Abstract
Objective To determine whether the antimicrobial nature of a fatty acid chelate temporary dental cement can be enhanced by the addition of 5% cetyl pyridinium chloride (CPC).
Materials and methods The temporary cement, Cavex Temporary was employed, and additions of CPC were made to either the base or the catalyst paste prior to mixing the cement. Release of CPC from set cement specimens was followed using reverse-phase HPLC for a period of up to 2 weeks following specimen preparation. Potential interactions between Cavex and CPC were examined by Fourier transform infrared spectroscopy (FTIR) and antimicrobial effects were determined using zone of inhibition measurements after 24 h with disc-shaped specimens in cultured Streptococcus mutans.
Results FTIR showed no interaction between CPC and the components of the cement. CPC release was found to follow a diffusion mechanism for the first 6 h or so, and to equilibrate after approximately 2 weeks, with no significant differences between release profiles when the additive was incorporated into the base or the catalyst paste. Diffusion was rapid, and had a diffusion coefficient of approximately 1 × 10−9 m2 s−1 in both cases. Total release was in the range 10–12% of the CPC loading. Zones of inhibition around discs containing CPC were significantly larger than those around the control discs of CPC-free cement.
Conclusions The antimicrobial character of this temporary cement can be enhanced by the addition of CPC. Such enhancement is of potential clinical value, though further in vivo work is needed to confirm this.
Introduction
The incorporation of antimicrobial compounds into dental restorative materials is a subject that is being increasingly studied.[Citation1] It was not necessary for silver amalgam, because this material is inherently antimicrobial, but it has been studied for both dental composite resins [Citation2–6] and glass-ionomer cements.[Citation7–11] In both cases, the aim has been to release the antimicrobial substance in a controlled way to inhibit or kill micro-organisms that make up the oral biofilm.
For composite resins, a typical study involved chlorhexidine,[Citation3] which was incorporated into the material at levels of a few percent by mass, and found to be successful in inhibiting microbial growth. However, it proved to be effective for short periods only.[Citation3] It also had the disadvantage of leaving behind micro-voids in the resin structure, and these adversely affected the mechanical properties of the material.[Citation2,Citation4]
Silver has also been used as an antimicrobial agent in composite resins. It was introduced to the material as part of a silica glass filler [Citation5] and was found to leach at concentrations that made it effective against Streptococcus mutans and related species.[Citation6] There was no adverse effect on mechanical properties using this approach but the antimicrobial effect was also of a somewhat limited duration.[Citation5] Also, the silver released had an adverse effect on the color of the composite.[Citation12] On balance, it, therefore, appears that organic antimicrobial compounds give more favorable results in composite resins.
Glass-ionomers have also been studied for the incorporation of antimicrobial compounds.[Citation7–11] They possess some slight antimicrobial properties even in the absence of additives, due to their ability to release fluoride.[Citation13] In principle, fluoride is toxic toward micro-organisms, since it deactivates enzymes that contain magnesium by forming the insoluble compound MgF2.[Citation14] However, the effect is slight with clinical glass-ionomers because the level of release is low.[Citation15]
Chlorhexidine compounds have been added to glass-ionomers and successfully promoted antimicrobial character. For example, chlorhexidine gluconate has been added and shown to enhance antimicrobial activity.[Citation16] Chlorhexidine diacetate has also been shown to be effective,[Citation7] with increased antimicrobial properties being determined for up to 50 days. A number of other antimicrobial compounds have been found to be effective in improving the antimicrobial character of glass-ionomers, including cetyl pyridinium chloride (CPC),[Citation17] cetrimide,[Citation17] benzalkonium chloride [Citation17] and tri-sodium citrate.[Citation18] Mixtures of chlorhexidine diacetate and cetrimide have also been used, with promising results.[Citation19]
The difficulty with all of these additives is their tendency to slow down the setting reaction of the glass-ionomer cement, to weaken the set cement [Citation7,Citation17] and reduce its hardness.[Citation8] Nonetheless, significant improvements in antimicrobial character have been demonstrated and these may be of importance clinically.
To date, there have been no studies which include antimicrobial compounds in temporary cements. In these materials, a relatively short-term release might be acceptable, and could improve clinical outcomes where temporary cements are required prior to the permanent fitting of crowns and bridges with more durable cements. One material of interest is the chelate cement formed from fatty acids with metal salts, and this is the subject of the present study. The antimicrobial compound CPC has been added to either the base or the catalyst paste, cement specimens prepared and release profiles determined. Potential chemical interactions between the cement and CPC were also investigated by Fourier transform infrared spectroscopy (FTIR).
Materials and methods
The study employed a commercial temporary cement, Cavex Temporary (Cavex BV, Haarlem, Netherlands), a eugenol-free fatty acid chelate cement based on zinc oxide. The antimicrobial compound used was CPC (Sigma Aldrich, St. Louis, Missouri, United States). The cement was mixed according to the manufacturer’s instructions at a ratio of 1 g of “base” paste to 0.5 g of “catalyst” paste. Mixing was performed on a glass plate using a metal spatula.
Two series of specimens were prepared, each comprising three samples. In one, CPC was added to the base paste; in the other it was added to the catalyst. In both cases, the amount added corresponded to 5% by mass of finished paste.
Freshly mixed pastes were transferred to silicone rubber molds and placed between glass microscope slides. Finished specimens were of size 6 mm diameter by 2 mm depth. They were allowed to cure fully in the molds at 37 °C for 1 h, then placed in 5 cm3 volumes of deionized water in polypropylene tubes. They were stored at 37 °C, then at designated time periods (1 h, 2 h, 3 h, 6 h, 24 h, 1 week, 2 weeks) a volume of 0.1 cm3 was removed from the storage solution and placed in a glass HPLC vial for analysis.
Concentrations of CPC released at various time intervals were determined by HPLC using an Agilent 1200 series chromatograph (Santa Clara, California, United States) fitted with a reverse-phase C-18 Primsep column of length 150 mm × 4.60 mm. Analysis used a 20-μl injection volume, at a flow rate of 1.0 cm3/min, with an isochratic mobile phase consisting of 55:45 acetonitrile–water with an added drop of concentrated sulfuric acid. Detection was by means of a variable wavelength detector set to 259 nm.
Once release had equilibrated, data were converted to values of Mt/M∞ and plotted against square root of time, t½, to give a straight line whose slope was used to determine the diffusion coefficient. The best-fit line was determined by least-squares regression, and differences in release data and diffusion coefficients were tested for significance using the Student t-test.
Attenuated total reflectance (ATR) FTIR spectra were obtained for cement components and for Cavex discs which had been cured for 24 h using a Perkin Elmer Spectrum One spectrometer (Waltham, Massachusetts, United States) with a Universal Diamond ATR attachment. Data were collected between 650 and 4000 cm−1 with 10 accumulated scans at a resolution of 2 cm−1.
The antimicrobial activities of the Cavex cement alone and of the cement with 5% CPC mixed into the base were evaluated using the semi-quantitative Kirby–Bauer inhibition zone method. The micro-organism employed was S. mutans NCIMB 13700, a Gram-positive bacterium. 100 cm3 of Nutrient Broth (Oxoid) was inoculated with 0.1 cm3 of an overnight culture of the bacterium. The culture was then incubated for 24 h, with shaking, at 37 °C. 0.2 cm3 of the resulting culture was spread in triplicate on nutrient agar plates. Two 6-mm cement discs were placed on each spread plate. After incubation at 37 °C for 24 h, the plates were examined for clear zones. Plate counts indicated that the final population density of the spread plates was 1.5 (±0.7) × 108 colony forming units per plate. A one-tailed t-test (at p = 0.01) was used to determine any statistical difference between the antimicrobial activities of the cement specimens with and without 5% CPC.
Results
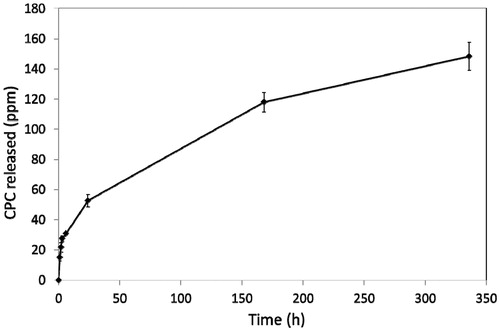
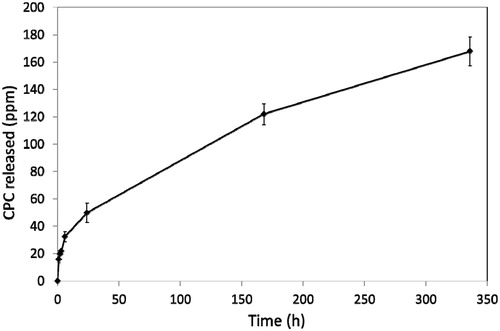
All samples were found to release CPC at all time intervals. After 2 weeks, release had equilibrated. Typical release profiles for CPC are shown in and for base-added and catalyst-added, respectively.
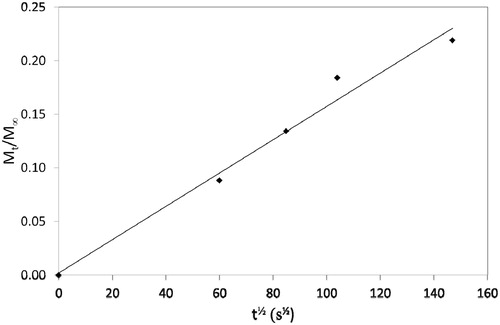
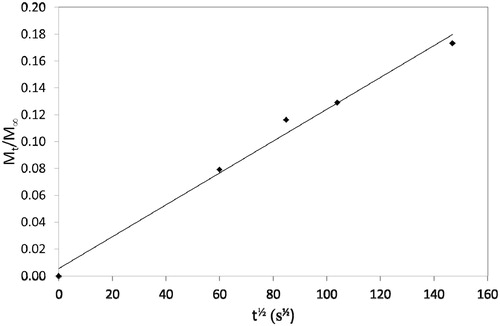
Release profiles were recalculated to determine values of Mt/M∞ and diffusion plots of Mt/M∞ versus t½ are shown in and . Diffusion coefficients were determined from the slope of these plots using the so-called Stefan approximation, i.e. substituting into the equation:
where s is the slope of the diffusion plot and l is the specimen thickness.
Release data are shown in . There were no significant differences between any of the values in this table, showing that release of CPC did not alter when the additive was incorporated into either the base or the catalyst component prior to mixing the overall cement.
Table 1. Release characteristics for CPC eluted from temporary cement added by incorporating into base or catalyst component (standard deviations in parentheses).
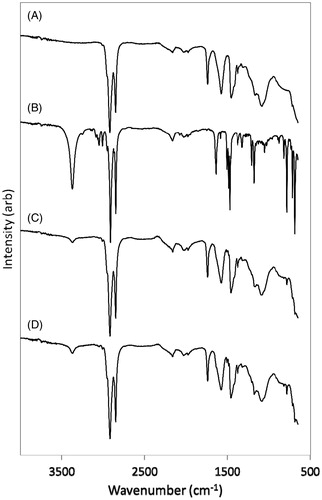
FTIR spectra of cement with and without 5% CPC are shown in , and assignments of bands in . No new bands appeared and none of the original bands were shifted by the presence of CPC, suggesting that there is no interaction between the cement components and CPC.
Table 2. FTIR assignments for the components of cavex temporary cement and CPC.
The antimicrobial effect of CPC released from Cavex discs is shown by the results in . The zone of inhibition was much larger in the discs formulated with CPC, and the t-calc value was greater than the t-crit value at p = 0.01, demonstrating that this difference was significant, and that sufficient CPC was released from the Cavex discs to inhibit the growth of the S. mutans culture.
Table 3. Inhibition zone data for cavex and CPC-cavex discs.
Discussion
FTIR spectroscopy showed that there was no chemical reaction between the components of the fatty acid chelate cement and CPC. For example, the band at 1637 cm−1 due to the nitrile (CN) group was visible in the spectra of the mixtures, showing that this functional group had not undergone a reaction. It occurred as a shoulder on the C–C stretching band of Cavex centered at 1577 cm−1, and could not be resolved further. Nonetheless, its appearance was clear enough to show the presence of the functional group, and hence to rule out a possible reaction. Overall, FTIR results confirmed in that there were no differences between the additive-free cement and the ones containing CPC, and this showed that CPC can be included without altering the essential properties of the cement.
Release of CPC from the cement was shown to occur by diffusion for the first 6 h or so, and to continue for several days until equilibrating after approximately 2 weeks. This behavior is similar to the release of a number of substances from composite resins and dental cements,[Citation20] including sodium fusidate from glass-ionomer [Citation21] and benzalkonium chloride from zinc polycarboxylate.[Citation22] Although diffusion appears to be the most straightforward of the possible release mechanisms, it is not followed in all cases and, for example, the release of gentamycin from acrylic bone cement in orthopedics does not have this release mechanism.[Citation23]
Release by diffusion can occur with a wide range of diffusion coefficients. The values, we have found in the present study are relatively high, being around 1 × 10−9 m2 s−1 and these were not influenced by whether the CPC was incorporated into the base or the catalyst component. Sufficient CPC was released to have a clear and significant effect on cultured S. mutans, as demonstrated by the much greater zones of inhibition around specimens containing CPC.
CPC was chosen for these studies because it is a useful broad-spectrum antimicrobial compound and is used in a variety of oral healthcare products, including mouth-rinses.[Citation24] Although it has a very low level of toxicity toward humans, oral care products typically contain such levels that there are no concerns over toxicity.[Citation25] Rather, the only drawbacks appear to be the possible staining of the tongue and teeth of the patient, following long-term use, and these are not considered severe from the medical point of view.[Citation25] Levels of CPC of only 0.05% are sufficient to be effective against normal oral flora [Citation24] and the substance is considered safe for inclusion in oral hygiene cosmetic products up to levels of 0.5%.[Citation26] Release levels in our experiments were well below this, not exceeding 200 ppm, which is equivalent to 0.02%. We, therefore, do not anticipate any problems for patients at the levels of CPC that we have used.
In dental materials, CPC does not necessarily have to be released to be effective. In one study, it was immobilized in an adhesive, where it showed an inhibitory effect on bacteria in contact with its surface.[Citation27] However, in the present study, it was clearly released, and was observed to have an antimicrobial effect some distance away from discs of chelate cement in which it was included. Cationic surfactants, such as CPC, are particularly useful antimicrobials because bacteria do not appear to develop resistance to them due to their nonspecific mode of action.[Citation28]
There are more than 700 types of bacteria that have been reported as capable of occurring in the oral cavity.[Citation29] S. mutans was used in the present study because it is a particularly common oral bacterium. It has been widely reported, and other species of the Streptococcus genus have also been found, including S. oralis, S. mitis and S. constellatus.[Citation30] These species typically occur as part of a biofilm that adheres strongly to surfaces within the mouth and such biofilms are known to contribute to the conditions of dental caries [Citation31] and periodontal disease.[Citation32]
A broad-spectrum antimicrobial, such as CPC, has the potential to deal with any of these micro-organisms that occur in the vicinity of a restoration, whether permanent or temporary. Indeed, the relatively short duration of the release of this substance from the fatty acid chelate cement in the current study, and from other cements previously reported [Citation20] suggests that it is particularly suited for temporary materials. As such, our findings indicate that this system has the potential to be beneficial in clinical situations in maintaining a relatively bacteria-free region in close proximity to the temporary repair, thus eliminating further damage due to caries.
Conclusion
The antimicrobial compound CPC has been shown to be capable of being included at satisfactory levels from a commercial fatty acid chelate temporary cement without affecting the setting reaction, and to be released by a diffusion process. Overall release levels represented 10–12% of the initial antimicrobial loading, and did not differ significantly when the additive was incorporated into the base or the catalyst paste. Diffusion coefficients were of the order of 1 × 10−9 m2 s−1 and also did not differ significantly when pre-mixed with either the base or the catalyst. Microbiological results show that release occurs at useful levels. Overall, temporary cements with added CPC have the potential to be clinically beneficial and act to reduce infection in the oral environment adjacent to the site of clinical treatment, however, further in vivo work is necessary to demonstrate this conclusively.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Young AM. Antibacterial releasing dental restorative materials. In: Lewis AK, editor. Drug Device combination products. Cambridge: Woodhead Publishing; 2011.
- Imatato S, Edi N, Takahashi Y, et al. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003;24:3605–3609
- Leung D, Spratt DA, Pratten J, et al. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials. 2005;26:7145–7153.
- Jedrychowski JR, Caputo AA, Kerper S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J Oral Rehabil. 1983;10:373–381.
- Kawashita M, Tsuneyama S, Miyaji F, et al. Antibacterial silver-containing silica glass prepared by sol-gel method. Biomaterials. 2000;21:393–398.
- Yamamoto K, Ohashi S, Aono M, et al. Antibacterial activity of silver ions implanted in SiO2 filler on oral streptococci. Dent Mater. 1996;12:227–229.
- Palmer G, Jones FH, Billington RW, Pearson GJ. Chlorhexidine release from an experimental glass ionomer cement. Biomaterials. 2004;25:5423–5431.
- Tüzüner T, Ulusu T. Effect of antibacterial agents on the surface hardness of a conventional glass-ionomer cement. J Appl Oral Sci. 2012;20:45–49.
- Takahashi Y, Imazato S, Kaneshiro AV, et al. Antibacterial effects and physical properties of glass-ionomer cements containing chlorhexidine for the ART approach. Dent Mater. 2006;22:647–652.
- Türkün LS, Türkün M, Ertugrul F, et al. Long-term antibacterial effects and physical properties of a chlorhexidine-containing glass ionomer cement. J Esthet Restor Dent. 2008;20:29–44.
- Hook ER, Owen OJ, Bellis CA, et al. Development of a novel antimicrobial-releasing glass ionomer cement functionalized with chlorhexidine hexametaphosphate nanoparticles. J Nanobiotechnol. 2014;12:3.
- Syafiuddin T, Himamitsu H, Toko T, et al. In vitro inhibition of caries around a resin composite restoration containing antibacterial filler. Biomaterials. 1997;18: 1051–1057.
- Forsten L. Fluoride release from a glass ionomer cement. Scand J Dent Res. 1977;85:5030504.
- ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Scand J Odont. 1999;57:325–329.
- Forss H, Jokinen J, Spets-Happonen S, et al. Fluoride and mutans streptococci in plaque grown on glass ionomer and composite. Caries Res. 1991;25:454–458.
- Marti LM, da Mata M, Ferraz-Santos B, et al. Addition of chlorhexidine gluconate to a glass ionomer cement: a study on mechanical, physical and antibacterial properties. Braz Dent J. 2014;25:33–37.
- Botelho MG. Compressive strength of glass ionomer cement with dental antibacterial agents. J South African Dent Assoc. 2004;59:51–53.
- Wren AW, Boyd D, Thornton R, et al. Antibacterial properties of a tri-sodium citrate modified glass polyalkenoate cement. J Biomed Mater Res B Appl Biomater. 2009;90:700–709.
- Tüzüner T, Kusgoz A, Kürsat ER, et al. Antibacterial activity and physical properties of conventional glass-ionomer cements containing chlorhexidine diacetate/cetrimide mixtures. J Esthet Restor Dent. 2011;23:46–55.
- Farrugia C, Camilleri J. Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements – a literature review. Dent Mater. 2015;31:e89–e99.
- Mulla Z, Edwards M, Nicholson JW. Release of sodium fusidate from glass-ionomer dental cement. J Mater Sci Mater Med. 2010;21:1997–2000.
- Ali MN, Edwards M, Nicholson JW. Zinc polycarboxylate dental cement for the controlled release of an active organic substance: proof of concept. J Mater Sci Mater Med. 2010;21:1249–1253.
- Baker AS, Greenham LW. Release of gentamicin from acrylic bone cement. Elution and diffusion studies. J Bone Joint Surg. 1988;70:1551–1557.
- Haps S, Slot DE, Berchier CE, Van der Weijden GA. The effect of cetylpyridinium chloride-containing mouth rinses as adjuncts to toothbrushing on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg. 2008;6:290–303.
- Namba N, Yoshida Y, Nagaoka N, et al. Antibacterial effect of bactericide immobilized in resin matrix. Dent Mater. 2009;25:24–30.
- Radford JR, Beighton DM, Nugent Z, Jackson RJ. Effect of use of 0.05% cetylpyridinium chloride mouthwash on normal oral flora. J Dent. 1997;25:35–40.
- EU Scientific Committee on Consumer Safety, Opinion on cetylpyridium chloride – Submission II, Colipa No. 97, SCCS/1548/15, 25 March 2105.
- Gao B, Zhang X, Zhu Y. Studies on the preparation and antibacterial properties of quaternized polyethyleneimine. J Biomater Sci Polym Ed. 2007;18:531–544.
- Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732.
- Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003;30:644–654.
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–59.
- Lovegrove JM. Dental plaque revisited: bacteria associated with periodontal disease. J NZ Soc Periodontol. 2004;87:7–21.