Abstract
Objective: The objective of this study was to estimate the prevalence of anaemia and its impact on healthcare utilisation in patients with rheumatoid arthritis (RA).
Methods: Patients with claims for moderate-to-severe RA (ICD-9 code 714.x) treated with disease-modifying antirheumatic drugs as well as controls without RA matched for age, gender and time in plan were selected from the MarketScan Research Database. Anaemia was identified by ICD-9 codes 280.x, 285.2x, 281.9, 285.9 and 284.8. The prevalence ratio and 95% confidence interval (CI) for anaemia among RA patients versus controls were estimated. Overall disease burden was measured using the Elixhauser Comorbidity Index (ECI).
Results: The prevalence ratio for anaemia in RA patients was 2.2 (95% CI 2.1–2.4). Mean ECI was higher in RA (2.26) compared with control (1.02) patients (p<0.001), and RA patients with anaemia had a higher ECI compared with those without anaemia (3.95 vs. 2.08; p<0.001). Total healthcare costs in RA patients with anaemia were approximately twice those of RA patients without anaemia.
Conclusions: The prevalence of clinically diagnosed anaemia in RA patients in the claims database was 2.2 times higher than that in the comparable non-RA control group. RA patients with anaemia had significantly higher levels of co-morbidity and healthcare costs than RA patients without anaemia.
Introduction
Anaemia is a common co-morbidity in patients with rheumatoid arthritis (RA), with approximately 30–70% of RA patients experiencing at least mild anaemia based on clinical observation studiesCitation1,Citation2. Although some cases of RA-associated anaemia can be attributed to iron deficiency caused by gastrointestinal bleeding due to the use of non-steroidal anti-inflammatory drugs, >50% of cases are characterised as anaemia of chronic disease or inflammation (ACD)Citation3. In a retrospective review of RA patients, anaemic patients, particularly those with ACD, had significantly greater disease activity, significantly more erosive joint damage and significantly higher levels of rheumatoid factor than patients without anaemiaCitation2. In a separate evaluation, RA patients with anaemia were found to have significant impairments in their physical disability compared with RA patients without anaemia, even after adjustments for demographics and clinical disease activityCitation4. Not surprisingly, RA patients with anaemia have also been reported to have a significantly poorer quality of life than their non-anaemic counterpartsCitation5. It is increasingly appreciated that the increased production of inflammatory cytokines (e.g. tumour necrosis factor-a, interleukin-1 and -6) in RA plays a role in ACD.
The considerably wide range of anaemia occurrences in RA patients could be related to differences in the anaemia definitions used and/or in the specific clinical characteristics of the population studiedCitation1. Nevertheless, data regarding the diagnosis and treatment of anaemia among patients with moderate-to-severe RA in general clinical practice are limited and there are few studies using epidemiological case-control methodology to quantitatively measure the risk of anaemia in RA patients compared with the general populationCitation6. Although a study in a privately insured population showed that healthcare costs of RA patients with anaemia were as much as twice those for non-anaemic patients with the same co-morbid conditionsCitation7, data comparing healthcare costs between RA patients with and without anaemia are limited.
In this analysis, the prevalence of diagnosed anaemia as well as overall co-morbidity and medical cost outcomes were calculated, using data from a claims database, in patients with moderate-to-severe RA who were treated with disease-modifying antirheumatic drugs (DMARDs). These outcomes were then compared between RA patients and age- and sex-matched non-RA controls. Comparisons were also made between RA patients with and without anaemia after adjusting for age, sex and co-morbidity.
Methods
Data source
The MarketScan Research Database is a longitudinal healthcare claims database that includes patients covered by employer-sponsored health plans for eligible employees, early retirees and dependants. Inpatient and outpatient diagnoses (International Classification of Diseases, 9th revision–Clinical Modification (ICD-9-CM) format) and procedures (Current Procedural Terminology, 4th Edition (CPT-4) and Healthcare Common Procedure Coding System (HCPCS) formats) are included in the dataset. Additional data elements include demographic variables (e.g. age, gender, geographic region) and start and stop dates for plan enrolment.
Selection criteria
Adults (age >17 years) continuously enrolled from the 1st January 2004 to the 31st December 2004 with medical and drug benefits were selected. Patients had to have had at least one interaction with the healthcare system to be included in the analysis. In this study, patients with claims for RA (ICD-9 code 714.x) and who had been treated with any DMARDs, including methotrexate, hydroxychloroquine, sulfasalazine, leflunomide, azathioprine and cyclosporine, in the year 2004 were selected. All patients who met the selection criteria were included in the analyses. A control group of individuals without RA claims was randomly selected and matched to the RA patients on age (within 1 year), gender and time in the plan prior to 2004. The following ICD-9 codes were used to identify anaemia: iron deficiency anaemia (280.x); anaemia in chronic illness (285.2x); other nutritional anaemia (281.9); unspecified anaemia (285.9); and other specified aplastic anaemia (284.8). ICD-9 procedure codes (v-codes), CPT-4 and HCPCS codes were used to identify transfusions. Actual laboratory values were not available to verify the presence of anaemia.
Measures and analyses
The prevalence ratio and 95% confidence interval (CI) for anaemia among RA patients were estimated and tested using Cochran–Mantel–Haenszel methodologyCitation8. The overall chronic disease burden was assessed via the Elixhauser Comorbidity Index (ECI) and compared between patient groups after adjusting for age and gender using a multiple linear regression modelCitation9.
In the analysis of medical (hospitalisation, outpatient service, emergency room visit, specialist visit and outpatient hospital service) and pharmacy costs during the year 2004, data were analysed and compared between RA patients and controls as well as between RA patients with and without anaemia. The cost data were transformed to a normal distribution using log transformation, and statistical tests were performed using multiple linear regression analysis adjusting for age, gender and co-morbidity.
Statistical analyses were performed using the SAS® system (SAS Institute Inc., Cary, NC). All statistical tests were two-sided and were performed at α=0.05.
Results
Patient characteristics
A total of 20,662 RA patients who met the inclusion criteria were identified in the year 2004; 75.5% were female and the average age of all RA patients was 57.6 years. As dictated by the composition of the database, patients and controls were most commonly residents of the South and North Central regions of the US. The majority of RA patients and controls had been enrolled in their health plan for >2 years ().
Table 1. Characteristics of the RA patients and controls.
Prevalence of anaemia in RA patients and controls
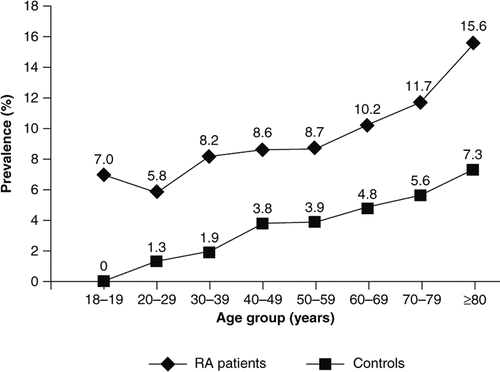
In the database, 9.7% (10.1% of females, 8.5% of males) of RA patients compared with 4.3% (4.4% of females, 4.0% of males) of controls had medical claims for anaemia. Anaemia increased with age from 5.8% in the age group 20–29 years to 15.6% in the age group ≥80 years in RA patients. Among control patients, anaemia increased from 1.3% in the age group 20–29 years to 7.3% in the age group ≥80 years ().
Among RA patients and controls, respectively, approximately 3.0 vs. 1.2% were diagnosed with iron deficiency anaemia, 6.9 vs. 2.7% were diagnosed with unspecified anaemia in chronic illness and 1.0 vs. 0.8% received blood transfusions. The prevalence ratio of any anaemia in RA patients compared with controls was 2.2 (95% CI 2.1–2.4) ().
Table 2. Prevalence of anaemia among RA patients and controls.
RA patients with anaemia were older (mean age 59.6 years) and had a greater proportion of females (78.5%) compared with RA patients without anaemia (mean age 57.4 years; 75.2% female).
Comparison of co-morbidities
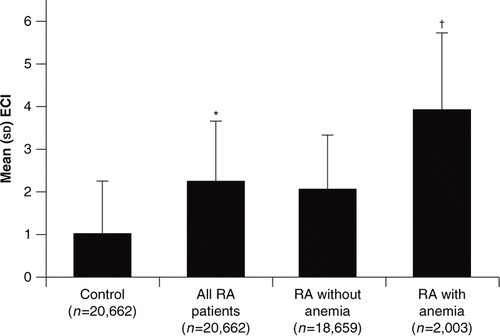
The overall chronic disease burden was highest in RA patients with anaemia (). The mean ECI scores were significantly higher in RA patients (2.26) compared with controls (1.02; p<0.001) and in RA patients with anaemia (3.95) compared with RA patients without anaemia (2.08; p<0.001) after adjustment for age and gender.
Comparison of medical costs
Total healthcare costs, including total medical and pharmacy costs, were significantly (p<0.001) higher in RA patients than in controls. In addition, results of the multiple regression analyses demonstrated that RA patients with anaemia had significantly higher total healthcare costs (p<0.001), including total medical costs (p<0.001) and pharmacy costs (p<0.001), than patients without anaemia even after adjusting for age, gender and co-morbidity index ().
Table 3. Healthcare costs among RA patients and controls.
Discussion
Anaemia has been shown to be associated with increased mortality and morbidity as well as with decreased physical functioning and quality of lifeCitation10,Citation11. Wolfe and MichaudCitation5 found that among 2,120 RA patients, 51.6% had haemoglobin levels of <12 g/dl and 13.7% had levels of <10 g/dl. In that analysis, lower haemoglobin levels were associated with increased clinical activity as assessed by swollen/tender joint counts, C-reactive protein, erythrocyte sedimentation rate, the Health Assessment Questionnaire (HAQ) and pain assessmentsCitation5. In a separate analysis of physical function in RA patients, Han et alCitation4 found that after adjustment for demographics, disease duration and disease activity, anaemia is an independent risk factor of physical disability as measured by the HAQ in RA patients, and improvement in anaemia after treatment is associated with significant improvement in physical function. Although these results suggest that anaemia has a significant negative impact both on RA symptoms and quality of life, little research on anaemia-related outcomes has been conducted and large-scale studies are needed to support the importance of anaemia screening and treatment in RACitation12.
Whilst clinical studies have shown that approximately 30–70% of patients with RA may experience at least mild anaemia based on haemoglobin levelsCitation1,Citation2, the prevalence of anaemia in RA patients compared with a general population has not been studied quantitatively. Data regarding the diagnosis and treatment of anaemia among patients with moderate-to-severe RA in general clinical practice are also very limited. In this study, anaemia prevalence as well as co-morbidities and healthcare costs were examined in RA patients with and without anaemia. These outcomes were then compared with an age-, gender- and time-in-plan-matched control group using data from a healthcare administrative claims database.
The prevalence of diagnosed anaemia in RA patients in the claims database was 2.2 times higher than that among comparable non-RA controls. Since RA patients enrolled in the analysis were patients with moderate-to-severe disease who had been treated with one or more DMARDs, it is possible that many of these RA patients were responding to their current DMARD treatment and therefore may have already experienced improvement in their inflammation. Although this factor could have contributed to the lower than expected prevalence of anaemia observed (i.e. 9.7%), this prevalence was still much lower than that based on clinical observational studies in RA patients with moderate-to-severe disease (30–70% as noted above), suggesting that anaemia in RA patients from general practice may be significantly underdiagnosed/undertreated.
In a similar claims-based data analysis in which healthcare costs of patients in a privately insured population who were predisposed to anaemia based on selected co-morbid conditions (chronic kidney disease, HIV, RA, inflammatory bowel disease, congestive heart failure and solid tumour cancers) were estimated, medical costs for anaemic patients were as much as twice those for non-anaemic patients with the same co-morbid conditionsCitation7. Results of the present analysis were consistent with this previous study in that the healthcare costs in DMARD-treated RA patients with anaemia were approximately two times those observed in DMARD-treated RA patients without anaemia.
The results of the analysis of ECI data indicated that the overall chronic disease burden was highest in RA patients with anaemia (3.95), followed by RA patients without anaemia (2.08) and then the control group (1.02). This is not surprising given the fact that previous studies have demonstrated a high occurrence of co-morbidities, including cardiovascular diseases and other inflammatory diseases, among RA patients compared with a non-RA populationCitation13. Whilst the authors were not able to monitor current disease activity in RA patients, previous studies of anaemia in RA have shown that RA patients with anaemia have greater disease activity, as manifested by fever, severe joint swelling and tenderness, weight loss and acceleration of the erythrocyte sedimentation rate, compared with RA patients without anaemiaCitation3,Citation14,Citation15 and that the severity of anaemia correlates with select inflammatory markers such as serum amyloid A and C-reactive proteinCitation16. Patients with more severe RA may be predisposed to a higher risk for other co-morbidities.
Taken together, the findings, as a useful resource for clinicians and healthcare providers, underscore the fact that anaemia has a significant impact on healthcare utilisation and overall co-morbidities in RA patients. Anaemia should be taken into account when choosing therapeutic options and should be monitored during RA treatment, especially in light of the possibility of significant underdiagnosis of anaemia. These results also highlight the importance of understanding the risk factors that increase the likelihood of anaemia in RA patients. Identification of ‘at-risk’ individuals is critical for improving the detection and treatment of anaemia in these already compromised patientsCitation12. In addition, the benefits of effective treatment of anaemia need to be better understood. Further studies are needed to examine whether treatment of anaemia can have a beneficial effect on other co-morbidities as well as a reduction in the costs of care among RA patients. Given that anaemia in RA is largely attributable to either ACD or iron deficiency anaemia, it is plausible that therapies capable of improving RA may also improve anaemia by decreasing the inflammatory burdenCitation17.
This study is limited by the fact that the data were derived from an administrative claims database in which disease severity and duration cannot be accurately assessed. The level of detail available in a claims database is restricted to that required for claims adjudication and internal/external reporting. This may lead to underidentification of anaemia since the diagnosis codes may not be recorded even though the patients had anaemia. However, the impact of this bias on the results may be minimised since a case-control study method was employed and a prevalence ratio was generated. Without the actual haemoglobin levels, the true occurrence and severity of anaemia cannot be determinedCitation7. Future research should include a prospective study that allows evaluation of actual haemoglobin levels as well as their relationship with disease activity.
In conclusion, the prevalence of anaemia in RA patients in the claims database was 2.2 times higher than that among the comparable non-RA control group but much lower than that reported based on clinical observational studiesCitation1,Citation2 in RA patients. Thus, anaemia in RA patients may be significantly underdiagnosed/undertreated. RA patients with anaemia had significantly higher co-morbidity and healthcare costs than RA patients without anaemia.
Acknowledgements
Declaration of interest: The research presented in this manuscript was funded by Centocor, Inc.
Staff members from Centocor, Inc. designed this study, analysed and interpreted the data, and wrote and submitted the manuscript for publication. All authors approved the content of the manuscript prior to submission.
The authors wish to acknowledge Michelle Perate MS, a consultant for Centocor, Inc., for her assistance in preparing the manuscript.
Notes
References
- Wilson A, Yu HT, Goodnough LT, et al. Prevalence and outcomes of anaemia in rheumatoid arthritis: a systematic review of the literature. American Journal of Medicine 2004; 116((Suppl 7A))50S–57S
- Peeters HR, Jongen-Lavrencic M, Raja AN, et al. Course and characteristics of anaemia in patients with rheumatoid arthritis of recent onset. Annals of the Rheumatic Diseases 1996; 55: 162–168
- Vreugdenhil G, Lowenberg B, Van Eijk HG, et al. Tumour necrosis factor alpha is associated with disease activity and degree of anaemia in patients with rheumatoid arthritis. European Journal of Clinical Investigation 1992; 22: 488–493
- Han C, Rahman MU, Doyle MK, et al. Association of anaemia and physical disability among patients with rheumatoid arthritis. Journal of Rheumatology 2007; 34: 2177–2182
- Wolfe F, Michaud K. Anaemia and renal function in patients with rheumatoid arthritis. Journal of Rheumatology 2006; 33: 1516–1522
- Ershler WB, Chen K, Reyes EB, et al. Economic burden of patients with anaemia in selected diseases. Value in Health 2005; 8: 629–638
- Nissenson AR, Wade S, Goodnough LT, et al. Economic burden of anaemia in an insured population. Journal of Managed Care Pharmacy 2005; 11: 565–574
- Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System, 2nd edn. SAS Institute Inc, Cary, NC 2000
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Medical Care 1998; 36: 8–27
- Goodnough LT, Dubois RQ, Nissenson AR. Anaemia: not just an innocent bystander?. Archives of Internal Medicine 2003; 163: 1400–1404
- Weiss G, Goodnough LT. Anaemia of chronic disease. New England Journal of Medicine 2005; 352: 1011–1023
- Swaak A. Anaemia of chronic disease in patients with rheumatoid arthritis: aspects of prevalence, outcome, diagnosis, and the effect of treatment on disease activity. Journal of Rheumatology 2006; 33: 1467–1468, [Comments in: Journal of Rheumatology 2006; 33: 1516–1522; and Journal of Rheumatology 2007; 34: 446, author reply 446–447.]
- Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Practice & Research. Clinical Rheumatology 2007; 21: 885–906
- Cartwright GE. The anaemia of chronic disorders. British Journal of Haematology 1971; 21: 147–152
- Birgegard G, Hallgren R, Caro J. Serum erythropoietin in rheumatoid arthritis and other inflammatory arthritides: relationship to anaemia and the effect of anti-inflammatory treatment. British Journal of Haematology 1987; 65: 479–483
- Pettersson T, Rosenlof K, Friman C, et al. Successful treatment of the anaemia of rheumatoid arthritis with subcutaneously administered recombinant human erythropoietin. Slower response in patients with more severe inflammation. Scandinavian Journal of Rheumatology 1993; 22: 188–193
- Doyle M, Rahman M, Han J. Improvement in hemoglobin in patients with rheumatoid arthritis treated with infliximab. Arthritis and Rheumatism 2006; 54((Suppl))S238