Abstract
Objectives: Glaucoma is a fairly common disease, however, little is known about the costs associated with prostaglandin analogues (PAs). The costs between the three available PAs (Lumigan® (bimatoprost), Xalatan® (latanoprost) and Travatan® (travoprost)) were compared as monotherapy and when adjunctive therapy was used.
* Lumigan is a registered trademark of Allergan Canada, Markham, ON, Canada.
† Xalatan is a registered trademark of Pfizer Canada, Kirkland, Québec, Canada.
‡ Travatan is a registered trademark of Alcon Canada, Mississanga, ON, Canada.
Methods: From the Québec drug claims database, all patients who used these drugs for 1 full year were identified. From the Ministry of Health (MoH) perspective, the average cost for all reimbursed costs (drug costs and pharmacist fees) were calculated. Those costs plus the patient out-of-pocket copayments were used for the payer + user (PU) perspective.
Results: Data from 4,653 patients were analysed (3,606 on monotherapy and 1,047 on combination treatment with adjunctive therapy), 59.7% were females, and the average age was 72.6 ± 10.4 years. MoH perspective costs were $410 ± $167 for bimatoprost, $381 ± $145 for latanoprost and $298 ± $121 for travoprost (all differences p<0.001), for patients on monotherapy. Costs of combination treatment with adjunctive therapy were $786 ± $416, $686 ± $313, and $623 ± $521, respectively (travoprost significantly lower than each of the other two p<0.001, others=not significant). Results from the PU perspective were comparable.
Conclusions: Travoprost had the lowest cost, both as monotherapy and in conjunction with other glaucoma treatments. Further comparative pharmacoeconomic evaluation is warranted.
Introduction
Glaucoma, all types combined, is one of the leading causes of blindnessCitation1,Citation2. Primary open angle glaucoma (POAG) is a chronic disease characterised by progressive optic nerve damageCitation3. Risk factors related to the progression of open angle glaucoma have been examined in a number of studies. Intraocular pressure (IOP) has been identified as one of the few modifiable risk factors for glaucoma progressionCitation4–8. It is one of the major factors implicated in the loss of optic nervesCitation3,Citation9, and is present in the majority of patientsCitation10.
Although vision loss and progressive neuropathy can occur without elevated IOP, traditional glaucoma treatments have focused on the reduction or control of IOP, the one risk factor that can be modifiedCitation11–13. Control of IOP has been associated with a delay of the progression of optic nerve damageCitation3,Citation13. IOP can be reduced or controlled either with medications, by laser surgery, by incisional surgery, or a combination of the aboveCitation3,Citation14. The goal of treatment is to reach an IOP level below which further optic nerve damage would be prevented, then to maintain the IOP at or below that target levelCitation3.
Although the β-blockers have been the mainstay of medical therapy in the last two decades, they are being replaced by the more recently introduced class of prostaglandin analogues (PAs)9,11,14,15. Included in that class are Lumigan® (bimatoprost), Xalatan® (latanoprost), Travatan® (travoprost) and unoprostone isopropyl. Only the first three PAs are licensed on the Canadian market as once daily treatments for POAGCitation16. PAs offer an improved safety profile over other topical ophthalmic drops, with no observed systemic effectsCitation17–19. Because the PAs offer safer (i.e. fewer systemic effects) and more effective therapy (in terms of lowering IOP), their popularity has increased to the point of becoming the medical treatment of choiceCitation9,Citation14,Citation18. In fact, some authors have proposed to use PAs as first-line treatment and b-blockers either as adjunctive or second-line therapyCitation14.
Although PAs offer improved safety and enhanced efficacy over older drugs, they also have higher acquisition costs. Within the Canadian publicly funded healthcare context, cost differences may have a significant impact. Yet, a literature search could not locate any comparative studies between individual PAs. Since PAs have already been in use in Canada for a number of years, various provincial medication claims databases would enable the estimation of the cost of their use as incurred in the respective provinces.
The primary objective of this study was to compare the drug cost to treat patients with each of the three PAs available in Canada, based on their actual use as monotherapy for POAG in a general population. A secondary objective was to compare costs between the three drugs when used in conjunction with other topical POAG medications.
Patients and methods
Source of data
Data from the administrative prescription claims database of the Régie de l’Assurance Maladie du Québec (RAMQ) were used. The RAMQ database is based on prescription claims from the publicly administered drug plan in the second largest province of Canada, Québec, which covers approximately 2.4 million residents of the province, including 885,000 ≥65 years of age. It contains information on patient age and gender, medication use, claim date, quantity dispensed, pharmacist's dispensing fee, co-payment, cost reimbursed and specialty of the physician who prescribed the medication. The database has previously been validated and found to be both reliable and accurateCitation20.
Cohort participants
The authors studied naive patients (i.e. people who, in the 12-month period prior to their first PA prescription, were not dispensed any non-PA glaucoma medication). Naive patients were classified into two groups: those who were not dispensed any other glaucoma medication in addition to their PA medication (PA patients) and those who were dispensed adjunctive therapy (adjunctive patients). Adjunctive therapy was defined as any non-PA medication indicated in the treatment of glaucoma, as specified by the Canadian Compendium of Pharmaceuticals and SpecialtiesCitation21. Only patients who received at least two non-PA glaucoma prescriptions during the studied period were considered to be using adjunctive therapy.
From the RAMQ database, pharmaceutical records of a random sample were obtained of all patients who, between 28th July 1997 and 28th February 2006, were dispensed at least one of the following three PAs: bimatoprost, latanoprost, or travoprost. Combinations of a PA and a non-PA medication, such as Xalacom® (latanoprost and timolol), were not included in the database. From this dataset, all patients who initiated PA therapy after 24th May 2003 were identified. This was 1 year after the latest PA (i.e. bimatoprost) was introduced onto the Canadian marketCitation22. In that way, the authors sought to minimise the effects of aggressive promotion of a new drug in the period immediately following its market introduction. It is the policy of the RAMQ to provide datasets with a random sample of 75% of the first 10,000 patients meeting the inclusion criteria, 50% for the next 70,000 eligible patients and 25% for the remaining eligible patients for a maximum of 125,000 patients, to maintain patient anonymity (RAMQ personal communication). Included in the analysis were patients with at least 1 full year of data after initiation of glaucoma therapy with PAs, as defined by a prescription for a PA dispensed at least 334 days (i.e. 1 year minus 31 days) after the first PA prescription. All data after 1 year of PA utilisation were censored in order to have exactly 1 year of resource utilisation for all patients. The date of the first dispensed PA for a patient was identified as his/her index date. All patients included in the cohort had at least 1 year of data prior to his/her index date and 1 year of continuous PA use. Only data from patients who had remained on the same PA for the entire year were analysed.
Outcome measurements
The resources used between patients using different PAs for both patient groups were compared (i.e. patients receiving only PAs as monotherapy and patients receiving PAs plus adjunctive treatment). First, the average number of PA bottles was assessed. Because not all PAs have the same bottle size, the use of 2.5 ml bottle equivalents was reported, which is the only size available for both latanoprost and travoprostCitation23,Citation24 and the smallest size for bimatoprostCitation25. Therefore, a patient using a 5 ml bottle of bimatoprost was considered to be using two 2.5 ml bottle equivalents. At the time of analysis, the cost per ml for all three PAs was similar. The cost per ml for bimatoprost and travoprost was $10.60, while it was $10.40 for latanoprost. The aggregated and disaggregated costs from the Ministry of Health (MoH) and the MoH + patient perspective, or the payer + user (PU) perspective were also compared. In this analysis, the MoH perspective amounted to that of the drug plan, as no other costs, such as physician fees, were included (only drug costs are available in the RAMQ database). From the MoH perspective, the medication cost reimbursed by the RAMQ, the pharmacists’ dispensing fees and the total costs were compared between treatment groups. From the PU perspective, patient co-payments were added to the cost reimbursed by the RAMQ for the medication. Costs were not inflated to 2006 values as all costs included in the analysis remained constant for the study time horizon.
Statistical analysis
For all continuous variables, including costs and number of bottles, the median and interquartile range were reported. The costs and number of bottles of the three PA categories were compared between groups using a Kruskal-Wallis (with Dunn's post hoc procedure, to identify specific PA category differences). Proportions between groups for demographic categories were assessed with a χ2 test for contingency tables and p values ≤ 0.05 were considered statistically significant. All statistical analyses were performed on patient level data using SAS v.9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Demographics
A total of 4,653 patients met the inclusion criteria, of which 3,606 (77.5%) used a PA as their sole glaucoma medication, whereas 1,047 (22.5%) used adjunctive therapy in addition to their PA. There were more females than males in the cohort (59.7%; χ2=174.5, df=1, p<0.001); furthermore, the proportion of females was different among the three PA categories (χ2=8.61, df=2, p=0.014) and the average age was 72.6 (standard deviation (sd) = 10.4) years. Demographic characteristics are presented in .
Table 1. Demographic characteristics of patients for each index therapy.
PA monotherapy patients
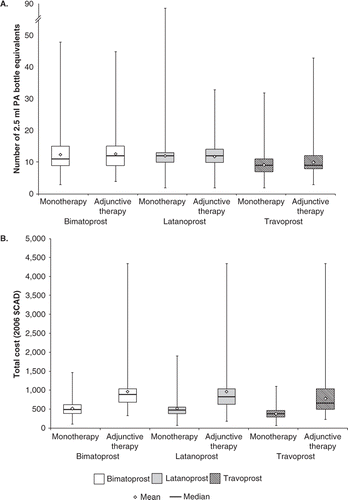
Patients on PA only used on average 12 (median = 11) × 2.5 ml PA-bottle equivalents in 1 year. There was a significant difference in the number of 2.5 ml PA-bottle equivalents used between patients on bimatoprost, latanoprost and travoprost (Kruskal-Wallis (K-W) χ2=199.97, df=2, p<0.001). Bimatoprost users needed a median number of 11 (interquartile range (IQR) = 6) bottles, whereas latanoprost users needed a median number of 12 (IQR=3) bottles and travoprost users only needed a median number of 9 (IQR=4) 2.5 ml PA-bottle equivalents. The total cost from the MoH perspective was highest for bimatoprost (median=$389.95, IQR=$213.14), followed by latanoprost (median=$365.14, IQR= $165.05) and then travoprost (median=$287.17, IQR=$150.77). Patient co-payments were highest for bimatoprost and lowest for travoprost. Total median PU costs were $496.52 (IQR=$244.85), $476.19 (IQR=$170.61) and $383.73 (IQR=$174.94) for bimatoprost, latanoprost and travoprost, respectively. Disaggregated costs associated with patients using only PAs as their glaucoma medication are presented in , and bottle utilisation and total cost per patient is depicted in .
Table 2. Disaggregated costs associated with patients of each index therapy group, for PA users only.
Patients with adjunctive therapy
Patients on PAs and adjunctive therapy also used on average 12 (median=11) × 2.5 ml PA-bottle equivalents. The highest number of 2.5 ml PA-bottle equivalents per patient was associated with bimatoprost users (median=12, IQR=6) and latanoprost (median=12, IQR=4), followed by travoprost (median=9, IQR=4). There was a significant difference in the number of bottles needed per patient between the groups (K-W χ2=65.23, p<0.001). Total MoH costs were lowest for travoprost users (median=$507.09, IQR=$388.10) and significant differences were found between groups (K-W χ2=23.31, df=2, p<0.001). Travoprost users also showed the lowest co-payment cost per patient (median=$161.04, IQR=$104.71), but significant differences were found only between latanoprost and travoprost users (χ2=8.21, df=1, p=0.004). Significant differences in the total PU cost per patient between all three medications were found. Travoprost costs were significantly lower compared to both bimatoprost and latanoprost. Detailed disaggregated costs are presented in , and bottle utilisation and total cost per patient is depicted in .
Table 3. Disaggregated costs associated with patients of each index therapy group, for PA and adjunctive therapy users.
Discussion
This is the first comparative ‘cost in use’ analysis of the PAs based on their actual use on a Canadian provincial drug plan. Travoprost treatment was found to be significantly less costly than the other two PAs. The mean yearly cost of PAs varied between $383 and $522 for monotherapy with PAs, as derived from the Québec provincial medication claims database, and between $783 and $966 for combination therapy. This compares to approximately $500 per year for a mix of monotherapy and combination therapy, previously estimated by this group in a study based on data extracted from patient chartsCitation26. Nonetheless, in an analysis of first year costs of newly diagnosed patients in the previous study, the average yearly cost increased to $793 per patient.
Seven major differences exist between the two studies: (1) this study was based on Québec data, while the previous one was performed in Ontario; (2) selection of patients was based on type of medication use in the present study instead of a diagnostic code as in the previous one (ICD-9 365.11); (3) the patients in this study had to have completed 1 full year of therapy without discontinuation, hence excluding those who may have used the medications less frequently and for shorter periods, as opposed to inclusion of all patients meeting the diagnostic codes in the previous study; (4) this study assessed PA users and combination users separately, while the previous study was on a mixed population; (5) the previous study was conducted 4 years earlier in a population mainly using b-blockers while the present study was on a population using PAs; a group of medications with higher costs, which is becoming the standard of care; (6) the present study only included medication costs, while the previous study also included cost of procedures and physician fees; and (7) the present study reported costs as incurred by the provincial public payer while the costing in the previous study was modelled with data from a chart extraction.
More recently, a study published by Lachaine and colleagues assessed the cost effectiveness of two of the three PAs available in Canada compared to treating IOP with timolol, dorzolamide and brimonidineCitation27. The authors reported a 3-month cost (in Canadian dollars) for latanoprost and travoprost of $127.41 and $123.28, respectively. This would translate into 1 year cost of $509.64 for latanoprost and $493.12 for travoprost, assuming healthcare resources consumed remained constant throughout the year. These costs are for PA monotherapy, but include costs other than drug costs. Therefore, the results are in accordance with those of Lachaine et alCitation27 as they are lower, and in the same order of magnitude.
Although care was taken to reduce bias as much as possible, many limitations apply to this study. First, the patient enrolment in the dataset was based on medication use and not on a diagnosis. In addition, the analysis was not differentiated by severity of the disease. Furthermore, this analysis only applies to newly diagnosed patients who took a PA for a full year. However, persistence rates of approximately 65–70% have been recently reported for PAs in the first year of utilisationCitation28,Citation29. In addition, the present analysis did not take into account variations in patient adherence (i.e. in the amount of medication applied by the patients). In order to address this concern, a post-hoc analyses on ‘non-completer’ patients was performed (i.e. those who did not continue one full year of index therapy). Examining the costs of medications dispensed for this latter group over the same study period, the direction of the results did not change, with travoprost still being associated with the lowest costs among the three PAs. The overall costs for the group of ‘non-completer’ patients were $535 (sd=$295) for travoprost, $568 (sd=$291) for latanoprost and $638 (sd=$359) for bimatoprost. Costs associated with travoprost were significantly lower than those associated with bimatoprost (p<0.001) and with latanoprost (p=0.032). A significant difference was also found between costs associated with the bimatoprost and latanoprost treatments (p=0.002).
It is worth noting that the difference in costs between monotherapy (PAs only) and combination therapy (PAs plus adjunctive therapy) was in the order of 45%. Hence, although travoprost was found to be associated with lower costs than the other two PAs in both monotherapy and combination therapy, there would still be, at least in theory, a chance that one of the other two PAs has on average lower costs than travoprost if it was associated with a much lower rate of adjunctive therapy. Analyses to estimate the rates of adjunctive therapy for patients starting a PA were performed, and rates of 20% for travoprost, 22% for latanoprost and 26% for bimatoprost in the first year of therapy were determined. Travoprost was significantly associated with a lower rate than bimatoprost (p=0.01), while rates associated with travoprost were not significantly different when compared to latanoprost (p=0.13). Covert and Robin also recently found a lower rate of adjunctive therapy in the first year for newly diagnosed patients with travoprost of 16.8% as compared to 17.7% for bimatoprost and 25.0% with latanoprostCitation30. These findings confirm that travoprost would still be associated with a lower cost. However, further research on the rates of adjunctive therapy use in newly diagnosed patients initiating PA therapy is warranted. Future research should also include adherence analyses within the persistent population.
With regards to generalisability of results, as this study was performed on a sample from the general population, as diverse as possible and from different geographic areas within the province of Quebec, results may apply to that province, but should be used with caution for the Canadian population at large.
Finally, as this research only estimated costs but not outcomes, further comparative pharmacoeconomic evaluations are warranted to simultaneously determine costs and outcomes as well as cost effectiveness.
Conclusion
In this first Canadian study comparing costs associated with the use of prostaglandin analogues at the population level, travoprost had the lowest costs, both as monotherapy and in conjunction with other glaucoma treatments, when compared to bimatoprost and latanoprost. Further comparative pharmacoeconomic evaluation is warranted.
Acknowledgements
Declaration of interest: This study was funded by Alcon Research Ltd, TX.
MI is the president of PharmIdeas, who have received funds from Alcon Research. JHW and TRE are both Principals of PharmIdeas, and OD is an employee of PharmIdeas. DC is an employee of Alcon Research Ltd.
Notes
* Lumigan is a registered trademark of Allergan Canada, Markham, ON, Canada.
† Xalatan is a registered trademark of Pfizer Canada, Kirkland, Québec, Canada.
‡ Travatan is a registered trademark of Alcon Canada, Mississanga, ON, Canada.
* Xalacom is a registered trademark of Pfizer Canada, Kirkland, Québec.
References
- Quigley H. Number of people with glaucoma worldwide. British Journal of Ophthalmology 1996; 80: 389–393.
- Fuchs J, Nissen K, Goldschmidt E. Glaucoma blindness in Denmark. Acta Ophthalmologica (Copenhagan) 1992; 70((1))73–78.
- American Academy of Ophthalmology. Primary Open-Angle Glaucoma. American Academy of Ophthalmology, San FranciscoCA 2000.
- Collaborative normal-tension glaucoma study group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. American Journal of Ophthalmology 1998; 126((4))498–505.
- The AGIS Investigators. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. American Journal of Ophthalmology 2000; 130((4))429–440.
- Gordon M, Beiser J, Brandt J, et al. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Archives of Ophthalmology 2002; 120((6))714–720.
- Leske M, Heijl A, Hussein M, et al. Factors for glaucoma progressioni and the effect of treatment. The early manifest glaucoma trial. Archives of Ophthalmology 2003; 121((1))48–56.
- Lichter P, Musch D, Gillespie B, et al. Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medications or surgery. American Journal of Ophthalmology 2001; 108((11))1943–1953.
- Soltau J, Zimmerman T. Changing paradigms in the medical treatment of glaucoma. Survey of Ophthalmology 2002; 47((Suppl 1))S2–S5.
- Leske M, Connell A, Schachat A, et al. The Barbados eye study: prevalence of open angle glaucoma. Archives of Ophthalmology 1994; 112: 821–829.
- Alward W. Medical management of glaucoma. The New England Journal of Medicine 1998; 339((18))1298–1307.
- Coleman A. Glaucoma. Lancet 1999; 354: 1803–1810.
- Schuman J. Antiglaucoma medications: a review of safety and tolerability issued related to their use. Clinical Therapeutics 2000; 22((2))167–208.
- Schwartz K, Budenz D. Current management of glaucoma. Current Opinion in Ophthalmology 2004; 15((2))119–126.
- Sherwood M, Migdal C, Hitchings R, et al. Initial treatment of glaucoma: surgery or medications. Survey of Ophthalmology 1993; 37((4))293–305.
- Health Canada. Notice of Compliance Database. Health Canada; 2006. http://www.nocdatabase.ca Last Updated 13th June 2005. Accessed 5th May 2006.
- Alexander C, Miller S, Abel S. Prostaglandin analogue treatment of glaucoma and ocular hypertension. The Annals of Pharmacotherapy 2002; 36((3))504–511.
- Camras C, Toris C, Tamesis R. Efficacy and adverse effects of medications used in the treatment of glaucoma. Drugs & Aging 1999; 15((5))377–388.
- Nguyen Q. The role of prostaglandin analogues in the treatment of glaucoma in the 21st century. International Ophthalmology Clinics 2004; 44((2))15–27.
- Tamblyn R, Lavoie G, Petrella L, et al. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. Journal of Clinical Epidemiology 1995; 48((8))999–1009.
- CPS. Compendium of Pharmaceuticals and Specialties (CPS). The Canadian Drug Reference for Health Professionals. Canadian Pharmacists Association, OttawaON 2006
- Health Canada. Notice of Compliance: Lumigan. Ottawa, ON: Health Canada; 2006. http://www.nocdatabase.ca Last Updated 13th June 2005. Accessed 5th May 2006.
- CPS. Compendium of Pharmaceuticals and Specialties. Travatan (Travoprost). Elevated Intraocular Pressure Therapy – Prostaglandin F2a Analogue. Canadian Pharmacists Association, OttawaON 2006.
- CPS. Compendium of Pharmaceuticals and Specialties. Xalatan (Latanoprost). Elevated Intraocular Pressure Therapy – Prostaglandin F2a Analogue. Canadian Pharmacists Association, OttawaON 2006.
- CPS. Compendium of Pharmaceuticals and Specialties. Limigan (Bimatoprost). Elevated Intraocular Pressure Therapy – Prostaglandin F2a Analogue. Canadian Pharmacists Association, OttawaON 2006.
- Iskedjian M, Walker J, Vicente C, et al. Cost of glaucoma in Canada: analyses based on visual field and physician's assessment. Journal of Glaucoma 2003; 12((6))456–462.
- Lachaine J, Hodge W, Steffensen I, et al. Prostaglandin analogues for ophthalmic use: a cost-effectiveness analysis. Canadian Journal of Ophthalmology February, 2008; 43((1))33–41.
- Schwartz G, Mozaffari E, Oates V, et al. Population-based persistency rates for topical glaucoma medications measured with pharmacy claims data. American Journal of Managed Care 2002; 8((Suppl))S255–S261.
- Wilensky J, Fiscella R, Carlson A, et al. Measurement of persistence and adherence to regimens of IOP-lowering glaucoma medications using pharmacy claims data. American Journal of Ophthalmology 2006; 141((1 Suppl))S28–S33.
- Covert D, Robin A. Adjunctive glaucoma therapy use associated with travoprost, bimatroprost and latanoprost. Current Medical Research and Opinion 2006; 22((5))971–976.