Abstract
Objective: Although atrial fibrillation (AF) is the most commonly sustained arrhythmia in adults, few studies have examined the direct treatment cost of AF.
Methods: A Medicare database of a 5% random national sample of all beneficiaries was used to identify patients diagnosed with AF in 2003 and to follow them for 1 year after diagnosis. These patients were matched on a 1:1 basis by age, gender and race. The incremental cost of treating AF was calculated with multivariate regression models adjusting for covariates.
Results: In total, 55,260 subjects developed new AF, of which 69% were ≥75 years old, 54% were female and 91% were White. The adjusted mean incremental treatment cost of AF was $14,199 (95% confidence interval $13,201–15,001; p<0.01). Some of this cost was attributable to the incidence of stroke and heart failure at the 1-year post-AF diagnosis. A significantly higher proportion of AF patients experienced stroke (23.1 vs. 13.3%; p<0.01) and heart failure (36.7 vs. 10.4%; p<0.01) compared with Medicare beneficiaries without AF.
Conclusions: Mean incremental direct treatment costs for Medicare beneficiaries with AF were higher than previously reported. Interventions that can reduce the incidence of AF and its complications may also reduce the national economic impact of AF.
Keywords::
Introduction
Atrial fibrillation (AF) was estimated to affect 2.5% of the adult population (5.1 million individuals) in the US in 2000 and is projected to affect between 12.1 million and 15.9 million by the year 2050Citation1. There is a general agreement that the prevalence of AF rises as age increasesCitation1–6, with prevalence rates estimated to be 6% in 2002 among Medicare beneficiaries aged ≥65 years with even higher prevalence rates in older subsets of this populationCitation7. In this older population, the prevalence of AF is higher among Whites compared with their Black counterpartsCitation8,Citation9 as well as among men compared with womenCitation3,Citation10.
AF is associated with several co-morbidities, many of which (such as hypertension, coronary artery disease and heart failure (HF)) are age-related cardiovascular conditionsCitation11,Citation12. Other risk factors for AF include cardiac conditions such as valvular heart disease (VHD), myocardial infarction (MI), cardiomyopathy, atrial septal defect, atrial myxoma and cardiac or other thoracic surgical procedures as well as non-cardiac conditions such as hyperthyroidism, diabetes, obesity, chronic obstructive pulmonary disease (COPD), alcohol intoxication, anaemia, sepsis and electrolyte disturbancesCitation13–17.
Not surprisingly, AF also has significant complications. Stroke and HF are the two most important medical complicationsCitation16,Citation18 and greatly contribute to the high cost of AF. The total incremental direct cost for treatment of AF in a privately insured population in the US was estimated to be $12,349, which is five times greater than the cost of treating patients without AFCitation19. Of the total costs associated with AF, hospitalisations account for 50–52%, whilst medications make up an additional 20–23%Citation20,Citation21. In general, there is an increasing trend in the number of hospitalisations related to AF in the USCitation11,Citation22. In particular, chronic dialysis patients had a high incidence of hospitalisation for AF, and patients undergoing coronary artery bypass graft (CABG) surgery who experienced AF had longer lengths of stay than CABG patients with no AFCitation23,Citation24.
Although data exist on the increase of hospitalisations due to AF both in the Medicare and commercially insured populations, limited data are available that comprehensively explore resource utilisation and associated costs of AF among Medicare beneficiaries as well as the specific costs in other healthcare settings such as the emergency room (ER), outpatient clinics and physician offices. In addition, assessment of treatment costs related to co-morbidities and complications associated with AF is lacking. Moreover, data on the incremental economic impact of AF tend to be scarce and not current. Therefore, the objectives of this analysis were to identify the total direct treatment costs of AF patients compared with patients without AF and, hence, the financial impact on the Medicare programme and to identify associated risk factors and complications that significantly contribute to the economic outcomes of AF.
Methods
Data source
This retrospective analysis employed medical claims and associated administrative data for a 5% random sample of Medicare beneficiaries followed longitudinally across time and across multiple settings of care. The Centers for Medicare and Medicaid Services (CMS; the agency responsible for Medicare) record data on the care and outcomes of patients who are Medicare beneficiaries due to their age (≥65 years) or disability status. Data on a 5% random sample of the total Medicare population are available to external researchers through the Medicare Public Use Files. These datasets have been widely used to understand healthcare costs, medical needs and associated clinical outcomes of the US elderly populationCitation25,Citation26.
Inpatient and outpatient medical care was assessed using multiple data files representing different healthcare settings to ensure comprehensive coverage of all medical care provided to AF patients. These data from different settings were linked longitudinally via encrypted unique patient identifier numbers. Information available included patient demographic characteristics (e.g. age group, gender, race), patient clinical characteristics (e.g. principal diagnoses, co-morbidities), medical care and associated costs (e.g. major procedures and services, Medicare reimbursements, length of hospital stay as well as length of stay within special care units, skilled nursing facility use and use of ambulatory care). Data from 2002–2004 were analysed.
Study design and identification of the study population
This study employed a longitudinal, matched cohort design from the Medicare perspective. The study population was selected on an incidence-based approach, based on records of new diagnosis for AF, along with a matched comparison group comprised of Medicare beneficiaries without AF. The study period was the 1st January 2002 to the 31st December 2004, with the possible index date being between the 1st January 2003 and the 31st December 2003. An analytical data file was created that extended for each patient from 1 year prior to the index date through to 1 year after the index date.
Patients in the AF cohort were identified by the presence of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code 427.3 (i.e. index event with the principal code in 2003 including 427.32 (atrial flutter) and 427.30 (atrial tachycardia) and were matched with patients without AF. Individuals in the group without AF were a cohort of Medicare beneficiaries without a diagnosis of AF in the time period studied (2002–2004). This cohort was matched on a 1:1 basis with individuals diagnosed with AF. Matching was performed on basic demographic characteristics including age group, gender and race. Patients were considered for the matching procedure if, in the year before the index AF diagnosis in the AF group or in the year before inclusion in the non-AF group, they did not have a diagnosis of AF or of any clinical consequences of AF such as stroke, HF, syncope, non-cerebral embolic events, chest pain/acute coronary syndrome and drug toxicities.
Statistical analysis
Descriptive statistics were calculated. For categorical variables, percentages were reported. Differences between groups were analysed using Pearson's χCitation2 test. For continuous variables, mean and standard deviation (sd) were reported. Mean differences between groups were analysed using Student's t-test or Wilcoxon rank-sum test. Median, minimum and maximum values were also reported for continuous variables. An a priori level of significance of 0.05 was set for the analyses.
Clinical characteristics in terms of patient co-morbidities and surgical interventions before AF were identified from the literature and were assessed over the 1-year pre-index period. These medical conditions, identified through appropriate primary and secondary ICD-9-CM diagnosis codes, included cerebrovascular disease (CBVD), hypertension, rheumatic heart disease, diabetes, COPD, ischaemic heart disease, obesity, obstructive sleep apnoea, sick sinus syndrome, VHD, hyperthyroidism, cardiomyopathy, anaemia, sepsis and alcoholism. Common cardiovascular procedures such as CABG surgery, cardiac catheterisation and percutaneous coronary intervention were also identified through appropriate ICD-9-CM procedure codes. Indicator variables (1 = yes, 0 = no) were created to signify the presence or absence of these medical conditions or surgical interventions during the pre-index period. Complications known to be caused by AF, notably stroke and HF, along with other common complications associated with AF, including fainting, non-cerebral embolic events, chest pain/acute coronary syndrome and drug toxicities, were also identified and examined through appropriate ICD-9-CM codes in the post-index period. These complications were ascertained as new diagnoses in the post-index period (i.e. study AF patients who had a diagnosis of any one of these AF-related complications in the 1-year pre-index period were excluded from the analyses) to minimise the potential effect that the pre-existence of these conditions may exert on follow-up costs after onset of AF.
Reimbursements made by CMS through the Medicare programme for healthcare utilisation were used to compute healthcare costs. Since claims and associated reimbursements occurred over 3 years, costs were adjusted to 2004 US dollars using the medical care consumer price indexCitation27. Total costs were also disaggregated into ER, hospitalisation, outpatient visit, physician visit, nursing home, hospice and durable medical equipment costs. Statistical significance was examined by looking at the difference obtained between post- and pre-index resource utilisation for each parameter for both cohorts (with and without AF) using the non-parametric Wilcoxon rank-sum test. The AF group was further stratified based on the presence of complications such as stroke and HF to quantify the economic impact of these complications on follow-up costs of AF.
A multivariate ordinary least squares model was employed for computing incremental 1-year costs of AF adjusting for differences in baseline demographic variables, clinical co-morbidities, total baseline cost and complications in the post-index period between the groups with and without AF. This multivariate analysis allowed for the identification of co-morbid conditions and complications that affected total follow-up costs of AF. Stepwise models were systematically built by beginning with age, gender, race, study cohort and geographic region (Model 1), and then extending the model by adding pre-index co-morbid conditions and surgical interventions as well as post-index complications (Model 2) and total baseline cost (Model 3). Total 1-year post-index cost was the dependent variable. Due to the skewness observed in total healthcare cost, a logarithmic transformation of total treatment cost was performed and used as the dependent variable. Adjusted incremental mean treatment costs were calculated by exponentiating the least squares means and then multiplying the result by a ‘smearing’ coefficient, the sum of the exponentiated residuals divided by the sample size, to produce unbiased estimates of the mean expendituresCitation28. All statistical analyses were performed using SAS version 9.1 (SAS Inc., Cary, NC).
Results
Patient characteristics
The study population consisted of 55,260 AF patients (and 55,260 matched non-AF individuals), of whom 69% (n=38,322) were aged ≥75 years and 54% (n=29,802) were female. Among those within the 65–69, 70–74 and ≥75 years age groups, males constituted 56.9, 53.8 and 42.31%, respectively. Most patients were White (91%; n=50,285), whilst 5.3% (n=2,936) were African–American and the remaining 3.7% were comprised of Asian, Hispanic or other races ().
Table 1. Baseline demographic characteristics and costs in the 1-year period prior to the index AF event.
Most AF patients (n=48,232; 87.3%) were coded in the outpatient setting, whilst 8.1% (n=4,471) were identified in the ER and the remaining 4.6% (n=2,557) in the inpatient setting.
There were significant differences between those with and without AF with respect to geographic location, with a higher percentage of AF patients living in the northeast compared with those without AF, and a higher percentage of those without AF residing in the Midwest and western regions of the country (p<0.01).
During the 1-year pre-index period, a significantly higher mean (sd) cost of hospital admissions ($4,372 ($12,943) vs. $1,741 ($6,471); p<0.01), a higher mean (sd) cost of ER visits ($3,114 ($11,540) vs. $1,110 ($5,618); p<0.01), a higher mean (sd) cost of outpatient visits ($1,037 ($3,512) vs. $250 ($2,011); p<0.01) and a higher mean (sd) cost of physician visits ($1,171 ($2,859) vs. $260 ($1,776); p<0.01) were found for the AF cohort compared with the cohort without AF (). The higher costs in each of these cost components for the AF cohort were consistent with more intense resource utilisation in the 1-year pre-index period compared with the cohort without AF: mean (sd) number of hospital admissions (0.5 (1.1) vs. 0.3 (0.7); p<0.01), mean (sd) hospital length of stay (3.5 (10.7) days vs. 1.5 (6.5) days; p<0.01), mean (sd) number of ER visits (0.2 (0.6) vs. 0.1 (0.4); p<0.01), mean (sd) number of outpatient visits (5.0 (7.2) vs. 0.9 (3.5); p<0.01) and mean number of physician visits (24.4 (35.8) vs. 4.6 (17.8); p<0.01). A significantly higher percentage of AF patients had three or more hospital admissions (5.9 vs. 2.0%; p<0.01), three or more ER visits (2.3 vs. 0.7%; p<0.01), three or more outpatient visits (49.0 vs. 9.9%; p<0.01) and three or more physician visits (78.5 vs. 14.5%; p<0.01) compared with patients without AF. The higher resource utilisation resulted in a higher mean (sd) total cost ($6,580 ($14,522) vs. $2,250 ($8,375); p<0.01) in the AF cohort compared with the cohort without AF ().
AF patients had a high baseline prevalence of CBVD (15.9 vs. 9.4%; p<0.01), hypertension (59.3 vs. 49.8%; p<0.01), ischaemic heart disease (36.6 vs. 20.3%; p<0.01), diabetes (25.5 vs. 18.2%; p<0.01), COPD (22.1 vs. 13.7%; p<0.01), anaemia (21.7 vs. 14.6%; p<0.01) and VHD (16.2 vs. 6.3%; p<0.01) (). Other co-morbidities, including rheumatic heart disease, cardiomyopathy, pulmonary embolism, hyperthyroidism, obesity, sepsis, alcoholism, depression and sick sinus syndrome, were also found to be significantly higher among patients with AF compared with those without (). Similarly, AF patients had a higher percentage of any prior cardiac interventions (7.2 vs. 2.6%; p<0.01), most notably cardiac catheterisation (5.6 vs. 2.2%; p<0.01), CABG (1.1 vs. 0.3%; p<0.01) and percutaneous transluminal coronary angioplasty (0.4 vs. 0.2%; p<0.01), as well as any abdominal surgical interventions (3.8 vs. 2.7%; p<0.01) ().
Table 2. Co-morbidities and invasive procedures in the 1-year period prior to the index AF event.
Complications in the post-index period
Stroke and HF were the two major complications of AF in the 1-year post-index period, with a higher percentage of AF patients experiencing stroke (23.1 vs. 13.3%; p<0.01) and HF (36.7 vs. 10.4%; p<0.01) compared with those without AF. Other notable complications, including acute MI (5.0 vs. 2.0%; p<0.01), palpitations (7.0 vs. 2.6%; p<0.01), tachycardia (11.4 vs. 2.5%; p<0.01), chest pain (22.8 vs. 12.5%; p<0.01), drug toxicity (3.0 vs. 0.9%; p<0.01) and non-cardiac embolic events (4.2 vs. 1.2%; p<0.01), were also significantly higher in the AF cohort compared with the cohort without AF.
Incremental treatment costs of AF
The mean 1-year post-index total healthcare cost in the AF cohort was $23,750 (sd $32,653) compared with $7,439 (sd $14,940) for the cohort without AF. Thus, the mean incremental 1-year total cost for treating AF patients was $16,311 (sd $25,391), which reflected significantly greater utilisation of multiple types of medical care. Specifically, a significantly higher percentage of AF patients had three or more hospital admissions (28.4 vs. 7.4%; p<0.01), three or more ER visits (13.6 vs. 2.8%; p<0.01), three or more outpatient visits (72.1 vs. 60.7%; p<0.01) and three or more physician visits (88.9 vs. 87.2%; p<0.01) compared with patients without AF.
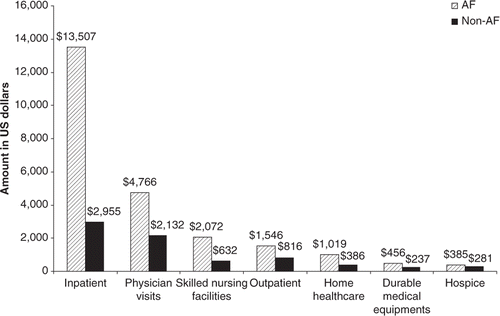
The difference in hospitalisation costs constituted the largest portion (65%) of the total incremental cost of AF (mean (sd) $13,507 ($24,788) vs. $2,955 ($8,959)). However, higher costs of all types among those with AF, including physician visits (mean (sd) $4,766 ($5,975) vs. $2,132 ($3,584)), skilled nursing facilities (mean (sd) $2,072 ($5,760) vs. $632 ($3,193)), outpatient care (mean (sd) $1,546 ($4,101) vs. $816 ($5,005)) home healthcare (mean (sd) $1,019 ($2,583) vs. $386 ($1,787)), durable medical units (mean (sd) $456 ($1,496) vs. $237 ($1,250)) and hospice care (mean (sd) $385 ($2,756) vs. $281 ($2,632)), were consistently observed compared with the group without AF ().
Incremental impact of stroke and HF on AF treatment costs
Stratification of the AF group based on the presence of complications highly prevalent in the AF group, such as stroke and HF, produced an incremental impact of stroke on AF treatment cost of $7,929 ($23,143 vs. $15,214; p<0.01), whilst HF had a much higher impact of $15,540 ($30,664 vs. $15,214; p<0.01) relative to an AF patient without stroke and HF, respectively. After adjusting for other covariates, the incremental costs of stroke and HF on 1-year healthcare costs were $7,907 ($7,824–8,232) and $12,117 ($11,878–12,702), respectively ().
Table 3. Multivariate ordinary least squares regression model: incremental AF total treatment costs in the 1-year post-index period*.
Multivariate analysis of adjusted incremental 1-year total cost of patients with AF relative to those without AF
Mean adjusted incremental costs of treating AF patients compared with individuals without AF were examined in an ordinary least squares regression context (). After adjusting for baseline differences in demographic characteristics only (Model 1), AF patients incurred a mean incremental total healthcare cost of $15,792 (95% confidence interval (CI) $14,234–16,949) compared with individuals without AF. Expansion of Model 1 by including invasive procedures as well as post-index complications associated with AF (Model 2) resulted in a mean incremental total healthcare cost of $14,772 (95% CI $13,294–15,340) among AF patients compared with patients without AF. Model 3 (expansion of Model 2 by including Medicare cost in the year prior to the diagnosis of AF) revealed a mean incremental healthcare cost of $14,199 (95% CI $13,201–15,001) in the 1-year post-index period for an AF individual compared with a patient without AF.
This multivariate analysis also showed that, although pulmonary embolism is present only in 1.4% of the AF population (), it has a significant impact on total costs ($3,938 (95% CI $3,743–4,189)), followed by diabetes ($3,490 (95% CI $3,177–3,603)) and rheumatic heart disease ($2,726 (95% CI $2,556–2,965)). Total treatment costs of those who underwent CABG prior to the onset of AF were significantly higher than those who developed AF without the presence of CABG at baseline ($9,696 (95% CI $8,725–10,190); p<0.01). If a patient had complications such as stroke, HF and MI, AF post-index costs would be even more significant, as HF, stroke and MI incurred mean incremental costs of $12,117 (95% CI $11,878–12,702), $7,907 (95% CI $7,824–8,232) and $12,162 (95% CI $11,747–12,757), respectively.
Discussion
This study examined resource utilisation and associated incremental total healthcare costs among newly diagnosed AF individuals in comparison with a matched cohort of individuals without AF in the Medicare population. The findings suggest that the cost to Medicare beneficiaries with AF is significantly greater than previously recognised and that these costs are affected by the incidence of stroke and HF. The mean incremental treatment cost of AF, adjusted for confounders, was $14,199 (95% CI $13,201–15,001; p<0.01). In the year following the AF diagnosis, stroke and HF were experienced by many AF patients (23.1 and 36.7%, respectively) and had a substantial effect on cost. Specifically, the incremental impact of stroke on AF treatment cost was $7,929 ($23,143 vs. $15,214; p<0.01), whilst HF had a much higher impact at $15,540 ($30,664 vs. $15,214; p<0.01).
Few studies19,21,29–32 have estimated the total treatment cost of AF, and the costs identified here are higher than earlier studies21,30–32. The lone previous Medicare cost analysis, conducted in the early 1990s from 1989 data, estimated the impact of AF on mortality, stroke and medical costsCitation29. Patients with AF incurred significantly higher costs compared with patients without AF (n=26,753; p<0.05) matched on the basis of age and sex. Mean 2-year treatment costs for AF patients were $12,229 compared with $10,682 for patients without AF, yielding an average incremental cost of $1,547. Of note, these costs were greater for male Medicare beneficiaries aged 65–74 years ($14,345 vs. $11,923; p<0.05) than those aged ≥75 years. It is also worth noting that the analysis plan for Wolf's study defined control subjects with one cardiovascular disease diagnosis. Thus, the incremental cost of AF was reduced because patients were already receiving ongoing care for cardiovascular disease. Moreover, Wolf's publication reflected costs and practice patterns more than 8 years old29.
More recent data on younger, commercially insured Americans have yielded findings similar to, but still lower than, these. For example, the total incremental costs (direct plus indirect costs) for treatment of AF was estimated to be $14,875 (p<0.01) in a privately insured US populationCitation19. These incremental costs were computed by comparing the treatment cost of patients with AF ($18,454 ± 47) with that of matched (1:1) patients without AF ($3,579 ± 15) (n=3,944). Of this total incremental cost of $14,875, the incremental annual direct cost of treating AF was found to be $12,349, five times more than the cost of treating a patient who does not have AF (p<0.01). However, this study was conducted among a considerably smaller population (n=3,944) than the present study and did not include patients >65 years of age. The average age of the population in this study was 55 years, a substantially healthier population than Medicare beneficiaries. Furthermore, although co-morbidities were included in the model, the severity of the conditions in this population may not be comparable with that in the present study.
As noted above, the costs identified were higher than those in other studies published recently using other methodologies21,30–32. A study released in 2006 by Coyne et al analysed three cross-sectional, federally funded and nationally representative medical encounter databases to determine costs attributable to treating AF in the USCitation30. This study estimated total annual costs for AF treatment to be $6.65 billion in 2005 US dollars, or approximately $3,000 per person (assuming 2.2 million people with AF in the US as reported in the study), which is well under the estimate found in this studyCitation30. However, the study also reports that the $6.65 billion per year is underestimated as not all encounters with AF as a co-morbidity were included in the study. Also, the analysis did not include fees for hospital-based physician services and did not include actual costs of care for reported services that might have underestimated the total costsCitation30.
A cost-effectiveness study estimated AF costs to be approximately $21,000 for rate-controlled patients and approximately $26,000 for rhythm-controlled patients over a period of 5 yearsCitation31. This estimate, when translated into an annual cost, was also notably smaller than the incremental cost estimate reported in this study, possibly due to limited data collection on resource use. Data on outpatient visits were not reported in this study. Also, hospitalisation costs (the major cost component) estimated reflected an assumption of fewer hospital days compared with the number observed in the Medicare dataCitation31.
The other mentioned studiesCitation21,Citation32 also report total direct costs of AF to be below the authors’ estimate but included sizeable populations <65 years of age. One of these studies might have possibly underestimated the overall costs (approximately $5,600 per patient per year) potentially due to non-estimation of initial hospital costs for approximately one-quarter of study patients whose first AF episode was in an inpatient settingCitation32. The other study estimated the total cost of managing patients with AF in France and reported the mean annual total cost to be €3,209Citation21. This study enrolled a much younger population, as 50% of the total population was <50 years, 12% were aged 50–59 years and approximately one-half of the reported 33% between 60–69 years would have been <65 yearsCitation21.
The incidence of stroke was higher in this study compared with othersCitation34,Citation35. This may be attributable to the inclusion of haemorrhagic stroke as well as ischaemic stroke in the capture of outcomes whereas the other studies only reviewed ischaemic strokeCitation34,Citation35. Also, the AF patients in this study appeared to have a higher degree of baseline CBVD, hypertension, rheumatic heart disease, ischaemic heart disease and VHD that could have potentially led to an increased incidence of follow-up stroke and HF. However, the results mirrored those reported in an Italian study that showed a 24.6% prevalence rate of AF among newly diagnosed ischaemic stroke patientsCitation36.
The authors’ goal was to calculate the total incremental costs to the US Medicare programme of medical care provided to beneficiaries with AF. By definition, because Medicare does not cover all medical expenditures for beneficiaries, some costs (such as deductibles and co-payments borne by supplemental health insurance or out-of-pocket beneficiary payments) are not included in the analysis. Accordingly, the direct medical costs to Medicare presented here are an underestimate of the total treatment costs of AF among those aged ≥65 years. Second, the elderly Medicare population studied is dissimilar to those younger than 65 years in terms of clinical characteristics as well as most likely access to coverage and access. Accordingly, these results should not necessarily be representative of costs for younger patients with AF. Specifically, care should be taken in any extrapolation of these results to younger cohorts. Third, this study was conducted of the care and costs between 2002 and 2004, before Medicare covered most oral medications. Correspondingly, the use and costs of oral medications do not appear in the data analysed. Given the goal was to calculate the costs to Medicare, that absence of data merely reflected the fact that medication costs were not covered by Medicare. However, their absence underestimated the true total cost of AF. Fourth, to identify subjects with an incident diagnosis of AF, a 1-year ‘wash-out’ period without a comparable or similar diagnosis was used to improve the probability that the observed index event was indeed the first such event. However, the authors are unable to confirm that such an event did not happen prior to the wash-out period. Given the chronic nature of care for AF, they would have expected to observe care with a stated diagnosis of AF if earlier events had occurred. Therefore, the authors doubt that they observed a recurrent event, but cannot rule this out. Similarly, they cannot rule out a similar effect in the non-AF cohort. Overall, if some patients in the AF cohort did indeed have earlier AF unobserved in this database and if that rate of earlier AF was greater than such a rate among those in the matching non-AF cohort, then the calculated costs for new AF would be overestimated. Finally, the retrospective, administrative data analysed do not contain the clinical detail captured in typical prospective randomised clinical trials or patient registries. This study could be replicated in greater clinical depth with such studies where the data collection form can be designed to capture such information of use in such an analysis.
Conclusions
Mean incremental direct treatment costs for Medicare beneficiaries with AF were greater than previously reported, even after adjusting for differences in baseline demographic, clinical and economic characteristics. A substantial portion of these costs was attributed to incident stroke and HF among AF patients in the first year following AF. Interventions that can reduce the incidence of AF and its complications may also reduce the national economic impact of AF by preventing ER visits, hospitalisations and other care for AF and its sequelae.
Acknowledgements
Declaration of interest: This study was funded by Astellas Pharma US, Inc.
GAL served as a paid consultant to Abt Associates Inc., which received funding from Astellas Pharma US, Inc. to complete the research presented in this report. GAL also serves as consultant and speaker to AstraZeneca, GSK, Novartis and CVT. WCL and CLP are investigators employed by Abt Associates, a research organisation that received funding from Astellas Pharma US, Inc. to complete the research presented in this report. JS is an employee of Astellas Pharma US, Inc. At the time of the study, SB was an investigator employed by Abt Associates.
Notes
References
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114: 119–125.
- Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997; 96: 2455–2461.
- Tsang TS, Miyasaka Y, Barnes MD, et al. Epidemiological profile of atrial fibrillation: a contemporary perspective. Progress in Cardiovascular Diseases 2005; 48: 1–8.
- Wolf PA, Benjamin EJ, Belanger AJ, et al. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. American Heart Journal 1996; 131: 790–795.
- Furberg CD, Psaty BM, Manolio TA, et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). American Journal of Cardiology 1994; 74: 236–241.
- Tsang TS, Petty GW, Barnes ME, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. Journal of the American College of Cardiology 2003; 42: 93–100.
- Lakshminarayan K, Solid CA, Collins AJ, et al. Atrial fibrillation and stroke in the general Medicare population. A 10-year perspective (1992 to 2002). Stroke 2006; 37: 1969–1974.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Journal of the American Medical Association 2001; 285: 2370–2375.
- Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. American Journal of Cardiology 1998; 82: 2N–9N.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation 2004; 110: 1042–1046.
- Khairallah F, Ezzedine R, Ganz LI, et al. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. American Journal of Cardiology 2004; 94: 500–504.
- Patel PJ, Keating RJ, Gersh BJ, et al. Outcome of patients with newly diagnosed atrial fibrillation at the Mayo clinic and residing in that area. American Journal of Cardiology 2004; 94: 1379–1382.
- Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. Journal of the American College of Cardiology 2004; 292: 2471–2477.
- Abusaada K, Sharma SB, Jaladi R, et al. Epidemiology and management of new-onset atrial fibrillation. American Journal of Managed Care 2004; 10: S50–S57.
- Johnson J, Napier KT. Atrial fibrillation. New theories, emerging treatments. Journal of the American Academy of Physician Assistants 2005; 18: 36–41.
- Snow V, Weiss KB, Le Fevre M, et al. Management of newly detected atrial fibrillation: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Annals of Internal Medicine 2003; 139: 1009–1017.
- van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Archives of Internal Medicine 2003; 163: 936–943.
- Greenlee RT, Vidaillet H. Recent progress in the epidemiology of atrial fibrillation. Current Opinion in Cardiology 2004; 20: 7–14.
- Wu EQ, Birnbaum HG, Mareva M, et al. Economic burden and co-morbidities of atrial fibrillation in a privately insured population. Current Medical Research and Opinion 2005; 21: 1693–1699.
- Stewart S, Murphy N, Walker A, et al. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004; 90: 286–292.
- Le Heuzey JV, Paziaud O, Piot O, et al. Cost of care distribution in atrial fibrillation patients: The COCAF Study. American Heart Journal 2004; 147: 121–126.
- Wattigney WA, Mensah GA, Croft JB, et al. Increasing trends in hospitalizations for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation 2003; 108: 711–716.
- Abbott KC, Trespalacios FC, Taylor AJ, et al. Atrial fibrillation in chronic dialysis patients in the United States: risk factors for hospitalization and mortality. BMC Nephrology 2003; 4: 1–10.
- Hravnak M, Hoffman LA, Saul MI, et al. Resource utilization related to atrial fibrillation after coronary artery bypass grafting. American Journal of Critical Care 2002; 11: 228.
- Lee WC, Christensen MC, Joshi AV, et al. Long-term cost of stroke subtypes among Medicare beneficiaries. Cerebrovascular Diseases 2007; 23: 57–65.
- Lamas GA, Pashos CL, Normand ST, et al. Permanent pacemaker selection and subsequent survival in elderly Medicare pacemaker recipients. Circulation 1995; 91: 1063–1069.
- US Department of Labor. Bureau of Labor Statistics. Consumer Price Index. Available at: http://www.bls.gov/cpi/home.htm [accessed 22 March 2007].
- Duan N. Smearing estimate: a nonparametric retransformation method. Journal of the American Statistical Association 1983; 78: 605–610.
- Wolf PA, Mitchell JB, Baker CS, et al. Impact of atrial fibrillation on mortality, stroke, and medical costs. Archives of Internal Medicine 1998; 158: 229–234.
- Coyne KR, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value in Health 2006; 9: 348–356.
- Marshall DA, Levy AR, Vidaillet H, et al. Cost-effectiveness of rhythm versus rate control in atrial fibrillation. Annals of Internal Medicine 2004; 141: 653–661.
- Reynolds MR, Essebag V, Zimetbaum P, et al. Healthcare resource utilization and costs associated with recurrent episodes of atrial fibrillation: The FRACTAL Registry. Journal of Cardiovascular Electrophysiology 2007; 18: 628–633.
- Miller PSJ, Drummond MF, Langkilde LK, et al. Economic factors associated with antithrombotic treatments for stroke prevention in patients with atrial fibrillation. European Heart Journal 2005; 7((Suppl C):)C41–C54.
- The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. New England Journal of Medicine 2002; 347: 1825–1833.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Time trends of ischemic stroke incidence and mortality in patients diagnosed with first atrial fibrillation in 1980 to 2000. Report of a community-based study. Stroke 2005; 36: 2362–2366.
- Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke. Results from a population-based study. Stroke 2005; 36: 1115–1119.