Abstract
Objective: This analysis was conducted to compare the direct medical costs of treatment with darbepoetin alfa every 3 weeks (Q3W) and epoetin alfa every week (QW) in patients with chemotherapy-induced anaemia (CIA) from the payer's perspective.
Methods: An analysis was conducted from a US health plan perspective to compare the annual budget impact for CIA with darbepoetin alfa Q3W and epoetin alfa QW over a 16-week treatment period. Dosing regimens were obtained from registration clinical trials.
Results: Mean doses, including dose adjustments, were 375.6 μg Q3W for darbepoetin alfa and 43,187 U QW for epoetin alfa. Costs of medical resources included drug acquisition and administration costs. The base case analysis resulted in a per-patient budget impact of $8,544 and $8,667 for darbepoetin alfa and epoetin alfa, respectively. Per member per month cost was $0.90 for darbepoetin alfa and $0.91 for epoetin alfa, based on an estimate of 2,735 CIA patients in a health plan population of 2.17 million. The analysis was most sensitive to drug dose, treatment period and drug price.
Conclusions: Results suggest that per-patient direct medical costs of CIA treatment, when initiated at labelled starting doses, are comparable for darbepoetin alfa Q3W and epoetin alfa QW.
Introduction
Anaemia is a common condition among patients with cancer receiving chemotherapy and can cause fatigue, nausea, dyspnoea, anorexia and cardiac dysfunctionCitation1–3. Anaemia-related fatigue is significantly associated with depression, anxiety and loss of productivityCitation4–6. Despite the significant negative impact on patients’ health-related quality of life, typically only two-thirds of patients with chemotherapy-induced anaemia (CIA) receive treatmentCitation2,Citation5
In addition to increased cost to the patient, anaemia is also associated with increased costs to the healthcare systemCitation7,Citation8. A US administrative claims analysis covering year 2000 data for 2.3 million adult health plan members found an anaemia prevalence of 3.5% among all patients in the health plan and 21.4% among those with cancer, with 86.5% of patients not receiving any treatment for their anaemiaCitation8. The average costs for patients with anaemia were more than twice those of their non-anaemic counterparts, even after accounting for age, sex, other co-morbidities and plan typeCitation8. The benefits of anaemia treatment are well established and the results of the analysis indicate that not treating anaemia is associated with considerable costs to payersCitation8
Darbepoetin alfa and epoetin alfa are erythropoiesis-stimulating agents (ESAs) that are approved by the US Food and Drug Administration (FDA) and recommended by the National Comprehensive Cancer Network guidelinesCitation9 for the treatment of anaemia in patients with non-myeloid malignancies, where the anaemia is due to the effect of concomitantly administered chemotherapy in the oncology setting. The two proteins have the same basic structure but are glycosylated differently. Darbepoetin alfa carries two additional sialic acid residues, which increases the half-life of the modified protein approximately three-foldCitation10–12. The two molecules are available in different doses and both have been shown to be safe and efficaciousCitation13–15
The recent approval of darbepoetin alfa for an every 3 weeks (Q3W) regimen in the treatment of CIA has resulted in a need for decision makers to draw on economic comparisons for formulary decision making. Although the most common regimens for darbepoetin alfa and epoetin alfa are every 2 weeks (Q2W) and every week (QW) dosing, respectivelyCitation16, darbepoetin alfa administered Q3W may be advantageous as it allows for synchronisation of patients’ anaemia treatment with ongoing cancer treatments, which may have implications not only for patients and providers but also for health plans. Synchronisation of treatments may reduce the number of visits and blood tests required for patients, reduce treatment-related opportunity costs for oncology clinics, and potentially reduce costs for health insurers without compromising patient care. Haematological outcomes have been shown to be comparable for ESAs administered synchronously and asynchronously with chemotherapyCitation17,Citation18
This study was designed to estimate the annual budget impact of ESA treatment associated with treating cancer patients receiving chemotherapy with either Aranesp® (darbepoetin alfa)Citation19 Q3W or Procrit®Footnote† (epoetin alfa)Citation20 QW from a US health plan perspective. The analysis used data from randomised registration trials as no head-to-head trials are available comparing these dosing regimens.
Patients and methods
Methods
A budget impact analysis was developed to evaluate the economic impact of ESA therapy from a US health plan perspective in patients with non-myeloid malignancies and CIA. The analysis focused on the estimation of direct medical costs that are relevant to a third party payer and compared the total direct medical costs of ESA treatments based on drug acquisition costs and drug administration costs (therapeutic injections and separately identifiable physician office visits). The analysis was designed to estimate the budget impact on a health plan in the US and therefore focused on the estimation of direct medical costs that are relevant to a third party payer.
Data sources
ESA dose and administration
This analysis compared a Q3W dose and administration schedule of darbepoetin alfa with a QW dose and administration schedule of epoetin alfa over a 16-week time horizonCitation19–22. The ESA regimens compared in this analysis were taken from registration studies referenced in the package inserts and the published literature, taking into account dose adjustment due to escalation, reduction or withholdingCitation19–23. The total dose for each ESA was calculated by multiplying the mean ESA dose per injection by the total number of injections over the time horizon.
The mean dose for darbepoetin alfa was obtained from an active-control registration trial comparing darbepoetin alfa initiated at 500 μg Q3W and darbepoetin alfa 2.25 μg/kg QWCitation21. The mean weekly dose reported by Canon et alCitation21 for the Q3W regimen was 125.2 μg, corresponding to a mean dose of 375.6 μg per Q3W scheduled administrationCitation21. The mean dose per scheduled administration for epoetin alfa (43,187 U) was obtained from a registration trial22, as reported by Cremieux et alCitation24. Dosing regimens of darbepoetin alfa and epoetin alfa were subject to dose adjustment based on haemoglobin response.
Time horizon
It was assumed that all patients would receive 15 weeks of ESA therapy and 1 week of observation over the course of 1 yearCitation21. The 16-week time horizon was based on randomised clinical trials that have evaluated the use of both darbepoetin alfa and epoetin alfa in CIA patients with similar ESA time horizonsCitation15,Citation17,Citation18–Citation21,Citation24–26. Discounting was not incorporated into the analysis because the time horizon in this study was less than 1 year.
Costs
Drug costs were based on the reported 2007 average wholesale price (AWP) from the September 2007 edition of the Drug Topics RED BOOK™Citation27. The AWP of darbepoetin alfa was $5.491/μg and the AWP of epoetin alfa was $15.024/1,000 U. Since the AWP may not necessarily reflect the actual purchase price, and AWP minus 20% is a common basis for reimbursement to payers, AWP minus 20% was used for the base case analysisCitation28
The analysis assumed that all patients require one injection per administration and one physician office visit (level two) per administration. To determine the cost of an office visit, the appropriate procedural codes for each type of visit were identified using Current Procedural Terminology (CPT) codes and were assigned costs for each code based on Medicare payment levels using the 2007 Medicare physician fee scheduleCitation29,Citation30. Using Medicare payment levels as a proxy for health plan costs is considered an acceptable costing method in the USCitation29. The cost of a physician office visit for basic evaluation and management (CPT code 99212) was $38.66 and the cost of an injection (CPT code 90772) was $20.09Citation30. The per-visit cost was calculated to be equal to the sum of the physician office visit cost and the injection cost ($58.75). The 99212 office visit code was considered separately identifiable from the 90772 injection code, following recent guidance from the Centers for Medicare and Medicaid Services (CMS)Citation31. Total administration costs were calculated by multiplying the per-visit administration cost by the frequency of visits over the time horizon of the analysis.
Budget impact
To estimate the annual budget impact for a typical health plan, the per-patient budget impact for the 16-week time horizon was determined and multiplied by the estimated number of CIA patients receiving ESA therapy within the plan. Specific assumptions for the following variables were made to estimate the size of the CIA population within a typical health plan: total enrollees; percentage of patients with non-myeloid malignancies; percentage of cancer patients receiving chemotherapy; percentage of chemotherapy patients with anaemia (CIA patients); and percentage of CIA patients receiving ESA therapy.
The total number of enrollees in a typical health plan (2,170,243) was estimated on the basis of the average enrolment for five major US health plansCitation32. To estimate the number of patients in the plan with non-myeloid malignancies (26,043), the prevalence of non-myeloid malignancies in the general US population (1.2%) was multiplied by the total number of enrollees in the planCitation33. The number of non-myeloid cancer patients was multiplied by the percentage receiving chemotherapy (31.3%) to estimate the number of non-myeloid cancer patients in a typical health plan receiving chemotherapy (8,151)Citation34. The number of CIA patients (4,483) was estimated by multiplying the number of chemotherapy patients by the percentage with anaemia (55%)Citation3. Finally, the number of CIA patients was multiplied by the percentage receiving ESA treatment (61%) to determine the total number of CIA patients receiving ESA therapy (2,735)Citation35. The per member per month budget impact was calculated by dividing the annual budget impact by 12 and dividing the resultant value by the total number of enrollees in the health plan.
Sensitivity analyses
One-way sensitivity analyses were performed on four variables: unit ESA drug cost; mean ESA dose per injection; time horizon; and visit costs (unit cost and frequency of visits). The ESA drug price was varied from the base case value (AWP minus 20%) up to the AWP and down to the average sales price (ASP) plus 6%, in accordance with the 2007 payment allowance limits (fourth quarter) set by CMS, which were $3.08/μg for darbepoetin alfa and $9.60/1,000 U for epoetin alfaCitation36
Mean ESA dose per injection was varied from the minimum to the maximum mean ESA dose per injection, as reported in the literature or calculated from the data presented in the studiesCitation15,Citation20–22,Citation24–26,Citation37–39. The minimum mean dose per injection for epoetin alfa was 38,044 U QW, calculated from the data presented in Waltzman et alCitation25, and the maximum mean dose per injection was 46,307 U QW, calculated from the data presented in Witzig et alCitation26. The minimum mean dose per injection for darbepoetin alfa Q3W was varied to 283.8 μg Q3W based on an efficacy study of the Q3W 200-μg regimenCitation38 (representing a 24.4% reduction in dose from the base case), and the maximum mean dose per injection was varied to 467.2 μg Q3W, representing a 24.4% increase from the base caseCitation21. The time horizon for receiving ESA therapy was varied between 12 and 24 weeks. For the time horizon sensitivity analysis, it was assumed that the mean dose per injection reported by Canon et al in a 16-week studyCitation21 was a valid approximation of the dose required over a 12- or 24-week treatment period (i.e. 11 and 23 weeks of ESA treatment with 1 week of observation, respectively). The cost of a physician visit was varied by adding the cost of a complete blood count panel (CPT 85027 = $9.04) and by removing the cost of an evaluation and management visit (CPT 99212 = $38.66). The frequency of physician office visits was varied by requiring a weekly physician office visit, irrespective of the ESA injection schedule.
Confidence intervals for total costs
Bootstrap and Monte Carlo methods were used to calculate 95% confidence intervals (CIs) for point estimates obtained in the base case analysis. The bootstrapping procedure was done with replication using patient-level data. Monte Carlo simulation was done using @Risk Version 4.5 software add-in to Microsoft Excel 2003. These calculations were based on the distribution of dose, since dose was identified as the single most influential cost driver.
The average per-patient dose per Q3W administration was calculated using patient level data from the study by Canon et alCitation21. A dose distribution for darbepoetin alfa Q3W was obtained by bootstrapping 1,000 iterations of 353 patients with replacement (the sample size for the Q3W darbepoetin alfa arm in the trial by Canon et alCitation21).
Data from five epoetin alfa studies were used to calculate CIsCitation15Citation,22,Citation25,Citation26Citation,40 using two different approaches. The first approach was more conservative and weighted the mean dose for each epoetin alfa trial by sample size. The second approach assumed a uniform dose distribution for epoetin alfa (ranging between the lowest dose of 38,044 U calculated from the trial by Waltzman et alCitation25 to 46,307 U, a calculated dose based on the trial by Witzig et alCitation26). Monte Carlo simulation was applied to the dose distribution of both drugs for 5,000 patients to obtain 95% CIs for total costs.
Results
Budget impact
Based on the 16-week per-patient direct medical costs for darbepoetin alfa Q3W (mean dose per injection of 375.6 μg) and epoetin alfa QW (mean dose per injection of 43,187 U), the per-patient impact was $8,544 and $8,667, respectively. The per member per month costs were $0.90 and $0.91 for darbepoetin alfa and epoetin alfa, respectively. The annual budget impact for CIA patients treated with either 100% darbepoetin alfa Q3W or 100% epoetin alfa QW was $23,367,840 and $23,704,245, respectively.
The per-patient budget impacts of darbepoetin alfa Q3W and epoetin alfa QW were similar, with total 16-week per-patient direct medical costs of $8,544 and $8,667, respectively. The CIs calculated on the basis of an epoetin alfa dose distribution weighted by sample size were $8,229–$8,669 for darbepoetin alfa and $8,087–$8,934 for epoetin alfa; when based on a uniform dose distribution for epoetin alfa (ranging between 38,044 and 46,307 U), the CIs were $8,230–$8,920 for darbepoetin alfa and $7,818–$9,157 for epoetin alfa. Results are summarised in . Total per-patient estimated drug acquisition costs for darbepoetin alfa and epoetin alfa were $8,250 and $7,786, respectively, using AWP minus 20%. Total per-patient visit costs for darbepoetin alfa and epoetin alfa were $294 and $881, respectively. The lower visit costs for darbepoetin alfa were due to fewer ESA injections over the 16-week time horizon (5 injections for darbepoetin alfa and 15 injections for epoetin alfa) and lower physician office visit costs for darbepoetin alfa.
Table 1. Base case analysis*: darbepoetin alfa Q3W vs. epoetin alfa QW.
Sensitivity analyses
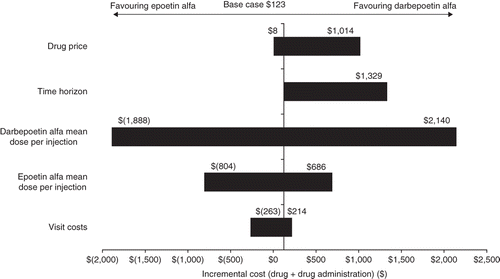
Sensitivity analyses showed that the per-patient budget impact over the 16-week time horizon was most sensitive to changes in assumptions of drug dose (mean dose per scheduled injection), time horizon and drug price, and least sensitive to the cost of an office visit (). For example, when the mean dose per injection for darbepoetin alfa patients decreased from 375.6 to 283.8 μg (as reported in a study by Taylor et alCitation38), the total per-patient incremental budget impact (difference) over the 16-week time horizon increased from $123 to $2,140, favouring darbepoetin alfa. Similarly, when the mean dose per injection for epoetin alfa patients decreased from 43,187 to 38,044 U (as calculated from the data presented in a study by Waltzman et alCitation25), the total per-patient incremental impact (difference) over the 16-week time horizon favoured epoetin alfa (incremental cost of $804). When the duration of the treatment cycle was increased from 16 to 24 weeks, the total per-patient incremental impact increased from $123 to $1,329, favouring darbepoetin alfa. When assumptions about the drug price methodology were changed from AWP minus 20% to ASP plus 6%, the total per-patient incremental impact increased from $123 to $1,014, favouring darbepoetin alfa. For drug administration, when the unit cost of an office visit was changed from $58.75 to $67.79 (due to adding a complete blood count panel at each visit), the total per-patient incremental impact increased from $123 to $214, favouring darbepoetin alfa. shows the impact range of each sensitivity analysis variable on the total incremental budget impact per patient.
Table 2. One-way sensitivity analyses: total per-patient budget impact of treatment.
Discussion
This analysis suggests that the budget impact of darbepoetin alfa Q3W and epoetin alfa QW are comparable in a health plan population with non-myeloid malignancies and CIA. The per-patient budget impact of CIA treatment for 16 weeks, using AWP minus 20% to estimate drug price, was found to be $8,544 (95% CI $8,229–$8,669) for darbepoetin alfa Q3W and $8,667 (95% CI $8,087–$8,934) for epoetin alfa QW, based on simulations using a dose distribution for epoetin alfa weighted by sample size. These results remained consistent when a uniform dose distribution for epoetin alfa (ranging between 38,044 to 46,307 U) was used to calculate CIs.
One-way sensitivity analyses showed that the results were most sensitive to changes in drug dose, time horizon and drug price, least sensitive to the cost of an office visit and moderately sensitive to the frequency of physician office visits. These findings are consistent with results from other clinical trial data and analyses based on claims dataCitation41–43. However, they are not consistent with the results of a meta-analysis estimating cost effectiveness carried out by Rosberg et al, which employed a dose-conversion ratio as an outcome measureCitation37. It is important to note that a dose-conversion ratio has uncertain clinical utility and is not considered an acceptable endpoint in clinical trials by the FDA.
In this analysis, all patients were assumed to require only one physician office visit (level two) and one injection per administration, with the number of visits equal to the number of administrations. However, the frequency of office visits may vary in clinical practice, with more frequent office visits for darbepoetin alfa than one visit every 3 weeks. Changing the frequency of office visits for darbepoetin alfa Q3W to one physician office visit every week, irrespective of ESA administration schedule, resulted in an increase of $386 for darbepoetin alfa administration costs; overall costs for darbepoetin alfa and epoetin alfa were $8,930 and $8,667, respectively, favouring epoetin alfa by a small margin.
These findings are consistent with an economic evaluation by Reed et alCitation44 of an open-label randomised trialCitation25 where patients received either darbepoetin alfa Q2W using a 200-μg injection or epoetin alfa QW. Reed et al found that over a duration of 16 weeks there were no significant differences in total costs, although total costs were higher by $900 for patients receiving epoetin alfa than for patients receiving darbepoetin alfaCitation44. The evaluation also found that the single largest component of total costs was the cost of ESAs and their administration. When a sensitivity analysis was conducted on the method of reimbursement, four out of five methods used resulted in lower drug cost point estimates for darbepoetin alfa than for epoetin alfa. In addition, when analytical perspectives were varied, the costs of darbepoetin alfa Q2W and epoetin QW were not significantly different; however, epoetin alfa tended to be more costly. Six different methods were used to assign costs to study medications and injection time administration. Epoetin alfa was found to be more expensive in all cases except when costs were based on the Veteran's Administration supply scheduleCitation44. The benefits of extended dosing with darbepoetin alfa (e.g. less frequent physician office visits) may not have been fully captured, as patients were required to return to the clinic every week for blood tests as part of the clinical trialCitation44. A more complete accounting of the benefits of the darbepoetin alfa extended dosing regimen may further increase the estimated cost difference between the two regimens.
Similarly, the budget impact analysis results for this study may not fully capture the economic benefits of Q3W dosing. In the analysis, costs for complete blood counts were assumed to be similar between the two products, a conservative assumption as an extended darbepoetin alfa dosing regimen may potentially reduce the need for weekly complete blood counts. In addition, extended dosing regimens allow for synchronisation with chemotherapy treatment; this is likely to reduce the number of required clinic visits, which may also be of economic benefit to payers, patients and caregivers, their employers and healthcare providersCitation45,Citation46. Benefits may also include less time spent by the patient and provider for drug administration and treatment; the average patient receiving any epoetin alfa regimen requires 17.6 hours more treatment time to complete a course of treatment than patients receiving any darbepoetin alfa regimen, as reported by Houts et alCitation47. Indirect benefits, such as member satisfaction with extended dosing, are also not captured. One study reported 72% of patients receiving epoetin alfa QW preferred to switch to a less frequent dosing regimenCitation48
To date, few economic comparisons of darbepoetin alfa and epoetin alfa have been conducted and, to the authors’ knowledge, only one study has been based on a head-to-head trialCitation44. Economic comparisons that are available have a few notable limitations, such as not fully accounting for factors that should be included in the analysis based on the analytical perspective. These factors include the omission of indirect costs, such as the costs associated with treatment-related travel, in an analysis from the societal perspectiveCitation37, relying on meta-analyses based on incomplete evidenceCitation49, and not addressing the effect of differences in treatment regimensCitation50. Therefore, there is an ongoing need for direct head-to-head comparisons to include a comprehensive analysis of budget impact of ESA treatments.
Finally, it is important to note the recent safety concerns regarding the use of ESAs. Revisions to labelling include changes to the US FDA label for ESAs (November 2007) and a recent announcement from the European Agency for the Evaluation of Medicinal Products (October 2007) regarding upcoming changes to product information on the ESA labelling in Europe. The availability of this new information is intended to provide updated information on the risks and benefits of ESAs and to facilitate a full benefit–risk discussion between the prescribing physician and patient. In July 2007, a National Coverage Decision, published by CMSCitation51, restricted coverage of ESAs in Medicare patients. Given that any potential actions will likely affect the entire class of ESAs, the authors anticipate that future changes will impact both darbepoetin alfa and epoetin alfa similarly.
Limitations
There are several limitations to this study that warrant further discussion. The present analysis was carried out from a US health plan perspective, with budget impact based on drug acquisition and drug administration costs. Indirect costs (e.g. lost productivity and travel costs) were not included because the analysis was based on a comparison of direct medical costs from the perspective of a third party payerCitation48. This analysis did not include medical costs related to the management of treatment-related or non-treatment-related adverse events, blood transfusions, laboratory tests, iron supplementation, potential improvement in long-term clinical outcomes or hospitalisations. However, this is not expected to affect the results of the budget impact analysis because these cost components may be impacted similarly by both medications. Additional research is needed to quantify the impact of these particular variables on overall costs. As there are no head-to-head clinical trials comparing these dosing regimens, mean doses were taken from registration trials for both products. In addition, the annual budget impact was estimated based on the assumption that a 16-week time horizon, in which all patients received 15 weeks of treatment and 1 week of observation, reflects the drug acquisition and drug administration costs to the health plan on an annual basis. This assumption is based on the 15-week treatment period in the Canon et alCitation21 study. Base case parameter estimates were varied in the sensitivity analyses and the results were found to be plausibly sensitive to a few key variables.
Conclusion
The per-patient budget impact of CIA treatment for 16 weeks was comparable from a US health plan perspective for darbepoetin alfa administered Q3W and epoetin alfa administered QW. The potential advantages to patients and caregivers of extended dosing regimens of darbepoetin alfa should be further investigated. Comparative trials between QW and Q3W dosing are needed to gain further insight on dosing equivalence. In addition, as new and more efficient ESA dosing regimens are adopted by oncology clinics, their potential impact on the cost of treatment should be calculated.
Acknowledgements
Declaration of interest: Research support was provided by Amgen Inc. The authors would like to thank Niall Harrison and Kirsten Bunch of Gardiner-Caldwell London (Maidenhead, UK) for writing and editorial assistance; Arie Barlev at Amgen Inc. for providing expertise on economic analysis techniques; Peter J Neumann of the Centre for the Evaluation of Value and Risk in Health, Tufts-New England Medical Centre, for comments on an earlier draft; and Jason Scharf of Covance Inc. (San Diego, CA) for economic research and analytical assistance.
Notes
* Aranesp® is a registered trademark of Amgen, Inc.
† Procrit® is a registered trademark of Ortho Biotech Products, LP.
References
- Cella D, Zagari MJ, Vandoros C, et al. Epoetin alfa treatment results in clinically significant improvements in quality of life in anemic cancer patients when referenced to the general population. Journal of Clinical Oncology 2003; 21: 366–373
- Birgegard G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology 2005; 68((Suppl 1))3–11
- Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. Journal of the National Cancer Institute 1999; 91: 1616–1634
- Glaspy J. The impact of epoetin alfa on quality of life during cancer chemotherapy: a fresh look at an old problem. Seminars in Hematology 1997; 34: 20–26
- Berndt E, Kallich J, McDermott A, et al. Reductions in anaemia and fatigue are associated with improvements in productivity in cancer patients receiving chemotherapy. PharmacoEconomics 2005; 23: 505–514
- Tchekmedyian NS, Kallich J, McDermott A, et al. The relationship between psychologic distress and cancer-related fatigue. Cancer 2003; 98: 198–203
- Littlewood T, Zambrowski JJ, Cornes P. Treating anaemia: deconstructing healthcare costs. Current Medical Research and Opinion 2006; 22((Suppl 4))S23–S33
- Nissenson AR, Wade S, Goodnough T, et al. Economic burden of anemia in an insured population. Journal of Managed Care Pharmacy 2005; 11: 565–574
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™, Version 2. Fort Washington, PA: NCCN, 2006. Available at: http://www.nccn.org/professionals/physician_gls/default.asp [accessed April 2006]
- Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP). British Journal of Cancer 2001; 84((Suppl 1))3–10
- Elliott S, Lorenzini T, Asher S, et al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nature Biotechnology 2003; 21: 414–421.
- Powell J, Gurk-Turner C. Darbepoetin alfa (Aranesp). Proceedings (Baylor University Medical Center) 2002; 15: 332–335
- Rearden T, Charu V, Saidman B, et al. Results of a randomized study of every three-week dosing (Q3W) of darbepoetin alfa for chemotherapy-induced anemia (CIA). Journal of Clinical Oncology 2004; 22((Suppl))745S
- Kotasek D, Steger G, Faught W, et al. Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy; results of a double-blind, placebo-controlled, randomised study. European Journal of Cancer 2003; 39: 2026–2034
- Glaspy J, Vadhan-Raj S, Patel R, et al. Randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia: the 20030125 Study Group Trial. Journal of Clinical Oncology 2006; 24: 2290–2297
- Herrington JD, Davidson SL, Tomita DK, et al. Utilization of darbepoetin alfa and epoetin alfa for chemotherapy-induced anemia. American Journal of Health-System Pharmacy 2005; 62: 54–62
- Glaspy J, Applebaum S, Henry D, et al. Darbepoetin alfa 6.75 mcg/kg every 3 weeks (Q3W) may be synchronized with Q3W chemotherapy to alleviate anemia in cancer patients. Annals of Oncology 2004; 15((Suppl))iii219–iii220
- Glaspy J, Henry D, Patel R, et al. Effects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alfa: a randomised clinical trial of synchronous versus asynchronous dosing of darbepoetin alfa. European Journal of Cancer 2005; 41: 1140–1149
- Amgen Inc. Aranesp® (darbepoetin alfa) prescribing information. Thousand Oaks, CA: Amgen Inc., 2006.
- Ortho Biotech Products L.P. Procrit® (epoetin alfa) prescribing information. Bridgwater, NJ: Ortho Biotech Products L.P., 2006.
- Canon JL, Vansteenkiste J, Bodoky G, et al. Randomized, double-blind, active-controlled trial of every-3-week darbepoetin alfa for the treatment of chemotherapy-induced anemia. Journal of the National Cancer Institute 2006; 98: 273–284
- Gabrilove JL, Einhorn LH, Livingston RB, et al. Once-weekly dosing of epoetin alfa is similar to three-times weekly dosing in increasing hemoglobin and quality of life. Proceedings of the American Society of Clinical Oncology 1999; 18: 574a
- Cremieux PY, Fastenau JM, Kosicki G, et al. Cost-minimization analysis of once-weekly versus thrice-weekly epoetin alfa for chemotherapy-related anemia. Journal of Managed Care Pharmacy 2004; 10: 531–537
- Gabrilove JL, Cleeland CS, Livingston RB, et al. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. Journal of Clinical Oncology 2001; 19: 2875–2882.
- Waltzman R, Croot C, Justice GR, et al. Randomized comparison of epoetin alfa (40,000 U weekly) and darbepoetin alfa (200 microg every 2 weeks) in anemic patients with cancer receiving chemotherapy. Oncologist 2005; 10: 642–650
- Witzig TE, Silberstein PT, Loprinzi CL, et al. Phase III randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. Journal of Clinical Oncology 2005; 23: 2606–2617
- RED BOOK™. Drug References. Thomson Healthcare, 2007. Available at: http://www.micromedex.com/products/redbook [accessed September 2007]
- Department of Health & Human Services. Report to the President: prescription drug coverage, spending, utilization, and prices. Department of Health & Human Services, 2000. Available at: http://aspe.hhs.gov/health/reports/drugstudy/ [accessed September 2006]
- Hsiao WC, Braun P, Dunn D, et al. Resource-based relative values: an overview. Journal of the American Medical Association 1988; 260: 2347–2353
- Federal Register. Medicare Program; Revisions to Payment Policies, etc.; Final Rule. Department of Health and Human Services, Centers for Medicare & Medicaid Services 2006; 71((231, part 2):)69623–70274
- Department of Health and Human Services: Office of the Inspector General. Use of Modifier 25. Available at: http://www.ouhsc.edu/bc/documents/oei-07-03-00470useofmod25_000.pdf [accessed November 2006]
- Barnor IF, Namovicz-Peat S. Highlights from AIS's database of health plans. . In: AIS's Directory of Health Plans: 2007 Atlantic Information Services, Inc., 2007
- Ries LAG, Melbert D, Krapcho M et al.SEER Cancer Statistics Review, 1975–2004. National Cancer Institute. 5-year limited duration prevalence. Bethesda, MD: NCI. Available at: http://seer.cancer.gov/csr/1975_2004/ (based on November 2006 SEER data submission, posted to the SEER website, 2007) [accessed November 2007]
- Amgen US Tandem Cancer Patient Audit, 1999–2001
- Amgen US Tandem Cancer Patient Audit, 2007.
- Centers for Medicare & Medicaid Services Medicare Part B Drug Average Sales Price: October 2007 ASP Pricing File (Q4). Available at: http://www.cms.hhs.gov/apps/ama/license.asp?file=/McrPartBDrugAvgSalesPrice/downloads/October07ASPbyHCPCS_091807.zip [accessed September 2007]
- Rosberg JH, Ben-Hamadi R, Cremieux PY, et al. Dose conversion and cost effectiveness of erythropoietic therapies in chemotherapy-related anaemia: a meta-analysis. Clinical Drug Investigation 2005; 25: 33–48
- Taylor K, Ganly P, Charu V, et al. Randomized, double-blind, placebo-controlled study of darbepoetin alfa every 3 weeks for the treatment of chemotherapy-induced anemia. Blood 2005; 106: 992A–993A (abstract 3556).
- Schwartzberg LS, Yee LK, Senecal FM, et al. A randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia in patients with breast, lung, or gynecologic cancer. Oncologist 2004; 9: 696–707
- Agency for Healthcare Research and Quality. Comparative Effectiveness Review Number 3. Comparative effectiveness of epoetin and darbepoetin for managing anemia in patients undergoing cancer treatment. Available at: http://effectivehealthcare.ahrq.gov/healthInfo.cfm?infotype=rr&ProcessID=4&DocID=35
- Berger A, Kallich J, Oster G. Use of darbepoetin alfa and epoetin alfa for cancer-related anemia in clinical practice. In: MASCC/ISOO 18th International Symposium; 22 June 2006; Toronto, Canada. Poster.
- Berger A, Edelsberg J, Kallich JD, et al. Use of darbepoetin alfa and epoetin alfa in clinical practice in patients with cancer-induced anemia. Clinical Therapeutics. 2008; 30: 206–218
- Song X, Long SR, Marder WD, et al. The impact of methodological approach on findings of average weekly dose and cost comparisons between epoetin alfa and darbepoetin alfa. Journal of Managed Care Pharmacy (in press)
- Reed SD, Radeva JI, Daniel DB, et al. Economic evaluation of weekly epoetin alfa versus biweekly darbepoetin alfa for chemotherapy-induced anaemia: evidence from a 16-week randomised trial. PharmacoEconomics 2006; 24: 479–494
- Ross SD, Allen IE, Henry DH, et al. Clinical benefits and risks associated with epoetin and darbepoetin in patients with chemotherapy-induced anemia: a systematic review of the literature. Clinical Therapeutics 2006; 28: 801–831
- Kallich JD, Tchekmedyian NS, Damiano AM, et al. Psychological outcomes associated with anemia-related fatigue in cancer patients. Oncology (Williston Park, N.Y.) 2002; 16: 117–124
- Houts AC, Loh GA, Fortner BV, et al. Patient and caregiver time burden associated with anaemia treatment in different patient populations. Supportive Care in Cancer 2006; 14: 1195–1204
- Meehan K, Tchekmedyian S, Ciesla G, et al. The burden of weekly epoetin alfa injections to patients and their caregivers. Proceedings of the American Society of Clinical Oncology 2003; 22: 543
- Cremieux P, Vekeman F, Lefebvre P. Dose conversion and cost effectiveness of erythropoietic therapies in chemotherapy-related anemia: a Canadian application. Journal of Oncology Pharmacy Practice 2006; 12: 165–178
- Morreale A, Plowman B, DeLattre M, et al. Clinical and economic comparison of epoetin alfa and darbepoetin alfa. Current Medical Research and Opinion 2004; 20: 381–395
- Centers for Medicare and Medicaid Services. Decision Memo for Erythropoiesis Stimulating Agents (ESAs) for Non-Renal Disease Indications. (CAG-0383N). 30 July 2007.