Abstract
Background: Pegylated interferon and ribavirin are at present the standard treatment for chronic hepatitis C virus (HCV) patients.
Objective: The present economic evaluation compared 12 vs. 24 weeks of peginterferon alfa-2b + ribavirin treatments for HCV genotypes 2 or 3. Shortening the period of antiviral therapy is important in terms of adverse events and costs.
Methods: Clinical evidence was based on the results of a multicentre, randomised controlled clinical trial (RCCT) conducted in Italy, which found that the shorter course of therapy was as effective as the 24-week course for patients with HCV genotypes 2 or 3 responding to treatment at 4 weeks. A cost minimisation analysis was performed. The analysis took the Italian National Health Service (INHS) point of view, thus only healthcare costs (drugs, medical consultations, diagnostic tests, hospital admissions) were considered. Healthcare activities were estimated by the RCCT principal investigators and were priced by applying the INHS tariffs and prices.
Results: The total mean cost per patient was estimated at €9,785 for the standard group and €7,508 for the variable-duration group. Sensitivity analysis confirmed the robustness of the baseline results.
Conclusions: This study showed that the variable-duration regimen can be recommended as an efficient use of resources for patients from the INHS perspective.
Introduction
Chronic hepatitis C disease is the primary reason for liver transplantation in developed countries. According to World Health Organization estimates, approximately 180 million people in the world are infected with chronic hepatitis C virus (HCV), 130 million of them being HCV carriers at risk of developing liver cirrhosis and/or liver cancerCitation1. According to the last available estimates referring to 1999Citation2, 3–4 million people worldwide are newly infected each year and 70% are likely to develop chronic hepatitis. The prevalence in Europe is 1.03% (approximately 8.9 million people) but all these figures may have grown during the last decadeCitation3,Citation4.
HCV infection is asymptomatic or paucisymptomatic in 90% of cases. The immune system is unable to eliminate the virus in 50–80% of adult cases, so the disease becomes chronic and extrahepatic manifestations may develop, such as vasculitis, mixed cryoglobulinaemia or glomerulonephritis, among others Citation1,Citation2,Citation5. Patients usually have high transaminases, but the disease often goes unrecognised even in the chronic phase. It has been estimated that no more than 50% of HCV-infected persons are diagnosed in most developed countries, with two-thirds of them needing antiviral treatmentCitation1,Citation2,Citation4,Citation6.
Several vaccines are currently under development. However, until they become available precautions must be taken to prevent infection (e.g. testing blood and donor organs, virus inactivation of plasma derivatives, implementation and maintenance of infection control practices in healthcare settings), and the treatment of infected patients is the only way to limit diffusion of the virus.
There are at least six HCV genotypes showing differences in viral nucleotide sequence. Their main clinical differences lie in the response to antiviral treatmentCitation7. Patients infected with genotypes 1 or 4 achieve a lower sustained virological response than genotypes 2 and 3Citation8. As a consequence, different doses and duration of antiviral treatments are required according to the viral genotype.
Pegylated interferon and ribavirin are at present the standard treatment for chronic HCV patients. According to current guidelinesCitation9, 48 weeks of combination therapy is required for genotypes 1 and 4, and 24 weeks for genotypes 2 and 3. Overall, peginterferon with ribavirin is effective in up to 50% of patients; >80% of genotypes 2 and 3, and 46% of genotypes 1 and 4Citation8,Citation10–12.
The present economic evaluation compared 12- and 24-week peginterferon alfa-2b + ribavirin treatments for HCV genotypes 2 or 3. Since combination treatment with peginterferon and ribavirin is expensive and not very well tolerated, shortening the period of antiviral therapy with these two drugs is important in terms of adverse events and costs. Several studies have attempted to shorten the treatment for genotype 2 and 3 patientsCitation13–17, but they all presented substantial limits. The first complete study was a multicentre, randomised controlled clinical trial (RCCT) conducted in ItalyCitation18, on which this economic evaluation is based.
Methods
Efficacy
Clinical evidence was based on the abovementioned RCCT conducted in ItalyCitation18, which found that a shorter course of therapy with peginterferon alfa-2b and ribavirin (12 weeks) was as effective as a 24-week course for patients with genotypes 2 or 3 responding to treatment at 4 weeks.
The study was a non-inferiority trial comparing the standard and variable-duration strategies. Since it was recognised that the standard group would have provided little new information and only experience gained with the new variable-treatment schedule might have been advantageous, a 3:1 randomisation ratio was considered in favour of the variable group. Randomisation was done centrally, without stratification according to genotype, and using a permuted-block method.
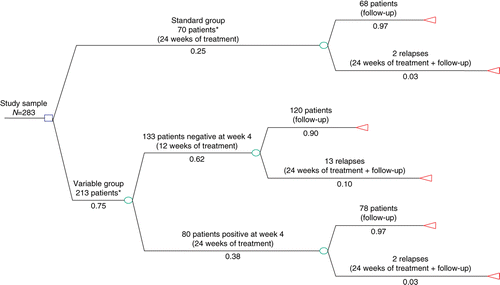
The 283 patients participating in the RCCT were assigned to two groups: 70 to the standard treatment group (24 weeks) and 213 to the variable-duration group, where the duration depended on a rapid virological response at week 4. Patients who were HCV RNA-negative at week 4 were treated for 12 weeks, whilst those still positive at week 4 received 24 weeks of combination therapy. Therefore, the variable-duration group comprised two subgroups: 133 patients HCV RNA-negative at week 4 and 80 patients positive at week 4. The main characteristics (age, sex, HCV genotype, HCV RNA levels) of the patients did not differ significantly (p>0.05) in the two arms.
The efficacy endpoint was measured in terms of sustained virological response (i.e. undetectable HCV RNA 24 weeks after the end of treatment): 76% (53/70 patients) in the standard group and 77% (164/213 patients) in the variable group. Relapses were slightly more frequent in the variable group and side effects were slightly higher in the standard group (p=0.049). These results supported the substantial equivalence of the two regimens.
Study design
In light of the RCCT findings of equivalence, a cost minimisation analysis was performed. The analysis took the Italian National Health Service (INHS) point of view, thus only healthcare costs were considered.
The diagnostic and therapeutic pattern according to the study protocol is depicted in . Additional information was requested to calculate the resource consumption of the two regimens from the RCCT principal investigators. The mean patient cost per group was then calculated.
The time horizon of the study, including the re-treatment of relapses, was: 18 months for the 12-week-treated patients; and 21 months for the 24-week patients.
Resource consumption
During the RCCT, patients received the following treatment for 12 or 24 weeks: (i) peginterferon alfa-2b 1.0 μg/kg weekly (mean weight 69 kg) plus (ii) oral ribavirin 1000 mg/day for body weight up to 75 kg or 1200 mg/day for higher body weight.
A weekly diagnostic and therapeutic schedule, derived from the RCCT protocol, was built up to estimate resource consumption (). At baseline (T0), all patients had a psychiatric and an ophthalmologic consultation before hospital admission; once admitted they had various diagnostic and laboratory tests (Set A in ). Two weeks later (T2), each patient had complete blood tests and a gastroenterologist consultation, then further laboratory tests at the end of the first month of therapy (Set B in ), followed by a further medical consultation (T4). The procedures at T4 were repeated at the end of the second (T8) and third month (T12). At this stage, the 133 patients in the 12-week group concluded their therapy and began a 6-month follow-up. The 24-week treatment patients (i.e. 70 of the standard group plus 80 of the variable group positive at week 4) repeated the laboratory tests (Set B) and the medical consultation as above at the end of the fourth (T16), fifth (T20) and sixth months (T24). After 6 months, these patients concluded their therapy and began follow-up (6 more months).
Table 1a. 24-week diagnostic/therapeutic scheme followed by patients during the clinical trial.
Table 1b. 12-week diagnostic/therapeutic scheme followed by patients during the clinical trial.
Both groups had a medical consultation and the same set of diagnostic tests carried out at the end of each month (Set C in ).
Table 1c. 24-week diagnostic/therapeutic scheme followed by patients during the clinical trial
Costs of treatment for adverse events (depression, thyroid dysfunction, anaemia and neutropenia) as well as relapses were included. Depression and thyroid dysfunction occurred in 27 patients, comprising 8 of those treated for 12 weeks and 19 patients treated for 24 weeks (9 in the standard treatment group and 10 in the variable treatment group).
Anaemia and low white cell counts (neutropenia) required a reduction in the ribavirin and peginterferon doses for 15 patients in the variable group (5 for the 12-week and 10 for the 24-week treatment groups) and 8 patients in the standard-duration group. Therefore, these side-effect costs were estimated as a drug cost reduction only.
In the variable-duration group, 5 patients (1 treated for 12 weeks and 4 treated for 24 weeks) dropped out because of side effects; in the standard treatment group 4 patients did not complete treatment because of poor compliance or side effects. These patients were not excluded from the cost analysis since an ‘intention-to-treat’ approach was adopted.
Starting from the first diagnostic visit (T4),depression, anaemia and neutropenia were treated until the end of treatment (12 or 24 weeks), whilst thyroid dysfunction was treated with thyroid hormones throughout the study in all groups, as this iatrogenic dysfunction unfortunately becomes chronic. Depression was treated with an antidepressant drug (paroxetine).
All relapses (2 patients in the standard group and 16 patients in the variable group, of whom 13 were treated for 12 weeks and 3 for 24 weeks) repeated the 24-week treatment course, including the related diagnostic procedures and laboratory tests and the 6-month follow-up. Re-treatment of relapses was assumed to start at the beginning of the fourth month of follow-up (T28 for 12-week and T40 for 24-week treatment).
Unit costs
Specialist consultations and laboratory tests were priced by applying the INHS outpatient tariffs, hospital admissions were priced by the national tariffs from the diagnosis-related group-like system, and drugs by dispensing prices (). All tariffs and prices refer to the year of the RCCT (2003).
Table 2. Italian National Health Service tariffs and drugs prices (Euros).
Sensitivity analysis
A sensitivity analysis was conducted to test the robustness of the resultsCitation19. The unit costs of medical consultations, diagnostic tests, drugs and hospitalisations were varied simultaneously by ± 10%, ± 20% and ± 50% to check the effects on the final resultsCitation20
Results
shows the mean total cost by group as well as splitting the costs of relapses.
Table 3. Average cost per patient by group (Euros).
The total mean cost per patient was estimated at €9,785 for the standard group and €7,508 for the variable group. Peginterferon plus ribavirin therapy was by far the largest component, accounting for >80% of the total costs in both regimens. The mean cost of relapses was lower in the standard group than the variable group; successfully treated patients accounted for >90% of total costs in both groups.
The sensitivity analysis confirmed the robustness of the baseline results ().
Table 4. Sensitivity analysis.
Discussion
An economic evaluation was designed based on the results of an Italian multicentre RCCT, conducted on a representative sample of patients with HCV genotypes 2 or 3, which supported the substantial equivalence of two different regimens of the same combination therapy aimed at eradicating HCV in chronically infected patients. Therefore, cost minimisation analysis was chosen as the appropriate type of economic evaluation.
The major limitation of the study is that resource consumption was not retrieved from the medical records of the patients enrolled in the RCCT, since the scientific board had not planned an economic evaluation when the study was launched. To estimate costs, data collection alongside a CCT is always preferable to a follow-up study, like in this case. However, these results should be based on reliable proxies of real data since all healthcare activities were estimated by the principal investigators of the RCCT. Moreover, combination therapy was by far the largest cost component in both groups and was directly retrieved from the RCCT. Another potential limit is that costs of medical and diagnostic tests might have been overestimated compared with real practice, since patients were probably seen more often than routine to scrutinise the innovative schedule. However, a negligible proportion (<10% of the total) of the costs of medical consultations and diagnostic tests was estimated in both groups, so this overestimate should have not affected the final results too much.
The results are unfortunately not comparable with other studies, since economic evaluations on peginterferon alfa in the literature all compare it with other active ingredientsCitation21–23.
This study identified the variable group as a cost-saving alternative to the standard group, even after a wide-range sensitivity analysis to test the robustness of the baseline results. Since combination therapy was by far the major cost component, it is worth noting that the weekly 1.0 μg/kg of peginterferon alfa-2b used in the Italian multicentre RCCT is lower than the standard of 1.5 μg/kg weekly. The lower dose was already shown to be as effective as 1.5 μg/kg in genotypes 2 and 3 patients in the original registration trial on combination treatment with peginterferon and ribavirinCitation11. If the standard dose had been adopted, as Dalgard et al did in a Norwegian pilot studyCitation17, savings might have been greater for the variable treatment schedule.
Although the duration of therapy is still open to discussionCitation24, current evidence to support individualised shortening of treatment after rapid virological response appears to be strong enoughCitation25. Rapid virological response at week 4 is the basis for the clinical decision to adopt the 12-week schedule rather than the traditional 24-week standard regimen. To increase the rate of negativity (i.e. the rate of responders) to the variable treatment, physicians should select patients according to baseline characteristics predictive of the response (e.g. low fibrosis scoreCitation26 or young age for genotype 1 short treatmentCitation27). Further research is needed to clarify this, with a view to individualising treatment in HCV patients. Since the total saving per patient in case of negativity was estimated at €2,277 in the variable group (cost at 24 weeks minus cost at 12 weeks), a routine targeting strategy for patients could lead to worthwhile savings from the health authorities’ perspective.
In conclusion, this study showed that the mean cost of management for patients with HCV genotypes 2 or 3 given a shorter course of therapy (12 weeks) with peginterferon alfa-2b and ribavirin was lower than the standard 24-week course. Therefore, the variable-duration regimen can be recommended as an efficient use of resources for patients from the INHS perspective.
Acknowledgements
Declaration of interest: The authors have declared no conflict of interest and have received no payment in the preparation of this manuscript.
Notes
References
- Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of Hepatology 2006; 45: 529–538.
- WHO. Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. Journal of Viral Hepatitis 1999; 6: 35–47.
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infectious Diseases 2005; 5: 558–567.
- Perz JP, Farrington LA, Pecoraio C, et al. Estimated global prevalence of hepatitis C virus infection. Boston: 42nd Annual Meeting of the Infectious Diseases Society of America (Abstract). 2004.
- Nocente R, Ceccanti M, Bertazzoni G, et al. HCV infection and extrahepatic manifestations. Hepatogastroenterology 2003; 50((52))1149–1154.
- National Institute of Health Consensus Development Conference Statement Management of Hepatitis C. Hepatology 2002; 36(suppl 1): S3–S20.
- Zein NN. Clinical significance of hepatitis C virus genotypes. Clinical Microbiological Reviews 2000; 13: 223–235.
- Shepherd J, Brodin H, Cave CB, et al. Clinical- and cost-effectiveness of pegylated interferon alfa in the treatment of chronic hepatitis C: a systematic review and economic evaluation. International Journal of Technology Assessment in Health Care 2005; 21((1))47–54
- National Institute for Health and Clinical Excellence. Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of chronic hepatitis C. Technology Appraisal Guidance 75. National Institute for Health and Clinical Excellence, London 2004.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine 2002; 347: 975–982.
- Manns MP, McHutchinson JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet 2001; 358: 958–956.
- Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. PEGASYS International Study group. Peginterferon alpha 2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Annals of Internal Medicine 2004; 140: 346–355
- Nomura H, Tsuchiya Y, Maruyama T, et al. The effects of a high dose, short course of interferon on hepatitis C. Journal of Gastroenterology and Hepatology 1999; 14: 85–89
- Ide T, Kumashiro R, Hino T, et al. Short term and two-step interferon therapy for chronic hepatitis C patients with low HCV RNA levels. Hepatology Research 2002; 22: 145–151
- Bjøro K, Bell H, Hellum KB, et al. Effect of combined interferon-alfa inductuion therapy and ribavirin on chronic hepatitis C virus infection: a randomized multicentre study. Scandinavian Journal of Gastroenterology 2002; 37: 226–232
- Nousbaum JB, Cadranel JF, Savary O, et al. Sustained virological response after a short course of treatment with interferon and ribavirin in two chronic hepatitis C patients. 2003; 39: 655–656
- Dalgard O, Bjøro K, Hellum KB, et al. Treatment with pegylated interferon and ribavarin in HCV infection with genotype 2 or 3 for 14 weeks: a pilot study. Hepatology 2004; 40: 1260–1265
- Mangia A, Santoro R, Minerva N, et al. Peginterferon Alfa-2b and Ribavirin for 12 weeks in HCV genotype 2 or 3. New England Journal of Medicine 2005; 352: 2609–2617
- Drummond MF, O’Brien BJ, Stoddart GL, et al. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press. 1997.
- Garattini L, Grilli R, Scopelliti D, et al. A proposal for Italian guidelines in pharmacoeconomics. Pharmacoeconomics 1995; 7: 1–6
- Buti M, Medina M, Casado MA, et al. A cost-effectiveness analysis of peginterferon alfa-2b plus ribavirin for the treatment of naïve patients with chronic hepatitis C. Alimentary Pharmacology & Therapeutics 2003; 17: 687–694
- Siebert U, Sroczynski G, Rossol S, et al. Cost-effectiveness of peginterferon alfa-2b plus ribavirin versus interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut 2003; 52: 425–432
- Malone DC, Tran TT, Poordad FF. Cost-efficacy analysis of peginterferon alfa-2b plus ribavirin compared with peginterferon alfa-2a plus ribavirin for the treatment of chronic hepatitis C. Journal of Managed Care Pharmacy 2005; 11((8))687–694.
- Schiffman ML, Suter F, Bacon BR, et al. PegInterferon Alfa-2a and Ribavirin for 16 or 24 weeks in HCV Genotype 2 or 3. New England Journal of Medicine 2007; 357: 124–134
- Mangia A. Short-duration therapy for hepatitis C: suitable for all?. Journal of Viral Hepatitis 2007; 14: 221–227
- Andriulli A, Dalgard O, Bjøro K, et al. Short-term treatment duration for HCV-2 and HCV-3 infected patients. Digestive and Liver Disease 2006; 38: 741–748
- Mangia A, Minerva N, Bacca D, et al. Individualized treatment duration for hepatitis C genotype 1 patients: a randomized controlled trial. Hepatology 2007; 46: 1–8