl Biomater. [E-pub ahead of print May 13, 2014] In press
- Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, Guillén Ade J, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 2008;4(3):237–240
- Lokina S, Suresh R, Giribabu K, Stephen A, Lakshmi Sundaram R, Narayanan V. Spectroscopic investigations, antimicrobial, and cytotoxic activity of green synthesized gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2014;129C:484–490
- Wang S, Lawson R, Ray PC, Yu H. Toxic effects of gold nanoparticles on Salmonella typhimurium bacteria. Toxicol Ind Health. 2011;27(6):547–554
- Cui W, Li J, Zhang Y, Rong H, Lu W, Jiang L. Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomedicine. 2012;8(1):46–53
- Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65:1803–1815