Abstract
Background: Multidetector computed tomography (MDCT) is a novel method for diagnosis and prognosis of coronary artery disease (CAD). The opportunity costs that favour MDCT over other CAD diagnostic methods is currently unknown.
Methods: This study used an episodes of care cost model based on epidemiologic and economic data evaluating individuals without known CAD undergoing MDCT or myocardial perfusion scintigraphy (MPS). It was a multicenter retrospective database review of medical and pharmacy-related claims linked by episodes of care from 2002 to 2005. CAD-related episodes of care costs were examined 1-year downstream for patients after initial MDCT that were matched to patients who underwent MPS.
Results: After adjustment for patient factors, 1-year total CAD-related episodes of care costs for MDCT were 16.4% lower than MPS, by an average of $682 (95% confidence interval $14, $1,350) per patient. While costs per CAD-related episode were similar between MDCT and MPS groups ($4,284 vs. $4,277, p=0.08).
Conclusions: Patients without known CAD who undergo MDCT as an initial diagnostic test, compared to MPS, incurred fewer CAD-related episodes of care and lower overall CAD-related costs.
Introduction
Cardiovascular disease is the leading cause of death and morbidity in the US, with more than 60 million individuals with some form of cardiovascular disease. The evaluation of patients with suspected coronary artery disease (CAD) beyond traditional cardiovascular risk factor assessment relies on the use of non-invasive imaging. Historically, stress testing by myocardial perfusion scintigraphy (MPS) imaging has been the most widely used method for the assessment of the extent and severity of CADCitation1–3. Recently, multidetector computed tomographic (MDCT) angiography has emerged with initial reports supporting its use as a novel non-invasive anatomic imaging modality that exhibits high accuracy for the detection and exclusion of obstructive coronary artery stenosis as well as cardiovascular prognosis, and is gaining widespread clinical acceptanceCitation4–6.
Reductions in CAD-related mortality and morbidity have been made possible, in part, by intervention and early detection of the disease, which have been supported through judicious use of non-invasive cardiac imaging. However, this success is accompanied by increased test utilisation and a consumption of a rising proportion of healthcare costs, without commensurate increases in diagnosis of diseaseCitation7–11.
Whether the introduction of MDCT into daily clinical cardiology practice will increase or decrease overall downstream or subsequent test utilisation and CAD-related healthcare costs is not yet known. Moreover, whether there are systematic utilisation differences of post-test CAD-related resources and costs in patients with suspected CAD undergoing MDCT compared to traditional methods of evaluation, such as MPS, is also unknown.
Analysis of downstream resource utilisation and costs of individuals undergoing MDCT or MPS may be affected by the prevalence of other co-morbidities, which may result in misrepresentation of overall post-test healthcare costsCitation12–15. Practical evaluations of the patterns of healthcare resource use following testing by MDCT or MPS may be more accurately examined by the assessment of costs specifically related to CAD episodes of care.
Thus, the authors sought to evaluate the downstream CAD-related episodes of care costs in individuals without known CAD undergoing initial diagnostic testing by MDCT, and to compare MDCT costs to those incurred by MPS testing.
Patients and methods
Data sources and study population
This study examined patient-level clinical and costs data from a proprietary data warehouse that was initiated over 12 years ago and has been maintained by Health Benchmarks® Inc. Health insurance claims and medical data were analysed from two large regional health plans, which encompassed an enrolled membership of more than 6.5 million lives from 2002 to 2005. Members were enrolled in a variety of health plans, including health maintenance organisations, preferred provider organisations, and Medicare Advantage plans. The average enrollment period was approximately 2 years per member. In order to show completeness of data, the study sample was restricted to individuals who were continuously enrolled during the sampling window. Individuals who did not have continuous eligibility in one of the two health plans throughout the entire study period were excluded.
The database included membership information (i.e. date of birth, gender, enrollment history and health insurance product type), pharmacy claims (i.e. generic and brand name, therapeutic class or generic product identifier, National Drug Code, and prescription fill date), and line-item inpatient and outpatient (i.e. principal and secondary diagnosis codes, procedure codes, and date of service or discharge date) service claims. Within the study period, individuals who had undergone MDCT or MPS as an initial CAD diagnostic test were identified. An index diagnostic test date was defined as the date an individual underwent either of these two tests. A sampling window for costs was created from 1 year prior to the diagnostic test date to 1 year after the diagnostic test date. This permitted an adequate period of time for downstream cost utilisation. While longer time periods might have captured additional costs, extending follow-up beyond 1 year was not possible with the available data.
Only patients without known CAD were included, as identified by no prior claims within 1 year of the diagnostic test for any CAD-related diagnosis code (International Classification of Diseases-9th revision - Clinical Modification (ICD-9) codes 413, 413.0, 413.1, 413.9, and 410). MDCT claims studied included only coronary heart disease-specific codes (ICD-9 codes 412, 413.0-.9, 414.0-7, 414.8, 786.05, 786.5, 786.69, 794.30, 794.31) for analysis. Claims relating to MPS included similar codes for coronary heart disease-specific states. Datasets included no patient identifying information.
Definition of disease and episodes of care
The database also included patient- and disease-specific episodes of care, which reflected a longitudinal picture of the resources consumed by continuously enrolled member populations. The use of analytic methodology developed by the Episode Treatment Group (ETG) (Symmetrical Health Data Systems, Phoenix, AZ) was employed to create the clinical database. The ETG grouping software permits linking of demographic information and patient-specific diagnostic, procedural and pharmacy claimsCitation16–18. Claims were grouped into CAD-related episodes of care according to diagnosis, and clinical algorithms were then used to make certain that the matching of downstream resources to medical claims was correct, and then to clinically define the appropriate initiation and termination dates of each type of episode.
Episodes of care were calculated from linked individual claim records for each insured person. Procedures and services linked to an individual and performed within 1 year of the index diagnostic test date (MDCT or single photon emission computed tomography [SPECT]) were considered as part of one episode. The authors used 1 year as their criteria to permit a reasonable downstream period by which an individual might utilise resources and costs. Further analyses were performed for episodes of care, including calculation of the actual duration of the episode as well as a breakdown of CAD-related episodes by clinical conditions, services and procedures. CAD-related clinical conditions included in the analysis were coronary heart disease, ischaemic heart disease, pulmonary heart disease, cardiovascular disease signs and symptoms, congestive heart failure, diabetes mellitus, hypertension and hyperlipidaemia. Service and procedural components of the episodes of care included management, surgery, facility room and board, inpatient service, outpatient service, and pharmaceutical charges. Whenever at least one inpatient service was performed within the 1-year follow-up period, it was included as an inpatient care episode.
Management charges were based on non-surgical records, and were often associated with claims submitted by clinicians for services related to patient evaluation. Surgical charges were for claims submitted by a physician for surgical procedures. Facility room and board charges were claims for hospitals or other treatment facilities for room and board charges. Inpatient service charges were claims associated with a hospitalisation (e.g. laboratory services or radiologic services). Outpatient service charges were claims not associated with a hospitalisation or for claims from a hospitalisation by a physician who was not the primary attending of record. Pharmaceutical charges were claims for prescription drugs.
Pre-test clinical risk estimates
Baseline demographic characteristics included age, gender, health plan type, screen year, household income and general co-morbidities. Baseline cardiac-related conditions were also recorded and included diabetes, hypertension, hyperlipidaemia, peripheral arterial disease and cerebrovascular disease. A baseline cardiac risk score was calculated for all individuals in a manner previously describedCitation19. Briefly, the cardiac risk score is comprised of a weighted average of the following: medication use including digitalis, anti-coagulants including aspirin, anti-arrhythmic agents, angiotensin-converting enzyme inhibitors, b-blockers, lipid-lowering drugs, anti-hypertensive medications, anti-diabetic medications; as well as clinical conditions including peripheral vascular disease and cerebrovascular disease.
Because the presence of coexisting co-morbid conditions may play an important role in increasing treatment costs, patients were stratified according to the presence of existing related co-morbidities. Overall pre-screen health condition was measured by the use of a baseline Charlson Co-morbidity Index, which incorporates both proprietary and Deyo and Romano modificationsCitation12. The Charlson co-morbidity score is a claims-based index of co-morbid conditions that is commonly used to adjust for baseline patient health statusCitation13–15. The index is a weighted measure of chronic diseases related to mortality and effectively identifies high-severity or resource intensive patients. Chronic disease states included in the Charlson co-morbidity score were: peripheral arterial disease, heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, dementia, paralysis, diabetes mellitus, diabetes with sequelae, chronic renal failure, various cirrhodities, moderate–severe liver disease, peptic ulcer disease, rheumatologic conditions, metastatic carcinoma, and other forms of cancer. Charlson co-morbidity scores calculated during the 12-month pre-test period were used to control for the influence of health status on post-test imaging costs.
Cost outcome measures
The primary outcome of this study was CAD-related episodes of care resource utilisation and costs for individuals without known CAD undergoing initial testing by MDCT or MPS. Primary cost-related outcomes were defined as actual insurance-paid claims of CAD-related episodes of care in the follow-up period after the index test in each cohort. All costs were expressed in 2005 US dollars. Total costs for all CAD-related episodes of care were computed for follow-up periods after the diagnostic imaging test. Furthermore, costs of downstream CAD-related costs per patient as well as costs per episode were calculated. Episodes of care were subdivided into costs associated with the following categories: management, surgical, facility, inpatient, outpatient and medication costs. Claims analyses of these parameters summated by an episode of care enabled consideration of costs from a third party payer's perspective.
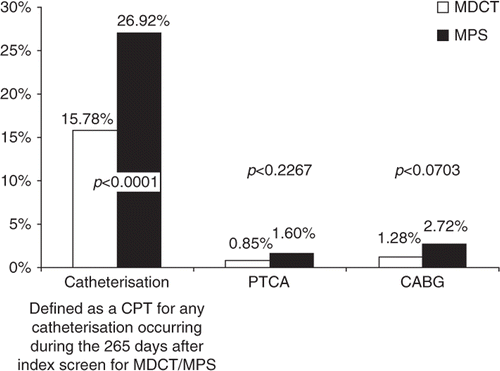
For each diagnostic test group, episode of care costs during follow-up were further analysed for high-cost procedures, including cardiac catheterisation, percutaneous transluminal coronary angioplasty, and coronary artery bypass surgery.
Statistical analysis
Descriptive statistics appropriate for the distribution of the variable were used to summarise all outcomes and covariates by cohort and to compare cohorts. Follow-up healthcare costs after the index diagnostic test in MDCT and SPECT cohorts were compared using a generalised linear model with a log link and gamma variance function. Model covariates included baseline cardiovascular risk factors, patient demographics, pre-test costs, pre-test cardiac risk (accounting for 10 cardiac risk states or cardiac-related medications) and the Charlson co-morbidity score (comprising 15 chronic disease states and conditions). Bootstrapping techniques were used to construct a 95% confidence interval (CI)around the cost difference between cohorts.
To account for differences in baseline clinical risk between the entire MDCT and MPS cohorts, a subgroup of MPS individuals was selected to have similar characteristics to individuals undergoing MDCT. Using traditional patient matching techniques via multilevel modeling, each MDCT patient was matched to four MPS patients on hyperlipidaemia, diabetes mellitus, coronary obstructive pulmonary disease, congestive heart failure, general co-morbidity, cardiac risk score, gender, screen year, age and health planCitation20,Citation21. While removing those patients of high cardiac risk who underwent MPS who could not be matched to patients who underwent MDCT may have resulted in a non-representative sample of those individuals who initially underwent SPECT, it provided a more valid comparison for estimating differences in post-screen CAD-related costs and clinical outcomes when compared to individuals undergoing MDCT.
Statistical analysis was conducted using SAS (v9.3 Cary, NC) or STATA (v8.0 College Station, TX) software and p-values less than 0.05 were considered statistically significant.
Results
Clinical characteristics of the study groups by initial diagnostic test
A total of 2,345 individuals, 39% men, comprised the study population. The average age was 54 years. Within the study group, 469 individuals underwent MDCT and 1,876 underwent SPECT (). No differences were noted between groups for hypertension, pulmonary heart disease and peripheral arterial disease.
Table 1. Clinical characteristics of the study registry by initial method of coronary evaluation.
MDCT patients were more likely to be taking aspirin and other anticoagulants, as well as loop diuretics and anti-diabetic medications (). No differences were noted between groups for other cardiac-related medications including angiotensen-converting enzyme inhibitors, statins, anti-arrhythmics, b-blockers, calcium channel blockers, digitalis, other anti-hypertensives and nitrates.
Table 2. Medication use.
Despite similar co-morbidity scores between groups, MDCT patients exhibited higher rates of certain components of the co-morbidity score such as COPD, dementia, paralysis, cirrhosis, rheumatoid arthritis and liver disease (). MPS patients were more likely to have renal failure.
Table 3. Co-morbidity score.
Downstream CAD-related costs by episodes of care
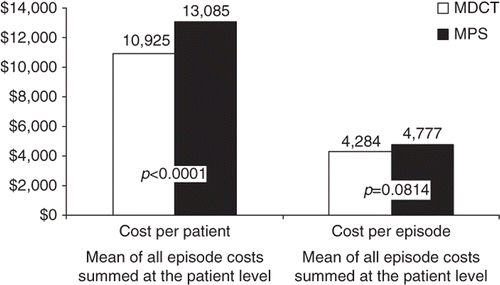
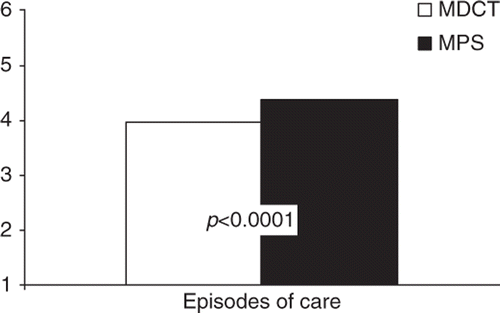
One-year downstream CAD-related episodes of care costs were lower for individuals undergoing MDCT compared to MPS, by an average of $682 per patient (95% CI, $14, $1,350) (). While costs per episode of care were not significantly different between MDCT and MPS patients ($4,284 vs. $4,777, p=0.08), individuals undergoing MDCT required fewer total numbers of CAD-related episodes of care (3.97 vs. 4.39, p<0.0001) ( and ).
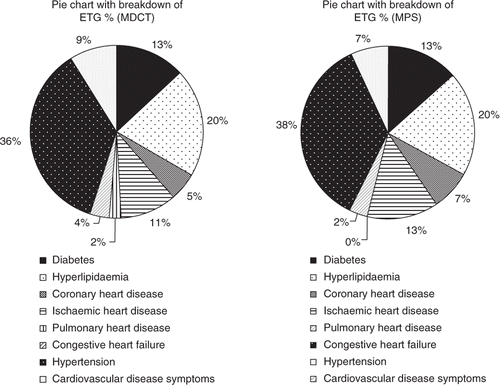
Components of CAD-related episodes of care comprising total downstream costs were similar between MDCT and MPS patients, with the highest proportion of individuals undergoing episodes of care for cardiovascular disease signs and symptoms, hyperlipidaemia and diabetes ().
Across high-cost cardiac-specific expenditures, MDCT exhibited lower utilisation rates for cardiac catheterisation (15.8 vs. 26.9%, p<0.0001). Rates of percutaneous transluminal coronary angioplasty (0.85 vs. 1.60%, p=0.23) and coronary artery bypass surgery (1.28 vs. 2.72%, p=0.07) were similar between groups ().
Discussion
This descriptive analysis was performed to provide new insight into the use of coronary MDCT in the evaluation of patients with suspected CAD. This observational cohort study of a large insured patient population undergoing initial coronary artery evaluation with MDCT demonstrates lower overall CAD-related episodes of care costs in a 1-year follow-up period compared to MPS, by an average of $682 per patient. Reduced downstream overall episodes of care costs are driven by fewer CAD-related episodes of care, as well as by lower rates of high-cost cardiac-specific expenditures, including cardiac catheterisation.
In this population of individuals with potentially lower CAD risk, MDCT patients exhibited episode costs and resource utilisation differences that were lower than those for MPS patients. This cost savings remained after additional multivariate adjustment for demographics, baseline cardiac risk and other factors. While the individual results of the MDCT and MPS tests are unknown in the current investigation, several potential explanations may be postulated to account for the present results.
Prior studies examining the sensitivity and specificity of detection of severe obstructive coronary stenosis have been favourable for MDCTCitation1–6. False positive results, findings which may incite further downstream testing, intervention and cost utilisation, occur less frequently than with MPS. It is possible that this lower rate of false positivity that occurs with MDCT contributed to the reduced numbers of episodes of care in the study follow-up period.
Furthermore, MPS is an imaging modality designed to identify severely stenotic flow-limiting coronary artery lesions, even though the majority of myocardial infarctions are caused by non-severe stenosesCitation1. In contrast, MDCT coronary angiography is able to detect mild and moderate coronary artery stenoses, which may identify a more optimal point in time to initiate favourable lifestyle and pharmacological modifications that affect cardiovascular outcome prior to the need for higher cost cardiac expenditures such as cardiac catheterisation, percutaneous transluminal coronary angioplasty, or coronary artery bypass surgeryCitation4,Citation22.
Finally, the negative predictive value for MDCT to exclude obstructive coronary artery stenosis has been demonstrated to be exceptionally highCitation4. Exclusion of severe stenosis by MDCT may have resulted in lower rates of downstream treatment, testing and intervention, which is reflected in the current data indicating that individuals undergoing MDCT require fewer CAD-related episodes of care.
Collectively, the current results support the concept that MDCT may be a useful test in clinical decision making, rather than inciting additional and potentially unnecessary tests and therapies.
To date, no direct head-to-head cost analysis has been performed comparing MDCT to MPS for the evaluation of individuals without known CAD. Thus, the findings of this study are of importance to healthcare providers, as well as public and private health insurers. As results of the current investigation reflect real-world practice patterns, they particularly apply to issues of interest of third-party payers.
However, the limitations of this study should be recognised. The present results were based on administrative claims data from a private payer mix, which precludes application of these results to other populations, including an exclusive Medicare population or other private payor mixes. Perhaps the primary limitation of the data was the absence of certain clinical information, such as pre-test symptoms and test results in the database. If available, these variables could have further strengthened the baseline similarity of the two groups. It remains possible that despite careful matching of MDCT and MPS cohorts on 10 patient variables including established clinical cardiovascular risk predictors, the groups may have remained different in other regards that were unobservable in the administrative claims database. It is possible for example, that those individuals who underwent MDCT may have possessed unobserved differences from MPS patients with regard to pre-test risk, type of physician ordering the examination, or ease of access to an MDCT scanner. Nevertheless, in the present study, a traditional matching approach was used to account for any potential biases in observed variables between MDCT and MPS cohorts. Future studies should focus on the use of different data sources with more clinical information to further address potential selection bias.
Furthermore, this analysis was focused on patients without known CAD. Thus, whether the current data apply to patients with a history of CAD would remains unknown.
Finally, the major outcomes of the present analyses were 1-year CAD-related episode costs. It remains possible that misclassification of CAD-related episodes of care or components of other episodes of care that were related to cardiovascular disease may have been excluded. In this regard, additional studies examining different classifications of CAD-related outcomes, longer follow-up time, cost effectiveness, and collection of clinical outcome data should be performed to provide further insight into clinical practice patterns and the cost of MDCT compared to traditional methods of coronary evaluation. Because of these limitations, the present results should be interpreted in the context of other data on the downstream clinical outcomes that relate to MDCT or MPS.
Conclusion
This analysis represents the first head-to-head comparison of two non-invasive cardiac imaging tests, MDCT and MPS, on the direct medical costs of evaluation of individuals with suspected CAD. Individuals undergoing evaluation by MDCT incurred fewer 1-year CAD-related episodes of care, which resulted in lower overall healthcare costs. Individuals undergoing evaluation by MDCT also underwent less downstream high-cost cardiac expenditure, including cardiac catheterisation, percutaneous transluminal coronary angioplasty and coronary artery bypass surgery. The study results suggest that MDCT may be a cost efficient alternative to MPS and suggests the need for further study comparing the two diagnostic modalities.
Acknowledgements
Declaration of interest: This study was funded by an educational grant from GE Healthcare. JKM has received research support and serves on the speaker's bureau for GE Healthcare.
Notes
References
- Klocke FJ, Baird MG, Lorrell BH, et al. ACC/AHA/ASNC Guidelines for the Clinical Use of Cardiac Radionuclide Imaging: Executive Summary—A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation 2003; 108: 1404–1418.
- Berman DS, Hachamovitch R, Kiat H, et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography. Journal of the American College of Cardiology 1995; 26: 639–647.
- Hachamovitch R, Shaw L, Berman DS. Methodological consideration in the assessment of noninvasive testing using outcomes research: pitfalls and limitations. Progress in Cardiovascular Diseases 2000; 43: 215–230.
- Hamon M, Giuseppe GB, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. Journal of the American College of Cardiology 2006; 48: 1896–1910.
- Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary CT angiography for prediction of all-cause mortality. Journal of the American College of Cardiology 2007; 50(12)1161–1170.
- Pundziute G, Schuijf JD, Wouter JJ, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. Journal of the American College of Cardiology 2007; 49: 62–70.
- Levit KR, Lazensby HC, Braden BR. National health expenditures. Health Affairs (Project Hope) 1996; 17: 35–51.
- Burner ST, Waldo DR, McKusick DR. National health care expenditures. Health Care Financing Review 1996; 14: 1–30.
- http://www.americanheart.org/presenter.jhtml?identifier=3000996.
- Ginzberg E. Health Services Research. Harvard University Press, Cambridge, MA 1991
- Fuchs U. The Future of Health Policy. Harvard University Press, Cambridge, MA 1993
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases 1987; 40((5))373–383.
- Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson Comorbidity Index using Canadian administrative databases: a perspective on risk adjustment in critical care research. Journal of Critical Care 2005; 20((1))12–19.
- de Groot V, Beckerman H, Lankhorts GJ, Bouter LM. How to measure comorbidity: a critical review of available methods. Journal of Clinical Epidemiology 2003; 56((3))221–229.
- Schneeweiss S, Maclure M. Use of comorbidity scores for control of confounding in studies using administrative databases. International Journal of Epidemiology 2000; 29((5))891–898.
- Forthman MT, Dove HG, Wooster LD. Episode treatment groups (ETGs): a patient classification for measuring outcomes performance by episode of illness. Topics in Health Information Management 2000; 21((2))51–61.
- Dang DK, Pont JM, Portmoy MA. Episode treatment groups: an illness classification and episode building system—Part II. Medical Interface 1996; 9((4))122–128.
- Dang DK, Pont JM, Portnoy MA. Episode treatment groups: an illness classification and episode building system—Part I. Medical Interface 1996; 9((3))118–122.
- Ray WA, Stein CM, Hall K, et al. Non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease: an observational cohort study. Lancet 2002; 359((9301))118–123.
- Carey K. A multilevel modelling approach to analysis of patient costs under managed care. Health Economics 2000; 9: 435–446.
- Raboud J, Breslow NE. Efficiency gains from the addition of controls to matched sets in cohort studies. Statistics in Medicine 1989; 8((8))977–985.
- Pohle K, Achenbach S, Macneill B, et al. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: comparison to IVUS. Atherosclerosis 2007; 190((1))174–180.