Abstract
Background: During pelvic and sacral tumor resection, important vessels, such as the inferior gluteal artery underneath the sciatic notch, are easily injured because of their invisibility. We employed a computer-assisted navigation system to preserve the inferior gluteal artery at the sciatic notch with visualization of the inferior gluteal artery at the sciatic notch, thus maintaining blood flow to the gluteus maximus.
Methods: We present the results of seven patients (five men and two women) with a mean age of 64.8 years (58.4–78.8) in whom computer-assisted navigation surgery had been carried out for pelvic and sacral tumor resections.
Results: Postoperative enhanced computed tomography (CT) confirmed the preservation of the inferior gluteal artery in all cases. At a mean follow-up of 21.3 months (3–39), the total postoperative complication rate was 25% (n = 2), including hematoma (n = 1) and wound necrosis (n = 1). There were no cases of deep infection.
Conclusion: This new application of computer-assisted navigation to pelvic and sacral tumor resection can contribute to reducing postoperative complications related to insufficient flap perfusion.
Background
Pelvic and sacral tumor resection is infrequently performed, and is associated with considerable morbidity and mortality.[Citation1–5] The complex anatomy, the often-hard consistency of the tumor, the close proximity of viscera, nerves and vascular structures and the need to change the position of the patient during the operation, all contribute to the difficulty of the procedure.[Citation2,Citation5–7] Vessels and other important structures are easily injured during these resections due to limited visibility, especially at the greater sciatic notch.
The greater sciatic notch is the key to pelvic tumor surgery because of its important bridging vessels. Injury to these vessels is associated with severe hemorrhage.[Citation8] The preservation of the inferior gluteal artery, which crosses the sciatic notch and supplies blood flow to the gluteus maximus muscle, is particularly important to reduce postoperative complications, especially flap necrosis. As these vessels are usually not visible, inadvertent injury to the gluteal arteries and subsequent hemorrhage or flap necrosis [Citation8] are common. To prevent subsequent hemorrhage, the ligation of internal iliac artery on the pelvic cavity side of the greater sciatic notch was frequently performed.[Citation9] In this case, the possibility of flap necrosis was not considered.
Computer navigation has been used for a number of years to aid surgical precision in spinal surgery, lower limb joint replacement and trauma.[Citation2] More recently, computer navigation-assisted surgery for the resection of pelvic tumors has demonstrated promising results, with more accurate resection, reconstruction and implant positioning.[Citation2] However, use of this navigation system to detect soft tissue structures during pelvic tumor resection has not been reported.
We describe a computer-assisted navigation system that uses preoperative computed tomography (CT) data to detect the inferior gluteal artery at the greater sciatic notch quickly and precisely during surgery, avoiding injury .The aim of this technical note is to describe the technique of preparation and usage of the system for intraoperative detection and preservation of the inferior gluteal artery during pelvic and sacral tumor surgeries.
Patients and methods
Patients
Between July 2010 and January 2014, we performed eight operations using computer-assisted navigation on seven patients (five men and two women) for bone neoplasms involving the pelvis or sacrum. All resections involved exposing the greater sciatic notch and encountering the inferior gluteal artery. One patient with a giant-cell tumor required two separate operations due to a large intraoperative hemorrhage. Patient ages at the time of surgery ranged from 58.4 to 78.8 years (mean, 64.8 years). We performed four internal hemipelvectomies (three patients with chondrosarcoma, one with osteosarcoma), two intralesional resections (giant-cell tumor of the sacrum) and one sacrectomy (chordoma and teratoma). The internal hemipelvectomies involved P1, P1/4, P1/2/4 and P2/3 resections. Patient background data are shown in .
Table 1. Patient characteristics.
Ethics
Institutional review board approval was not required because all data were collected from clinical records and imaging systems for routine preoperative planning and follow-up.
Preoperative planning
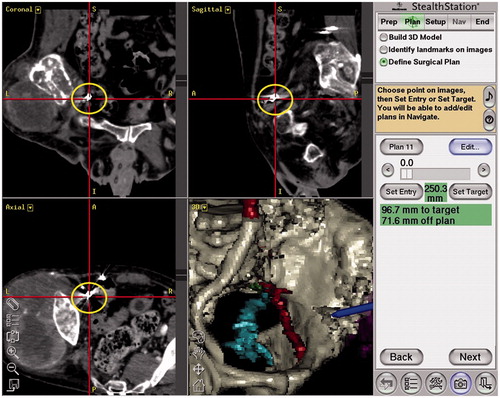
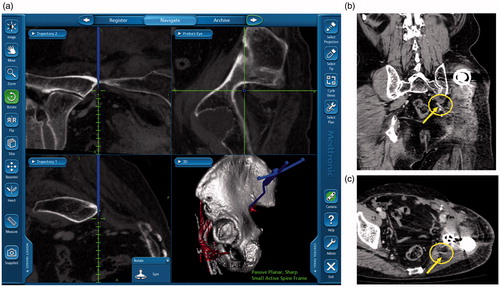
We used a guidance system StealthStation TRIA plus Treatment Guidance System® (Medtronic-Sofamor Danek Co., Ltd., Osaka, Japan) with navigation software (Synergy Experience® version 2.0.1 [] or Cranial version 5.2.5 [, used in Case 4]). No more than 5 d before surgery, we acquired a CT scan using a 64-slice scanner Aquilion 64® (Toshiba Medical System Co., Tochigi, Japan), set at 120 kVp, 250 mAs, 1 mm slice thickness and 1 mm interslice interval. Patient data were transferred to the navigation computer and reconstructed as three-dimensional (3D) images on a monitor. Seven to ten specific anatomical landmarks were preselected as potential registration points before operation (). In three operations, previously deployed embolization coils () were preselected as potential registration points.
Intraoperative navigation and surgical resection
The system is designed so that the surgeon is alerted and the computer navigation system will not allow surgery to begin if landmark localization accuracy is not less than 1 mm. After exposure of the preselected landmarks, the reference arc for the StealthStation is fixed to the ilium or sacrum according to the location of the tumor. Image-to-patient registration to match the patient’s real anatomy to the preselected points on the CT images is first performed using paired points. A navigation probe is touched to the real anatomical points to match them with the preselected points. Surface point matching further refines the registration. During this process, multiple points (a minimum of 30) are selected on the exposed surface of the normal pelvic or sacral bone.
These data are incorporated into the navigation system to calculate the registration error, which provides the magnitude of mismatch between the preoperative multiplanar and 3D CT images and the real anatomy of the patient. We achieved a registration error of less than 1 mm for all patients. The preselected landmarks for the registration points and the overall time to registration were recorded for analysis. Time to registration was defined as the time from fixing the registration arc until the finishing time of the last point for surface matching to obtain a registration error of less than 1 mm.
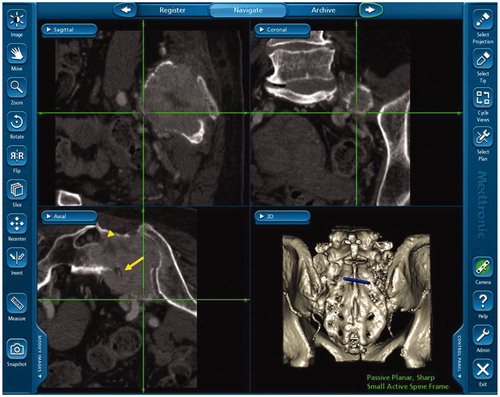
We utilized navigational guidance not only to achieve a proper margin at the resection site but also to locate and preserve the inferior gluteal artery at the greater sciatic notch. The navigation instruments allowed the identification of inferior gluteal artery underneath muscles or soft tissues at the greater sciatic notch ( and ). This navigation system also detected sciatic nerve roots encased by tumor in cases 2, 5 and 7 ().
Postoperative follow-up
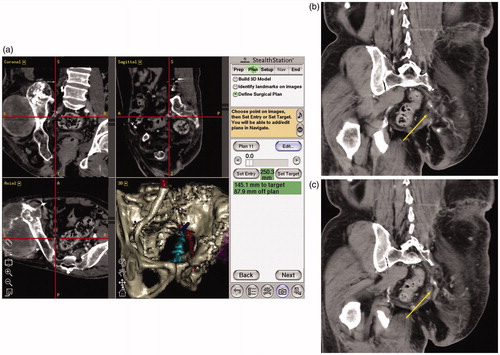
All patients were seen at our postoperative outpatient clinic every three months for the first two years, every four months for the next three years and every six months thereafter. At each visit, radiographs of the pelvis (anteroposterior and lateral views) were taken, and CT of the chest and pelvis was performed. Preservation of blood flow in the inferior gluteal artery was confirmed by postoperative CT or MRI three months or more after surgery ( ).
Results
Patient outcomes are summarized in . In all cases, the minimum global error of less than 1.0 mm was achieved. We felt that Cranial version 5.2.5 required more preparation time because of lower sensitivity to detect fine and complicated skeletal structures. We therefore used it only once, in Case 4, and used version 2.0.1 for the seven other surgeries. The time required for registration was measured from when the handheld wand was verified, through the registration of successive anatomic landmarks, until the final registration point was entered and the minimum global error was achieved. The median time required for registration was 29 min (range, 22–43 min). The median volume of intraoperative hemorrhage was 2176 mL (range, 300–6100 mL). Preservation of the inferior gluteal artery was confirmed by postoperative CT (). shows images of the intact artery at the greater sciatic notch. Mean operating time was 5 h and 50 min (3 h and 00 min–10 h and 10 min). Postoperative complications consisted of one case of wound necrosis requiring treatment in the operating room and one hematoma. The wound necrosis reached the superficial fascia of the external oblique muscle, and had an area of 2 × 10 cm. It was successfully treated by debridement and vacuum-assisted closure VAC® (KCI KK, Tokyo, Japan). The hematoma was removed and debrided in the operating room. There was no deep infection. All surgical specimens except for Case 2 achieved a negative margin. No local recurrence or distant metastasis has yet been observed in any of the cases.
Table 2. Outcomes.
Discussion
Previous reports on surgery with computer navigation [Citation2,Citation10,Citation11] have focused on precise bone tumor resection with accurate visualization of the extent of the tumor, without focusing on important soft tissue structures. The assistance of a computer navigation system in pelvic and sacral tumor resection has been reported to be a safe technique which allows adequate surgical resection under complex anatomical situations.[Citation2,Citation10,Citation11] The ability of the computer navigation system to perform precise and complex tumor resection makes it possible to create customized pelvic prostheses preoperatively.[Citation10,Citation11] We focused on vulnerable soft tissue structures and visualized important arteries with the computer-assisted navigation system, concentrating particularly on identification of the inferior gluteal artery at the greater sciatic notch.[Citation8] Operators usually have to release or cut the inferior gluteal artery from its attachments to the greater sciatic notch during pelvic tumor resection in order to admit a finger into the pelvis via the notch. Without navigational assistance, the inferior gluteal artery was frequently transected. In our series, preservation of the inferior gluteal artery was achieved in all cases. Using the computer-assisted navigation system, we were able to predict the exit points of the inferior gluteal artery before exploring the greater sciatic notch. This navigational technology has great potential benefit in allowing surgeons to confirm the location of otherwise invisible structures during surgery.
Postoperative complication rates following pelvic and sacral tumor surgeries are high,[Citation4,Citation6,Citation11] particularly with regard to wound complications:[Citation3,Citation4,Citation13] reported flap necrosis rates range from 16 to 38%,[Citation3,Citation4,Citation12] and wound infection rates from 35 to 79%.[Citation3,Citation4,Citation12,Citation14] Previous studies have demonstrated the importance of maintaining perfusion of the gluteus maximus to prevent flap necrosis.[Citation3,Citation15] Senchenkov et al. [Citation3] reported the adverse influence of ligation of the common iliac artery on flap integrity following external hemipelvectomy compared to ligation of the external iliac artery, and concluded that the level of vascular ligation strongly influences the posterior hemipelvectomy flap necrosis rate. The importance of the attachment of the gluteus maximus muscle to the fasciocutaneous part of the flap was reported in 1943.[Citation15] Detachment of the fasciocutaneous hemipelvectomy flap from the gluteal muscle was associated with a flap necrosis rate of 60%. Local tissue necrosis is a serious potential complication following pelvic and sacral tumor resection, and is one of the primary risk factors for the development of wound infection. One of the most common causes of in-hospital death following pelvic tumor operation is deep infection and subsequent sepsis, with perioperative mortality rates reaching up to 8%.[Citation3,Citation4,Citation12] Thus, the preservation of flap perfusion is important not only to reduce postoperative complications but also to reduce mortality.
In our series, we experienced one case of flap necrosis, which was treated successfully with debridement and vacuum-assisted closure. None of our patients experienced postoperative infection. This low rate of flap necrosis and wound infection may have been facilitated by the preservation of the inferior gluteal artery afforded by the computer navigation system.
To our knowledge, this report is the first in the English literature to demonstrate the intraoperative detection and intentional preservation of important vessels with computer-assisted navigation. We were also able to detect the obturator artery and tumor-encased sacral nerve roots in several cases.
Our procedure did not reduce the incidence of intraoperative bleeding; intentional preservation of small arteries does not lead to a reduction in intraoperative hemorrhage. Areas prone to bleeding in the pelvis include not only the greater sciatic notch but also the venous plexuses around the base of the bladder, the obturator vessels and the osteotomy surface.
In this article, we only showed bone tumor cases. The intra-operative localization of the bone tumor during surgery was easy and reliable because the CT demonstrated the bone tumor very clearly. The attachment of the inferior gluteal artery to the greater sciatic notch means that it anchors to the pelvis at the greater sciatic notch. However, one can expect a reduced reliability of navigation to detect the intra-operative position of the soft tissue tumor. All this may happen because a part of the tumor and the surrounding soft tissue shift when undergoing positioning and surgical manipulation. Actually, the median global error was 10.0 mm for recurrent colorectal cancer operations,[Citation16] although we achieved a registration error of less than 1 mm for all the patients. We cannot demonstrate the practical solutions to this problem. Thus, we recommend that the current computer imaging navigation system should be applied to detect the inferior gluteal artery at the greater sciatic notch and the bone tumor position.
In conclusion, our study introduces a new application for computer imaging navigation in pelvic tumor surgery that facilitates detection of the inferior gluteal artery at the greater sciatic notch. This technique is easily learned and applied, and shows promise in reducing postoperative complications following pelvic and sacral tumor resection.
Disclosure statement
The authors declare that they have no competing interests.
The authors did not receive any outside funding or grants in support of their research or for the preparation of this work. Neither they nor any member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from any commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or any member of their immediate families, are affiliated or associated.
References
- Angelini A, Drago G, Trovarelli G, et al. Infection after surgical resection for pelvic bone tumors: an analysis of 270 patients from one institution. Clin Orthop Relat Res. 2014;472:349–359.
- Jeys L, Matharu GS, Nandra RS, et al. Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Joint J. 2013;95-B:1417–1424.
- Senchenkov A, Moran SL, Petty PM, et al. Predictors of complications and outcomes of external hemipelvectomy wounds: account of 160 consecutive cases. Ann Surg Oncol. 2008;15:355–363.
- Baliski CR, Schachar NS, McKinnon JG, et al. Hemipelvectomy: a changing perspective for a rare procedure. Can J Surg. 2004;47:99–103.
- Akiyama T, Clark JC, Miki Y, et al. The non-vascularised fibular graft: a simple and successful method of reconstruction of the pelvic ring after internal hemipelvectomy. J Bone Joint Surg Br. 2010;92:999–1005.
- Yuen A, Ek ET, Choong PF. Research: is resection of tumours involving the pelvic ring justified?: A review of 49 consecutive cases. Int Semin Surg Oncol. 2005;2:9.
- Ogura K, Miyamoto S, Sakuraba M, et al. Immediate soft-tissue reconstruction using a rectus abdominis myocutaneous flap following wide resection of malignant bone tumours of the pelvis. Bone Joint J. 2014;96-B:270–273.
- Sim FH, Choong PFM, Weber KL. Orthopaedic oncology and complex reconstruction. In: Sim FH, editor. Master techniques in orthopaedic surgery. Philadelphia: Lippincott Williams & Wilkins; 2010. p. 3–82.
- Malawer MM, Sugarbaker PH. Musculoskeletal cancer surgery: treatment of sarcomas and allied diseases. Dordrecht/Boston/London: Kluwer Academic Publishers; 2001. p. 423–436.
- Wong KC, Kumta SM, Chiu KH, et al. Computer assisted pelvic tumor resection and reconstruction with a custom-made prosthesis using an innovative adaptation and its validation. Comput Aided Surg. 2007;12:225–232.
- Wong KC, Kumta SM. Computer-assisted tumor surgery in malignant bone tumors. Clin Orthop Relat Res. 2013;471:750–761.
- Apffelstaedt JP, Driscoll DL, Spellman JE, et al. Complications and outcome of external hemipelvectomy in the management of pelvic tumors. Ann Surg Oncol. 1996;3:304–309.
- Hillmann A, Hoffmann C, Gosheger G, et al. Tumors of the pelvis: complications after reconstruction. Arch Orthop Trauma Surg. 2003;123:340–344.
- Masterson EL, Davis AM, Wunder JS, et al. Hindquarter amputation for pelvic tumors. The importance of patient selection. Clin Orthop Relat Res. 1998;350:187–194.
- King D, Steelquist J. Transiliac amputation. J Bone Joint Surg Am. 1943;25:17.
- Kocak E, Al-Saif O, Satter M, et al. Image guidance during abdominal exploration for recurrent colorectal cancer. Ann Surg Oncol. 2007;14:405–410.
- Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60:731–746.