Abstract
Background: Metastatic colorectal cancer (mCRC) is one of the most common malignancies worldwide. The availability of new chemotherapeutic agents have modified the treatment of mCRC over the years creating the need to evaluate the financial impact of treatment. The aim of this study was to establish and quantify the financial resources needed during the first-line treatment of mCRC in Brazil.
Methods: The authors began by reaching expert consensus using a modified Delphi panel with oncologists working at public and private services in Brazil. Costs were calculated using official databases and the microcosting technique.
Results: The panel reached consensus on six regimens used in the first-line treatment of mCRC, as well as the resources involved in the administration of these regimens. All the regimens contain either fluorouracil (5-FU)/leucovorin or capecitabine, combined with either oxaliplatin or irinotecan. The analysis showed that, when compared with intravenous 5-FU/leucovorin, the cost of capecitabine was offset by administration costs.
Conclusion: The panel concluded that regimens containing capecitabine, especially capecitabine plus oxaliplatin (XELOX) are less expensive than those containing 5-FU/leucovorin. Given the comparable efficacy and good tolerability of the XELOX regimen, it may be an attractive choice for the first-line treatment of Brazilian patients with mCRC.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies in the world, accounting for approximately 945,000 new cases each year. It is also the second most frequent cancer in developed countriesCitation1. In Brazil, the estimated number of new cases of CRC in 2006 was 11,390 in men and 13,970 in womenCitation2. Although the disease is curable when detected at an early stage, approximately 25% of patients present with metastatic CRC (mCRC), and such patients have a 5-year relative survival rate of only 10%. This poor prognosis is associated with high direct, indirect and intangible costs for patients, payers and the society in general.
Advances in diagnostic and therapeutic techniques, and also the availability of several new active chemotherapeutic agents, have modified the treatment of mCRC over the last 15 years. Fluoropyrimidines, represented by fluorouracil (5-FU), remained the only agents used by medical oncologists in this setting for almost four decades. In an attempt to increase efficacy, 5-FU has been modulated and administered in various ways, with only modest improvementsCitation3,Citation4. This paradigm has changed significantly in recent years, due in large part to the introduction of multiple drugs that have demonstrated activity in the setting of mCRCCitation5. The currently available active agents include the fluoropyrimidines 5-FU and capecitabineCitation6,Citation7, the topoisomerase I inhibitor irinotecanCitation8, and the alkylating platinum oxaliplatinCitation9. In addition, biological therapies, such as the angiogenesis inhibitor bevacizumabCitation10 and the epidermal growth factor receptor-targeting monoclonal antibodies cetuximabCitation11 and panitumumabCitation12, have also shown efficacy in treating advanced disease; however, they have been approved only recently in Brazil and thus were not considered in the analysis. The current standard treatment for mCRC includes the use of a chemotherapy doublet in the first line, followed by a single agent or combination in the second and subsequent lines; whenever possible, biological therapies are combinedCitation5.
Capecitabine is an oral fluoropyrimidine developed to overcome the difficulties associated with intravenous administration of 5-FU and to increase the efficacy and tolerability of 5-FU-based chemotherapyCitation13. Thymidine phosphorylase is the final step in a three-step enzymatic process that converts capecitabine into 5-FUCitation14. During the time when capecitabine was undergoing investigation in Phase III trials, data were emerging on the superior efficacy of doublet regimens of 5-FU/leucovorin (LV) in combination with oxaliplatin or irinotecan compared with 5-FU/LV aloneCitation13.
A number of Phase III trials comparing 5-FU/LV/oxaliplatin (FOLFOX) with capecitabine/oxaliplatin (XELOX) have been conducted. In the TREE 1 trial, the median overall survival was 19.2 months for patients treated with FOLFOX, 17.9 months with bolus 5-FU/oxaliplatin and 17.2 months with XELOXCitation15. The Phase III NO16966 trial showed that the median progression-free survival achieved with XELOX with or without bevacizumab was not inferior to that with FOLFOX-4 (8 vs. 8.5 months)Citation16. That study also showed equivalence between XELOX and FOLFOX-4 in terms of overall survival (19.6 versus 19.8 months, respectively)Citation17. Moreover, the ML16987 Phase III trial disclosed that XELOX is non-inferior to FOLFOX-6, since it has a higher response rate with a comparable safety profileCitation18. The FOLFOX-4 regimen has well-known efficacy and toxicity profiles, but the FOLFOX-6 regimen is one of the most used due to its convenience in administration. XELOX is an alternative to FOLFOX-4 and -6 as first-line therapy in mCRC and offers the option of oral fluoropyrimidine administrationCitation17,Citation18. Finally, the combination of capecitabine plus irinotecan (XELIRI) has demonstrated significant efficacy but has overlapping gastrointestinal toxicityCitation14.
Clinicians are now faced with a number of different questions, including what is the most appropriate way to administer these various agents and what is the optimal combination of the many active therapies. The answers to these questions are central to improving treatment outcomes for metastatic disease, since the primary goal in treating these patients is prolonging life while minimising toxicity. The authors believe that besides efficacy and toxicity, economic issues assume utmost importance when choosing a chemotherapy regimen, since drug costs for treating mCRC may now vary up to 500-foldCitation19. Therefore, they attempted to evaluate the treatment costs for commonly available chemotherapy doublets used in the first-line treatment of patients with mCRC.
Methods
The Delphi panel
A modified Delphi technique was used, in order to reach consensus regarding the current approach to mCRC in BrazilCitation20,Citation21. The goal was to capture the reality of the Brazilian healthcare system. Consequently, oncologists who worked in the public and/or the private healthcare service, with large experience in CRC and who follow a substantial number of patients were targeted. First, a sample with more than 30 expert medical oncologists was randomly assigned for the first phase of the study. The experts were addressed from the associated oncologists from the Brazilian Society for Clinical Oncology (SBOC). The experts answered a structured questionnaire about clinical patterns for mCRC treatment, which was developed in cooperation with a market research team with recognised expertise on this type of survey. Second, the results were gathered, analysed and submitted to a validation panel. Finally, a validation panel was conducted.
The validation panel was composed of six medical oncologists with recognised expertise among their peers in Brazil. These medical oncologists, who represented public and private services, saw at least 15–30 patients with mCRC per week, had worked in this setting for 5–19 years, and had consistent knowledge about clinical research in mCRC, reflected by meeting presentations, scientific papers, and/or membership in groups with special interest in this area. The panel was conducted in March 2006.
The panel aimed at establishing and quantifying the financial resources needed during the first-line treatment of patients with mCRC, including the disease itself and of the major complications derived from the use of a central venous device (CVD), which is typically required in patients using continuous infusions of 5-FU. The authors attempted to explore and to reach consensus regarding procedures, laboratory tests, drug use, and number and type of different health professionals involved in treatment. A contract research organisation was selected to act as moderator/facilitator for the questionnaire and panel meeting exercise. A two-round modified Delphi approach was used. First, a set of tables displaying mCRC regimens was provided to each panel member, along with the first-round questionnaire. These regimens, shown in , were selected initially from a review of the literature9, 22–24. Since one of the panelists suggested the addition of one new regimen that was considered valuable (FLOX, bolus 5-FU, LV and oxaliplatin), this regimen was disseminated to the other panelistsCitation25. Questionnaires were based on the collected literature, current practice patterns and clinical experience with mCRC. In the second round, the oncologists met for a consensus meeting, which was conducted by a facilitator with previous experience in consensus-building strategies.
Table 1. Regimens currently used in the first-line treatment of metastatic colorectal cancer.
Cost calculation and data analysis
Public databases were used to obtain the unit costs of each resource used in the first-line treatment of patients with mCRC. Costs of medications used were taken from Brasíndice, which publishes the official price list in BrazilCitation26; the National Fees List of the Brazilian Medical AssociationCitation27 was the basis for the costs of medical fees; costs of materials were taken from the Simpro MagazineCitation28; and hospital rates were taken from the survey conducted by PROAHSACitation29. Costs related to the use of equipment and chemotherapy preparation were provided by the physicians in the Delphi Panel as there is no official source for these.
To determine the cost of a certain procedure or complication the microcosting techniqueCitation30 was applied: every resource (physician time, medicines, materials, equipments, hospital structure etc.) was multiplied by its unit cost and then summed, resulting in the total value per therapy. Costs were presented in Brazilian reais (US$1 = R$2.12374, from 3rd January 2006).
Results
Chemotherapy regimens
Even though the frequency of use of the regimens shown in varied according to the service, there was a greater trend towards the use of either FOLFOX-4 or FOLFOX-6 among the panel members. These are biweekly regimens, and their efficacy had already been demonstrated in clinical trials by the time the panel was held. Health insurance companies in Brazil typically cover both of these regimens. The FOLFIRI regimen, although used less often by the panel members, has the same characteristics as FOLFOX-4 in terms of treatment setting and coverage. Most patients receiving the FOLFOX-4, FOLFOX-6 and FOLFIRI regimens could theoretically be treated on an outpatient basis if portable infusion pumps were available. However, this is not always the case in Brazil, and a large proportion of such patients will need hospital admission when using regimens (assumed as 80%). This assumption has rather slight impact on total cost for these regimens. In the XELOX and XELIRI regimens chemotherapy is administered every 3 weeks, saving precious time for the patient. They also have the advantage of substituting the continuous infusion of 5-FU by the oral administration of capecitabine, thus avoiding the portable infusion pump issue; however, many health insurance companies in Brazil do not pay for oral drugs. Finally, the FLOX regimen requires no admission, since it employs only bolus administration of 5-FU, and is typically covered by health insurance companies. Despite these characteristics, the consensus concluded that this is the most expensive of the six regimens.
Central venous devices
One important point investigated in this study pertains to the use and complications of Port-a-Cath CVD. The panel members considered that the regimens FOLFOX-4, FOLFOX-6 and FOLFIRI require a CVD for their use in 100% of patients. With the XELOX, XELIRI and FLOX regimens, however, the panelists agreed that only between 20 and 30% of patients would need a CVD. Despite the use of a CVD in some patients treated with the XELOX and XELIRI regimens, the panelists concluded that catheter manipulation in these cases would be infrequent, with a resulting low rate of complications. It was considered that patients with a CVD would use it for a minimum of 12 months.
Ancillary drugs
According to the panel members, the antiemetic regimens used before intravenous administration of chemotherapeutic agents was the same for all six regimens. Such regimens typically include dexamethasone and ondansetron. On the other hand, the panel estimated that oral use of antiemetics at home would be required in only approximately 40% of the patients. In such cases, the choice of antiemetic depends on physician/service preference and on cost considerations, since these drugs are not covered by health insurance companies in Brazil.
For the regimens containing irinotecan (FOLFIRI and XELIRI), there was no consensus on the use of subcutaneous or intravenous atropin for diarrhoea prophylaxis, with rates varying among panel members from 30 to 50% in both regimens. On the other hand, there was consensus on the use of drugs for anaemia prophylaxis, with panel members prescribing erythropoietin (40.0000 IU/week) in 10% of the patients with all six regimens. Ferrous sulfate was used in 20% of the patients, and folic acid in only 10%, regardless of the chemotherapy regimen chosen. Likewise, there was consensus about the prophylaxis of neutropenia. For all regimens, the rate of use of filgrastim (300 mg/day for 5–10 days) was 5%, generally after 5 months from the beginning of treatment.
The intravenous use of magnesium sulfate and calcium gluconate was considered necessary in 80% of patients receiving three oxaliplatin-containing regimens (FOLFOX-4, FOLFOX-6 and XELOX) regimens. For the FLOX regimen, the use of these supplements was judged by the panel to be necessary in only 20% of the patients.
Diagnostic procedures
The diagnostic procedures required during chemotherapy were considered the same by panel members for all six regimens. Such procedures include blood tests for complete blood counts, renal function and hepatic enzymes. Imaging studies included a chest radiograph and computed tomography (CT) of the chest and abdomen. Imaging with CT was repeated every 2 months. Bone scan was not considered a routine examination, and was indicated only for patients with bone pain.
Costs
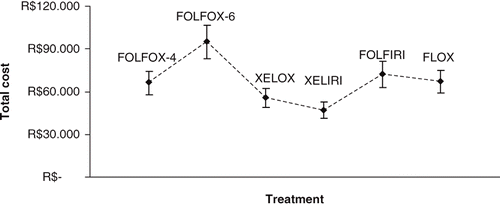
shows the professional resources consumed by each of the six first-line treatment regimens. lists all hospitalisations and materials used in a typical chemotherapy administration, including the use of gloves, syringes, saline/dextrose solutions, intravenous filters and lines, adhesive tapes and gauze. The total drug cost per regimen is shown in . depicts the estimated cost associated with the most common complications resulting from a CVD (FOLFOX-4, FOLFOX-6 and FOLFIRI), and the estimated total cost for each of the six regimens is shown in , assuming a 6-month timeframe for first-line therapy. Costs of adverse events were not included in the analysis as negligible differences were addressed. Results were most susceptible to drug costs variation, both chemotherapic and non-chemotherapic drugs, which were set at public list prices. Other variables, such as procedures and body surface area were tested within a multi-way sensitivity analysis, depicted in . Tested variables were complication costs, body surface area, acquisition costs and hospital costs, which were varied within ranges identified by the Delphi Panel and a 1.5–1.9m2 interval for body surface area.
Table 2. Professional costs for each first-line treatment regimen used in metastatic colorectal cancer in Brazil for 6 months.
Table 3. Hospitalisation and material costs for each treatment and for 6 months.
Table 4. Drug costs for each first-line treatment regimen for 6 months.
Table 5. Estimated costs of the main complications resulting from the use of a central venous catheter and estimated rate of occurrence.
Table 6. Estimated total cost for 6 months of treatment with each first-line regimen in Brazil.
Discussion
The results of the panel were in line with local guidelines from SBOCCitation31, suggesting a good accuracy of the methodology used, especially considering that SBOC guidelines were published in 2007. The main difference between the panel and society guidelines was the under use in clinical practice of biological therapies, and as stated before, the access to these medications is limited for the vast majority of patients in Brazil.
Hence, the financial costs involved in the management of mCRC in Brazil were assessed, comparing six identified regimens used for the first line in Brazil. The cost analysis suggests that, despite the higher cost of oral capecitabine, this is mostly compensated for by other costs incurred with regimens containing continuous infusions of 5-FU. Such costs are due in part to hospitalisations for drug administration, a particular feature of the Brazilian health insurance system. In addition, the regimens containing continuous infusions of 5-FU may require portable infusion pumps. Nevertheless, the most significant cost difference suggested by the panel is the high cost resulting from the use of a CVD. In addition to the costs of insertion and the catheter itself, a major point to consider involves the costs related to complications inherent in the use of a CVD in this clinical setting.
Advanced malignancies such as mCRC have a significant impact on health-related quality of life. Such impact is of particular importance in light of the fact that prognosis is generally poor and gains from chemotherapy are modest. The management of patients with mCRC is associated with significant costs to patients, insurers, the government and society in general. Major cost determinants in this setting include diagnostic procedures, monitoring/surveillance, hospitalisation, surgery, radiation therapy, chemotherapy and supportive care. The cost of treating mCRC has been estimated to be 2–3 fold higher than for patients with localised disease. Drug costs constitute a relatively small proportion of the overall cost of cancer care: the costs of hospital stays, outpatient care and other medical encounters generally accounted for >75% of the imputed costs in a retrospective analysis of resource use in patients with mCRC in five European countriesCitation32.
With the increasing number of new treatments available, there is an urgent need to determine the most cost-effective options in mCRCCitation33. Several pharmacoeconomic studies have been conducted to determine if the comparative clinical efficacy and superior tolerability of capecitabine over 5-FU/LVCitation6,Citation7, as well as the improved convenience and patient preference regarding oral administrationCitation34, translate into cost savings in the treatment of mCRC. Phase II clinical trials and interim results of one Phase III study showed that combination therapy with capecitabine and oxaliplatin (XELOX) is effective and well tolerated, thus representing an acceptable alternative to 5-FU/LV in the first-line treatment of mCRCCitation16. In addition, some economic analyses have indicated that the XELOX regimen will result in cost offsets compared with the FOLFOX regimen, primarily as a result of the higher incidence of treatment-related adverse events associated with the use of 5-FU/LVCitation35,Citation36.
More recently, the use of medical resources was analysed in the context of the NO16966 trialCitation37,Citation38. The use of CVD was higher in the group of patients treated with FOLFOX-4, in comparison with those treated with XELOX. The number of patients with at least one hospitalisation was similar with FOLFOX-4 and XELOX, while the use of XELOX led to a slightly higher number of admissions and an increased length of hospitalisation (half a day/patient higher). The two regimens were similar in terms of the amount of resource use needed to treat adverse events. The authors concluded that substituting oral capecitabine for continuous 5-FU reduces the number and duration of infusion visits and patient time costs, thus providing a more convenient regimen for themCitation37. In a companion report, the authors have shown that total direct medical costs were similar for FOLFOX-4 and XELOX (US$45,800 vs. US$44,500). However, XELOX had higher drug costs, while FOLFOX-4 had higher drug administration costsCitation38. On the other hand, the overall impact on medical resource use will vary depending on local patterns of care, especially with regard to the use of portable pumps and alternative FOLFOX regimens. Indeed, a cost-minimisation analysis conducted in France suggests that XELOX decreases hospital resource consumption, in comparison with FOLFOX-6, with approximately 30% lower number of total hospitalisations and a projected cost saving of approximately €4,889Citation18.
Analyses such as the one presented herein are subject to the premises adopted and to regional variations in patterns of care and costs of healthcare resources. Nevertheless, the panel composed of 30 members is thought to represent the view of Brazilian medical oncologists with recognised expertise in the management of mCRC. According to this panel, the XELOX and XELIRI regimens are less expensive than those containing intravenous infusions of 5-FU. This result, coupled with the confirmation of the comparable clinical efficacy between capecitabine- and 5-FU-containing regimens, suggests that the XELOX regimen in particular may become an attractive first-line choice for the treatment of Brazilian patients with mCRC. Further research with different evaluation methods, such as prospective data collection alongside a clinical trial, would be valuable for confirming results found in this medical resource use study, therefore improving database available in the country.
It is important to reinforce that, as there is an evident lack of database material in developing countries such as Brazil, performing cost studies, such as the one presented, is essential for aiding decision making where scarcity of resources is somewhat evident.
Acknowledgements
Declaration of interest: This study was funded by F. Hoffmann-La Roche Ltd. ES, MS and AC have all received financial sponsorship by Roche Pharmaceuticals. RC and RAR have no financial relationships to disclose.
References
- Stewart BJ, Kleihues P. World Cancer Report. IARC Press, Lyon 2003
- Brasil. Ministério da Saúde. Instituto Nacional de Câncer. Estimativa 2006: Incidência de Câncer no Brasil. Accessed 5 July 2007 from: http://www.inca.gov.br/estimativa/2006/.
- Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced Colorectal Cancer Meta-Analysis Project. Journal of Clinical Oncology 1992; 10: 896–903.
- Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group in Cancer. Journal of Clinical Oncology 1998; 16: 301–308.
- Saad ED, Hoff PM. Chemotherapy of metastatic colorectal cancer. Current Treatment Options in Gastroenterology 2005; 8: 239–247.
- Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. Journal of Clinical Oncology 2001; 19: 2282–2292.
- Van Cutsem E, Twelves C, Cassidy J, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large Phase III study. Journal of Clinical Oncology 2001; 19: 4097–4106.
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. New England Journal of Medicine 2000; 343: 905–914.
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology 2004; 22: 23–30.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine 2004; 350: 2335–2342.
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. New England Journal of Medicine 2004; 351: 337–345.
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. Journal of Clinical Oncology 2007; 25: 1658–1664.
- Kelly C, Cassidy J. Capecitabine in the treatment of colorectal cancer. Expert Reviews in Anticancer Therapy 2007; 7: 803–810.
- Terstriep S, Grothey A. First- and second-line therapy of metastatic colorectal cancer. Expert Reviews in Anticancer Therapy 2006; 6: 921–930.
- Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): Final analysis of the TREE-Study. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (20 June Supplement) 2007; 3510.
- Cassidy J, Clarke S, Diaz-Rubio E, et al. First efficacy and safety results from XELOX-1/NO 16966, a randomized 2x2 factorial Phase III trial of XELOX vs. FOLFOX4 + bevacizumab or placebo in first-line metastatic colorectal cancer (mCRC). Annals of Oncology 2006; 17, (Suppl 9, late breaking abstract 3).
- Cassidy J, Clarke S, Diaz-Rubio E, et al. XELOX compared to FOLFOX4: Survival and response results from XELOX-1/ NO16966, a randomized phase III trial of first-line treatment for patients with metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2007, ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (20 June Supplement), 2007: 4030.
- Perrocheau G, Bennouna J, Ducreux M, et al. Cost-minimization analysis of a phase III study of capecitabine + oxaliplatin (XELOX) vs. infusional 5-FU/LV + oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer (mCRC) in the French setting. Journal of Clinical Oncology 2007, ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (20 June Supplement), 2007: 4083.
- Schrag D. The price tag on progress – chemotherapy for colorectal cancer. New England Journal of Medicine 2004; 351: 317–319.
- Evans C. The use of consensus methods and expert panels in pharmacoeconomic studies. Practical applications and methodological shortcomings. Pharmacoeconomics 1997; 12: 121–129.
- Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technology Assessment 1998; 2: i–iv; 1–88.
- Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. Journal of Clinical Oncology 2004; 22: 229–237.
- Cassidy J, Tabernero J, Twelves C, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. Journal of Clinical Oncology 2004; 22: 2084–2091.
- Bajetta E, Di Bartolomeo M, Mariani L, et al. Randomized multicenter Phase II trial of two different schedules of irinotecan combined with capecitabine as first-line treatment in metastatic colorectal carcinoma. Cancer 2004; 100: 279–287.
- Wolmark N, Wieand HS, Kuebler JP, et al. A phase III trial comparing FULV to FULV + oxaliplatin in stage II or III carcinoma of the colon: Results of NSABP Protocol C-07. Journal of Clinical Oncology 2005; 23: 246S, (Suppl, late breaking abstract 3500).
- Guia Farmacêutico Brasíndice, Novembro 2006. São Paulo: Andrei Publicações Médicas, Farmacêuticas e Técnicas Ltda, 2006.
- Associação Médica Brasileira. Classificação Brasileira Hierarquizada de Procedimentos Médicos – CBHPM. Brasília: AMB – Associação Médica Brasileira, 2004.
- Revista Simpro, n. 45, 2006.
- Programa de Estudos Avançados em Administração Hospitalar e de Sistemas de Saúde - PROAHSA. Composição dos custos hospitalares. Indicadores PROAHSA. 41, 2006.
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes, 3rd ed. Oxford University Press, Oxford 2005
- Manual Geral de Condutas SBOC (16/11/2007). Available at http://www.sboc.org.br/ [in portuguese].
- Wiseman LR, Lyseng-Williamson KA. Management of metastatic colorectal cancer: defining the role of capecitabine. Disease Management & Health Outcomes 2005; 13: 137–149.
- Matasar MJ, Sundararajan V, Grann VR, et al. Management of colorectal cancer in elderly patients: focus on the cost of chemotherapy. Drugs & Aging 2004; 21: 113–133.
- Liu G, Franssen E, Fitch MI, et al. Patient preferences for oral versus intravenous palliative chemotherapy. Journal of Clinical Oncology 1997; 15: 110–115.
- Jansman FG, Postma MJ, van Hartskamp D, et al. Cost-benefit analysis of capecitabine versus 5-fluorouracil/leucovorin in the treatment of colorectal cancer in the Netherlands. Clinical Therapy 2004; 26: 579–589.
- Twelves C, Boyer M, Findlay M, et al. Capecitabine (Xeloda) improves medical resource use compared with 5-fluorouracil plus leucovorin in a Phase III trial conducted in patients with advanced colorectal carcinoma. European Journal of Cancer 2001; 37: 597–604.
- Scheithauer W, Cassidy J, Figer A, et al. A comparison of medical resource use for 4 chemotherapy regimens as first-line treatment for metastatic colorectal cancer (mCRC): XELOX vs. FOLFOX4 ± bevacizumab (A). Journal of Clinical Oncology 2007, ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (20 June Supplement), 2007: 4098.
- Garrison L, Cassidy J, Saleh M, et al. Cost comparison of XELOX compared to FOLFOX4 with or without bevacizumab (bev) in metastatic colorectal cancer. Journal of Clinical Oncology 2007, ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (20 June Supplement), 2007: 4074.