Abstract
Objective: To determine factors associated with the achievement of optimal lipid values (OLVs) and subsequent impact on clinical and economic outcomes.
Methods: An observational managed care database analysis was conducted among treatment-naïve adults with elevated cardiovascular (CV) risk, ≥12 months follow-up and full lipid panel from the 1st January 2002 to the 28th February 2005. Achievement of guideline-based levels for low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglycerides was evaluated via laboratory data. Annual CV-attributable resource utilisation was assessed via medical and pharmacy claims data. Clinical and economic outcomes associated with the achievement of OLVs were assessed using multivariate regression.
Results: A total of 52,778 patients were followed for a mean (standard deviation) of 27 (10) months with 13% achieving combined OLVs at baseline and 23% after 4 years. Of patients, 69% did not initiate lipid-modifying medication. The achievement of combined OLVs reduced the risk of CV event (odds ratio = 0.86; 95% confidence interval 0.78–0.95), resource utilisation (inpatient visits: 3.36 vs. 4.41 per 100 patient years, p<0.0001; emergency department visits: 1.1 vs. 2.4 per 100 patient years, p<0.05) and costs: $703 vs. $903 per patient year, p<0.0001.
Conclusions: Simultaneous achievement of OLVs was rare in this patient population. Physicians should be encouraged to manage multiple risk factors aggressively to improve clinical and economic outcomes associated with CV disease.
Introduction
Cardiovascular (CV) disease remains the leading cause of death in the US and is responsible for nearly half of all deaths in EuropeCitation1,Citation2. Hypertension, cigarette smoking, diabetes mellitus (DM) and dyslipidaemia are the major modifiable risk factors for CV morbidity and mortalityCitation1. An elevated level of low-density lipoprotein cholesterol (LDL-C) has been identified as a significant risk factor for CV diseaseCitation3,Citation4. In addition to LDL-C, low levels of high-density lipoprotein cholesterol (HDL-C) and elevated levels of triglycerides (TG) are also important independent risk factors for CV diseaseCitation3–6.
Evidence-based guidelines for the modification of lipid risk factors specify optimal values for LDL-C, and suggest desired levels for HDL-C and TG among various patient subgroups; however, these and more recent studies suggest a primary focus on lowering LDL-C with the use of statinsCitation3,Citation7–11. While the reduction of LDL-C is an important component of current therapeutic practice to lower CV risk, recent evidence suggests an incremental benefit in residual CV risk reduction with the inclusion of simultaneous elevation of HDL-C and lowering of TG levels in addition to lowering LDL-C. The Helsinki Heart StudyCitation12 and the Veterans Affairs High-Density Lipoprotein Intervention Trial (VA-HIT)Citation13 found that fibrate therapy was associated with reductions in CV morbidity and mortality in patients with low HDL-C and elevated TG. In addition, evidence-based guidelines, such as the National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III) report suggest the use of fibrate or niacin therapy when confronted with low HDL-C or when non-HDL-C is elevated in high-risk individualsCitation3,Citation8.
Previous research has demonstrated that simultaneous or combined achievement of acceptable levels for LDL-C, HDL-C and TG is uncommon in routine clinical practice in the US and suggest that this may be related to under-utilisation of guideline recommended pharmacotherapy, particularly those that target low HDL-C and high TG levelsCitation14–17. Furthermore, a recent study estimated up to a 45% increase in CV risk among patients not achieving desired levels of all three lipids simultaneously compared with patients who doCitation18.
While previous research provides important insight regarding the prevalence and clinical consequences of unacceptable levels of combined LDL-C, HDL-C and TG values, it does not describe the potential economic benefits of CV risk reduction derived from simultaneous optimisation of all three lipid parameters. The goals of this paper are to evaluate the impact of lipid-modifying medication on the achievement of desired lipid values, to estimate the risk of CV events in patients who achieved combined desired lipid values compared with those who did not, and to evaluate the potential economic benefit of the corresponding reduction in CV event risk in terms of CV attributable resource utilisation and costs.
Methods
Study design and data source
This was a longitudinal observational study that utilised administrative pharmacy, medical claims and laboratory result data. Data were collected from two health plans located in the Southeastern region of the US representing 4.3 million covered lives. Investigators were blinded to patients’ identities throughout the study. All study materials were handled in compliance with Health Insurance Portability and Accountability Act of 1996 regulations, and the analyses were conducted using a limited dataset. Institutional review board approval was not necessary because the study did not involve a patient intervention, the data were de-identified, and protected health information was not used.
Cohort selection
Patients aged 18 and older with the laboratory results from a complete lipid panel (total cholesterol, LDL-C, HDL-C and TGs) during the period spanning the 1st January 2002 to the 28th February 2005 were selected. The date of the first complete lipid panel in that timeframe was defined as the baseline laboratory date. Patients were excluded if they did not have continuous health plan eligibility for at least 12 months before and at least 12 months after the baseline laboratory date. Patients whose records included a Generic Product Identifier (GPI) code for a lipid-modifying medication (bile acid sequestrants, fenofibrates, ezetimibe, statins, nicotinic acid derivatives, miscellaneous antihyperlipidemics and combination antihyperlipidemics) in the 6 months prior to the baseline laboratory date were excluded.
The blinded medical, pharmacy and laboratory administrative claims records were extracted for all eligible patients. Data were extracted for all patients over the duration of the health plan eligibility within a study period spanning from the 1st January 2000 to the 28th February 2006. Individual lipid laboratory results observed over the study follow-up that did not occur on the same day as part of a full lipid panel evaluation were excluded from the analyses.
Patient risk stratification and optimal lipid value assessment
Patients were categorised into two risk groups based on the criteria for CV event risk described in the NCEP ATP III report and the information available in the claims database. The claims information in the 12 months prior to and on the baseline laboratory date were used to identify patients at risk for a CV event: (1) primary risk or (2) coronary heart disease (CHD) or a CHD risk equivalent/secondary risk, based on their age, gender, baseline lipid values, prior CV-related diagnoses and procedures, and medication usage. Patients with an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) or Current Procedural Terminology (CPT) code for CHD or a CHD risk equivalent as defined by any diagnosis or procedure relating to ischaemic heart disease (IHD), cerebrovascular accident/transient ischaemic attack (TIA), coronary artery bypass graft, angioplasty, aortic aneurysm, peripheral vascular disease or diabetes mellitus () occuring on any claim within an emergency department, inpatient or outpatient setting were classified in the secondary risk group. The primary risk group included patients with no evidence of CHD or a CHD risk equivalent and two of three CV risk factors based on age, HDL-C and prior hypertension. Patients whose risk status could not be clearly identified as primary or secondary risk based on the administrative claims and laboratory result data, and those who may have had unobserved risk criteria (e.g., patients who smoked or had a family history of cardiovascular disease) were excluded from the study.
Table 1. Classification of patients by cardiovascular prevention group factors.
The achievement of optimal lipid values (OLVs; ) was determined based on each patient's CV risk as per NCEP ATP III, American Heart Association, or American Diabetes Association evidence-based guidelinesCitation3,Citation8,Citation19 for LDL-C, and suggested acceptable levels for HDL-C and TG values.
Table 2. Optimal lipid values as defined by NCEP ATP III, AHA and ADA guidelines.
Comorbidity assessment
Baseline burden of comorbidity was assessed using the Deyo-Charlson co-morbidity indexCitation20 and calculated using medical claims in the 12-month, pre-baseline period. A history of hypertension, CHD, peripheral arterial disease (PAD), ischaemic stroke/transient ischaemic attack stroke (IS/TIA) or TIA, DM and metabolic syndrome were obtained using ICD-9-CM, CPT, or pharmacy records from the 12 months prior to the baseline lipid panel date. Metabolic syndrome was defined as the presence of a medical diagnosis of metabolic syndrome (ICD-9-CM code 277.7) or the presence of any three of the following conditions: obesity (ICD-9-CM code 178.xx), TG value ≥1.65 mmol/l (150 mg/dl), hypertension (ICD-9-CM codes 401.xx and GPI codes for antihypertensive medications), fasting blood glucose ≥6.05 mmol/l (110 mg/dl), and/or HDL-C <1.04 mmol/l (40 mg/dl) for men and <1.30 mmol/l (50 mg/dl) for women.
Identification of lipid therapy
Evaluation of lipid-altering pharmacotherapy was initially conducted on an intent-to-treat basis. Lipid-altering pharmacotherapy was characterised by the type of therapy initiated after the baseline laboratory measurement, and the time to initiation of therapy. In order to evaluate the effect of lipid-modifying medications on the achievement of OLVs, laboratory results were linked longitudinally to lipid-modifying medications based on an algorithm developed from a review of pharmacy and laboratory claims data. Prior to the date of each full lipid panel laboratory visit, pharmacy claims were scanned for fills for lipid-modifying medications. The study assumed that lipid-modifying medication would not be likely to affect lipid values within the first 4 weeks of therapy. At the time of each full lipid panel measurement, patients were considered to be receiving a lipid therapy only if their prescription was filled at least 4 weeks prior to the follow-up lipid panel but no longer than twice the number of days of supply for that prescription.
Identification of cardiovascular events
CV events were identified as any emergency department or an inpatient hospitalisation with ICD-9-CM or CPT codes for IHD, PAD, or IS/TIA. If multiple event types (IHD, PAD and/or IS/TIA) occurred during the same emergency department or inpatient hospitalisation, each event was recorded separately.
Resource utilisation and costs
Resource utilisation and costs were evaluated by a review of medical claims for encounters occurring within inpatient, outpatient and emergency department settings, as well as pharmacy claims for prescription medication. All emergency department visits occurring 1 day prior to an inpatient hospitalisation were considered to be part of the inpatient visit. Resource utilisation and costs were considered CV attributable if they were linked with medical encounters identified by any ICD-9-CM and/or CPT codes for CV disease or pharmacy fills with a GPI code for lipid altering therapy. These resource utilisations and costs were accrued over the duration of each patient's follow-up period. The total costs were annualised by aggregating the costs over time and dividing the total by the number of years of follow up. Initial presentation of total resource utilisation and cost was segmented by type of resource: (1) medical encounters and (2) fills for pharmacological lipid-altering therapy. Total resource utilisation and costs for medical encounters were further segmented via place of service (inpatient, emergency department and outpatient) in order to characterise the significant drivers of total medical resource utilisation and costs within each place of service. In subsequent multivariate analysis of CV event costs, pharmacy costs were incorporated as part of total costs in order to evaluate the full cost benefit associated with achievement of combined OLVs. Specifically of interest was whether the reduction in CV event attributable costs associated with the achievement of combined OLVs was sufficient to overcome the increased costs associated with pharmacological treatment associated with successful lipid optimisation. Since the purpose of the cost analysis was to compare CV-related expenditures between patients achieving and not achieving combined OLVs, inflation adjustment of costs to present-day dollars was not necessary as any inflation adjustment would be uniformly applied between both lipid achievement groups.
Statistical methods
Descriptive statistics were used to describe demography of the study sample, type of lipid-altering therapy initiated, achievement of OLVs during follow-up, frequency of CV events, and annual CV attributable resource utilisation and costs. Means and standard deviations were calculated for continuous variables, and relative frequencies were calculated for categorical variables. Statistical difference between groups was assessed using Pearson's Chi-square test for categorical variables and two sample t-test for continuous variables.
The association between the attainment of combined OLVs and lipid-modifying therapy was evaluated in an exploratory manner via generalised estimating equation (GEE) modified logistic regression. The dependent variable in the GEE logistic model was combined OLV attainment at each lipid laboratory visit. Independent variables were selected in a forward step-wise exploratory manner starting from a base model, which included the main covariate of interest (niacin or fibrate therapy), age and gender. In addition to fibrate or niacin therapy, additional covariates associated with the achievement of combined OLVs were also included: age at lab date, gender, statin therapy, hypertension, DM and CV disease prior to lab visit, baseline LDL-C, HDL-C and TG levels, and comorbidity index. To date there are no established or generally accepted summary statistics available for assessing the adequacy of fit of a GEE modified logistic regression model. Therefore, given the exploratory nature of GEE model development, further assessment of overall GEE model fit was withheld for this study. Kaplan-Meier curves were used to assess the unadjusted risk of CV event during the study follow-up period between patients achieving combined OLVs and those not achieving combined OLVs. In addition, a multivariate logistic regression model was developed to estimate the adjusted risk of CV events. The main covariate of interest was combined attainment of LDL-C, HDL-C and TG goals in the last full lipid panel prior to the first event or censorship. In addition to the attainment of combined OLVs, additional covariates associated with CV event risk were also included: age, gender, prior CV disease, DM, hypertension and duration of follow-up. Finally, a gamma distribution generalised linear model with log link function was developed to evaluate the association between the achievement of OLVs and total annual costs attributable to CV disease including pharmacy costs for lipid altering medication. In addition to combined OLVs, additional covariates associated with CV event risk were also included: age, gender, baseline lipid profile, DM, hypertension and comorbidity index.
All statistical analyses were conducted using SAS version 9.1 (Version 9.1 for WINDOWS. Copyright © 2006 SAS Institute Inc., Cary, NC, USA).
Results
Demographics and subject characteristics at baseline
A total of 52,778 patients met all the inclusion criteria for the study. The mean follow-up time for the entire patient population was 27 (standard deviation (sd) 10) months. More than half of the patients met the criteria for the primary risk subgroup (n=30,707; 58%) and 42% of the population (n=22,071) were identified in the secondary risk subgroup. The results of 132,279 full lipid panels were available for the study population during the study period. In the total study population, 18,178 patients received only one full lipid panel during the study period.
shows the demographics and characteristics of the study sample stratified by CV risk. The mean age of the entire study sample was 54.1 (sd 11.4) years and 53.6% were male. Primary risk patients were older and more likely to be male compared to secondary risk patients. The mean total cholesterol level for the total population at baseline was 5.29 mmol/l or 203.3 mg/dl. The baseline mean LDL-C was 3.22 (sd 0.92) mmol/l or 123.9 (sd 35.3) mg/dl, and baseline mean HDL-C was 1.22 (sd 0.38) mmol/l or 46.9 (sd 14.6) mg/dl. The baseline mean TG level was 1.78 (sd 0.88) mmol/l or 161.5 (sd 79.6) mg/dl. Primary risk patients had significantly worse mean lipid profiles than secondary risk patients.
Table 3. Demographics and patient characteristics.
The majority of patients (69%, n=36,366) did not have a pharmacy claim for any lipid-modifying medication during the follow-up period (). Among the 16,412 patients (31%) initiating lipid-modifying therapy, only 2.5% (n=404, less than 1% of the total population) received a lipid-modifying medication to improve HDL-C and/or TGs (fibrate or extended release niacin) in combination with therapy lowering LDL-C. Patients who received lipid-modifying therapy initiated treatment an average of 9.9 (sd 9.4) months after the first lipid panel during the study period. Secondary risk patients were significantly more likely to receive any lipid therapy than primary risk patients (34.3 vs. 28.8%; p<0.0001).
Table 4. Lipid-altering treatment.
Optimal lipid value achievement
At baseline, 13% (n=6,980) of the total population had achieved combined OLVs for LDL-C, HDL-C and TGs; 37% (n=19,679) had only one lipid fraction at a non-optimal level; 34% (n=18,108) had two lipid fractions at non-optimal levels; and 15% (n=8,011) were non-optimal for all three lipid fractions.
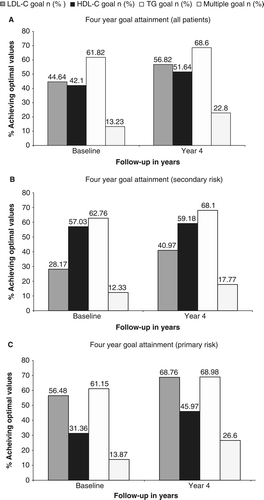
Overall, achievement of combined OLVs increased from 13% at baseline to 23% by the fourth year of follow-up (). This was mostly driven by increases in optimal achievement of LDL-C values (45% at baseline to 57% by year four) as both achievement of optimal HDL-C (42% to 52%) and TG (62% to 69%) values increased marginally from baseline. Among secondary risk patients (), the disparity in achievement of OLVs for HDL-C (57% to 59%) and TG (63% to 68%) was more dramatic relative to that of LDL-C (28% to 41%) from baseline to the fourth year of follow-up, contributing to a lower achievement of combined OLVs from baseline (12%) to follow-up (18%). Since low levels of HDL-C were among the criteria for identifying primary risk patients, it is not surprising that achievement of optimal HDL-C values was greatest from baseline (31%) to follow-up (46%) compared to LDL-C (56% to 69%) and TG (61% to 69%) ().
GEE model results show that the odds of multiple goal attainment were 42% greater in patients who received either fibrate or niacin therapy at the date of their laboratory visit compared with those patients not on fibrate or niacin therapy (95% confidence interval (CI) 1.22–1.66) (). Other factors that had a significant impact on the likelihood of lipid goal attainment included age at each laboratory visit, statin therapy on the date of laboratory visit, male gender, previously diagnosed hypertension and Deyo-Charlson comorbidity index.
Table 5. Summary of multivariate model results: likelihood of obtaining optimal lipid values for all measures.
Cardiovascular event risk
In the total population, patients who achieved combined OLVs were significantly less likely to be diagnosed with angina (3.8% vs. 4.7%; p<0.0001), undergo a revascularisation procedure (1.0% vs. 1.5%; p<0.0001), or experience myocardial infarction (1.1% vs. 1.6%; p=0.0002) during the follow-up period compared with patients who did not (data not shown). Secondary risk patients who achieved combined OLVs were significantly less likely to experience coronary events relating to angina (6.4% vs. 7.7%, p=0.0042) or revascularisation (1.43% vs. 1.88%, p=0.0008). Primary risk patients who achieved combined OLVs were significantly less likely to experience myocardial infarction compared with patients who did not (0.69% vs. 0.84%, p=0.0043). The frequency of a PAD diagnosis was similar between the OLV and non-OLV groups, overall and by CV risk groups.
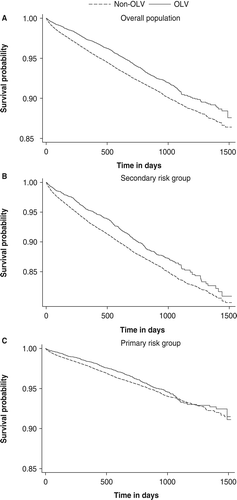
depicts the Kaplan-Meier curves describing the risk of CV events over the follow-up period for patients who achieved combined OLVs compared with those who did not for the total population and stratified by risk status. The curves show an elevated risk of CV events among patients who did not achieve combined OLVs over time compared to patients who did.
Consistent with unadjusted analysis, multivariate results () indicate that patients who attain combined OLVs were 14% less likely to experience a CV event compared with patients who did not attain combined OLVs (relative risk (RR) = 0.86; 95% CI 0.78–0.95).
Table 6. Cardiovascular events by risk of a cardiovascular event.
Resource utilisation and costs
As shown in , patients achieving combined OLVs had a significantly lower number of annual CV attributable inpatient visits (3 vs. 4 visits per 100 patient years; p<0.0001) and emergency department visits (1 vs. 2 visits per 100 patient years; p=0.047). Among secondary risk patients, those not achieving combined OLVs had a greater mean number of annual CV attributable inpatient visits (7 vs. 6 visits per 100 patient years; p=0.0002), but fewer outpatient visits (82 vs. 90 visits per 100 patient years; p=0.041). Patients achieving combined OLVs had more prescription claims than those not achieving combined OLVs overall and across risk levels.
Table 7. Annual cardiovascular attributable resource utilisation and costs by last lipid goal status.
In the overall study population and by CV risk group, patients not achieving combined OLVs incurred significantly greater total annual CV attributable healthcare costs than patients that did (). In particular, all study patients who did not attain combined OLVs incurred higher annual costs for CV disease related inpatient stays (US$683 vs. US$470; p<0.001) and emergency department visits (US$220 vs. US$23; p<0.0001). These results were consistent among secondary risk patients with the exception of annual CV attributable emergency department costs. In addition, inpatient costs were much lower among secondary risk patients attaining combined OLVs compared to non-OLV patients (US$778 vs. US$1127; p=0.0002). In the entire study sample and risk groups, patients achieving combined OLVs incurred statistically significantly higher prescription drug costs, although the mean values were not substantially higher.
Multivariate economic analysis indicate a 10% reduction in annual CV attributable costs (including costs associated with lipid modifying medications) among patients who achieve combined OLVs compared to patients who do not (RR=0.89; p=0.002) ().
Table 8. Summary of multivariate model results: costs attributable to cardiovascular disease.
Discussion
The results of this observational study suggest that simultaneous achievement of optimal LDL-C, HDL-C and TG values was rare in this commercially insured patient population. Specifically, of the three lipid parameters, patients were most likely to achieve optimal LDL-C levels over 4 years of follow-up compared to HDL-C or TG. Although all patients in this analysis were at elevated CV risk, initiation of lipid-altering pharmacotherapy of any type occurred in less than one-third of patients and was delayed by approximately 10 months from the first lipid panel until initiation of treatment. This under-treatment and delay may have been the result of one or more factors. Prescribers may have recommended a prolonged period of treatment with diet and exercise. Also, prescribers and patients may have been concerned about myopathy or gallstones as possible side effects of lipid-modifying medicationsCitation10, Citation21–23.
The emphasis on LDL-C lowering therapy is evident in this study and may be a direct result of guideline recommendations. Previous studies have shown that lowering LDL-C with statin therapy has been linked with improved CV outcomes and a reduced risk of deathCitation24–26. Statin monotherapy effectively lowers LDL-C, but has a modest impact on lowering TGs, and a minimal impact on raising HDL-CCitation27. The predominance of lipid-modifying pharmacotherapy that targets the reduction of LDL-C, in combination with under-utilisation of therapies that simultaneously raise HDL-C and lower TG levels, suggests a relationship between current treatment practices and suboptimal achievement of combined lipid values. While approximately half of the total study population had more than one baseline lipid parameters at suboptimal levels, less than 1% of patients were treated with combination therapy to target more than one lipid parameter. These results suggest a tremendous opportunity for improvement of patients’ lipid profiles beyond current treatment practices that target the optimisation of LDL-C alone.
Focused treatment targeting HDL-C and TG levels is of increasing interest yet not well defined in existing treatment guidelines. Results of this study, which indicate a 42% increased likelihood of OLV achievement with the use of fibrate or niacin therapy, in addition to the use of statin therapy, are promising and are consistent with other studies that have shown combination therapy of a statin with niacin or fibrate to be associated with increases in HDL-C, and reductions in LDL-C and TG levelsCitation28,Citation29.
The results demonstrate a consistent relationship between the achievement of combined optimal values for LDL-C, HDL-C and TG, and reduced risk of CV events. In addition, this study demonstrates a direct translation of this reduction in CV event risk among patients achieving combined OLVs in terms of reduced annual CV attributable resource utilisation and costs. Patients achieving combined OLVs had lower mean annual CV disease-related inpatient and emergency department visits, which correspondingly lead to lower total medical costs. While patients achieving combined OLVs were observed to incur slightly higher annual prescription drug costs for lipid-altering medication, it is interesting to note that multivariate analysis showed an overall decrease in annual CV disease expenditure among patients achieving combined OLVs. These results suggest that the cost benefit derived from reducing CV risk may offset the increase in cost of treatment, and further supports the health productivity of lipid therapy in these patients.
Many of the results in this study are similar to those found in previous publications. Other investigations have found that less than half of patients with known risk factors for CV disease have met their optimal LDL-C levelsCitation30–32. In addition, a retrospective chart review in high-volume prescribers of lipid-modifying drugs found that more than half of the patients met target LDL-C levels, but that HDL-C and TG levels were less likely to be metCitation16. In addition to the recommendations from expert panels such as the NCEP ATP III and American Heart Association, trial data have demonstrated that combination therapy for dyslipidaemia can improve lipid levels and CV outcomesCitation18,Citation28,Citation33,Citation34. Three meta-analyses have confirmed the findings that using medications to lower LDL-C reduces the risk of CHD in men and womenCitation26,Citation27,Citation35. Another meta-analysis has concluded that raising HDL-C in addition to lowering LDL-C has an additive effect on reducing the risk of CHDCitation4. Results from a meta-analysis of prospective trials have also shown that TGs are a risk factor for CHD independent from HDL-CCitation5. Long-term outcomes studies have also demonstrated the effectiveness of lipid-modifying medications on CV disease. In a study of more than 20,000 adults in the UK, CHD death and vascular event rates were significantly lower in patients treated with simvastatin for both men and women, and primary and secondary risk groupsCitation25. Frick et al documented the effectiveness of fibrate therapy in reducing CHD events in middle-aged men in the Helsinki Heart StudyCitation36. The VA HIT study found that gemfibrozil reduced the number of CV events in men with CHD and DM or a high plasma fasting glucoseCitation37 as well as in the total study populationCitation13.
The results of this study suggest that the healthcare costs related to CV disease incurred by patients who achieved OLVs were lower than the costs for patients who did not. Other studies have found that the costs of treating dyslipidaemia in some patient populations may be totally or partially offset by reductions in healthcare expenditures for future heart disease. The Scandinavian Simvastatin Survival Study (4S) found that most of the cost of simvastatin in patients with a history of CHD was offset by the reduction in the cost of hospital services during the approximately 5-year follow-up periodCitation38. Prosser et al found that therapy with pravastatin was cost effective for all secondary prevention patients and for primary prevention patients with high LDL-C, hypertension and low HDL-CCitation39. Scuffham and Chaplin developed a Markov model that demonstrated fluvastatin as a cost-effective option for preventing cardiac events in patients with and without DM who have recently undergone percutaneous coronary interventionCitation40,Citation41.
A cost-effectiveness analysis using data from VA-HIT found that therapy with gemfibrozil was highly cost effective in males with pre-existing CHD, low HDL-C and normal LDL-CCitation42. The results of an economic model populated with data from long-term outcomes studies found that fibrate therapy was cost effective for primary prevention of CHD in patients with low HDL-CCitation43. Another economic model using multinational outcomes data and costs from the UK also concluded that fibrates were more cost effective than statins in patients with DMCitation44. A study conducted in France found that nicotinic acid therapy was cost effective when added to statin therapy in CHD patientsCitation45.
The results of this study should be interpreted in the context of its key limitations. First, a computerised database of medical histories was used to define patient risk, treatment patterns and clinical outcomes. Although retrospective databases provide a blinded source of data, some exposure misclassification is likely. Selection bias is generally a concern with retrospective database studies because patients cannot be randomised. The results provide evidence of treatment patterns, but because of the retrospective study design, the reasons for the driving forces behind the treatment patterns cannot be ascertained. Second, the patients included in this study were enrolled in one of two commercial health plans in the Southeastern US. The results of this study may not be generalisable to other locations. Third, compliance with lipid-modifying medications was not an objective of this study, and pharmacy dispensing records were not accessed. Fourth, the possibility exists that patients obtained lipid-altering medication not identified in pharmacy claims data, but it is unlikely unless patients had prescription drug coverage from more than one healthcare plan. Currently lipid-lowering medications are not available without a prescription in the US. In addition, the definition for primary and secondary prevention was modified from treatment guidelines to reflect the information available in a claims database. The database did not capture all socioeconomic and demographic characteristics that are likely to impact lipid values, treatment patterns and CV risk such as race, education, income, smoking status, diet and exercise routines, or family history. The study population was limited to patients with the results of a lipid panel in their medical record who qualified for primary or secondary prevention strategies. At risk patients who had not been referred for laboratory assessments, or patients with risk factors that could not be captured in their electronic record were not included. It is likely that the results of this study reflect a conservative estimate of unmet need. Finally, only a subset of patients received more than one lipid panel per year during the study period. Patients who did not receive follow-up lipid panels may have achieved their lipid goals. This potentially underestimates the annual rate of combined goal attainment.
The results of this retrospective database study imply that physicians may not be considering their patients’ complete lipid profiles when making decisions about lipid-modifying medications. At year four, less than a quarter of patients who met the criteria for primary or secondary CV prevention achieved combined OLVs for LDL-C, HDL-C and TGs. Nearly 70% of patients had no prescription claim for a lipid-modifying medication during the study period. The majority of patients who did receive a lipid-modifying medication were treated with statin monotherapy. The results from this study should be confirmed with a prospective, long-term, randomised controlled trial. Although the NCEP ATP III report recommends that LDL-C should be the primary target of therapy for patients with dyslipidaemia, these and other treatment guidelines also cite the results of clinical trials that strongly support a lower risk of CV events and a reduction in atherosclerosis when HDL-C raising and TG lowering strategies are also incorporated in treatment planningCitation3Citation8,Citation19. Physicians should be encouraged to manage multiple risk factors aggressively as per the evidence-based guidelines to improve clinical outcomes and to minimise medical care utilisation and costs of CV disease.
Acknowledgements
Declaration of interest: This study was funded by Abbot Laboratories. RAQ is an employee of HealthCore, Inc. and has received research grants from Abbott Laboratories, AstraZeneca PLC, Bristol-Myers Squibb, Merck & Co., Inc., Novartis International AG and Pfizer Incorporated; MJC is the Vice President of Research Development & Operations of Health Outcomes for HealthCore, Inc. and has received the same research grants as RAQ; RTB is Assistant Director of Global Health Economics Outcomes Research for Abbott Laboratories; and RJS is Director of Global Health Economics Outcomes Research for Abbott Laboratories.
Notes
References
- Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics–2006 Update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation Journal of the American Heart Association 2006;; 113((6))e85–e151.
- Petersen S, Peto V, Rayner M, Leal J, Luengo-Fernandez R, Gray A. European cardiovascular disease statistics, 2005 edition. Available at:. http://www.ehnheart.org/files/statistics%202005-092711A.pdf, Accessed 10 March 2008.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Journal of the American Medical Association 2001;; 285: 2486–2497.
- Brown B, Stukovsky K, Zhao X. Simultaneous low-density lipoprotein-C lowering and high-density lipoprotein-C elevation for optimum cardiovascular disease prevention with various drug classes, and their combinations: a meta-analysis of 23 randomized lipid trials. Current Opinion in Lipidology 2006;; 17: 631–636.
- Hokanson J, Austin M. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. Journal of Cardiovascular Risk 1996;; 3((2):)213–219.
- Lamarch B, Després J-P, Moorjani S, Cantin B, Dagenais G, Lupien P-J. Triglycerides and HDL cholesterol as risk factors for ischemic heart disease: results from the Québec cardiovascular study. Atherosclerosis 1996;; 119: 235–245.
- American Diabetes Association. Standards of Medical Care in Diabetes–2008. Diabetes Care 2008;; 31((Suppl. 1):)S12–54.
- Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation Journal of the American Heart Association 2004;; 109((5):)672–693.
- Mosca L, Banka C, Benjamin E, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Journal of the American College of Cardiology 2007;; 49((11):)1230–1250.
- Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. Journal of the American Medical Association 2004;; 292((21):)2585–2590.
- Grundy SM, Cleeman JI, Bairey Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Journal of the American College of Cardiology 2004;; 44((3):)720–732.
- Manninen V, Elo M, Frick M, et al. Lipid alterations and the decline in incidence of coronary heart disease in the Helsinki Heart Study. Journal of the American Medical Association 1988;; 260: 641–651.
- Robins S, Collins D, Wittes J, et al, VA-HIT Study Group. Veterans Affairs High-Density Lipoprotein Intervention Trial: relation of gemfibrozil treatment and lipid levels with major coronary events. Journal of the American Medical Association 2001;; 285: 1586–1589.
- Mosca L, Merz NB, Blumenthal RS, et al. Opportunity for intervention to achieve american heart association guidelines for optimal lipid levels in high-risk women in a managed care setting. Circulation Journal of the American Heart Association 2005;; 111((4):)488–493.
- Sarawate C, Cziraky M, Stanek E, Willey V, Corbelli J, Charland C. Achievement of optimal combined lipid values in a managed care setting: Is a new treatment paradigm needed?. Clinical Therapy 2007;; 29((1):)196–209.
- Stacy T, Egger A. Results of retrospective chart review to determine improvement in lipid goal attainment in patients treated by high-volume prescribers of lipid-modifying drugs. Journal of Managed Care Pharmacology 2006;; 12((9):)745–751.
- Alsheikh-Ali A, Lin J, Abourjaily P, Ahearn D, Kuvin J, Karas R. Extent to which accepted serum lipid goals are achieved in a contemporary general medical population with coronary heart disease risk equivalents. American Journal of Cardiology 2006;; 98((9):)1231–1233.
- Stanek E, Sarawate C, Willey V, Charland S, Cziraky M. Risk of cardiovascular events in patients at optimal values for combined lipid parameters. Current Medical Research Opinion 2007;; 23((3):)553–563.
- American Diabetes Association. Standards of Medical Care in Diabetes - 2007. Diabetes Care 2007;; 30((S1):)S4–S41.
- Deyo R, Cherkin D, Ciol M. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology 1992;; 45: 613–619.
- Ballantyne C, Corsini A, Davidson M, et al. Risk of myopathy with statin therapy in high-risk patients. Archives of Internal Medicine 2003;; 163: 553–564.
- Thompson P, Clarkson P, Karas R. Statin associated myopathy. Journal of the American Medical Association 2003;; 289: 1681–1690.
- Caroli-Bosc F, Gall P, Pugliese P, et al. Role of fibrates and HMG-CoA reductase inhibitors in gallstone formation. Digestive Disease Science 2001;; 46((3):)540–544.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;; 344((8934):)1383–1389.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo controlled trial. Lancet 2002;; 360((9326):)7–22.
- Cheung BMY, Lauder IJ, Lau C-P, Kumana CR. Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. British Journal of Clinical Pharmcology 2004;; 57((5):)640–651.
- LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. Journal of the American Medical Association 1999;; 282((24):)2340–2346.
- Brown BG, Zhao X-Q, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. New England Journal of Medicine 2001;; 345((22):)1583–1592.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial biology for the investigation of the treatment effects of reducing cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation Journal of the American Heart Association 2004;; 110((23):)3512–3517.
- McBride P, Schrott HG, Plane MB, Underbakke G, Brown RL. Primary care practice adherence to national cholesterol education program guidelines for patients with coronary heart disease. Archives of Internal Medicine 1998;; 158((11):)1238–1244.
- Pearson TA, Laurora I, Chu H, Kafonek S. The Lipid Treatment Assessment Project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Archives of Internal Medicine 2000;; 160((4):)459–467.
- Qureshi AI, Suri MFK, Guterman LR, Hopkins LN. Ineffective secondary prevention in survivors of cardiovascular events in the US population: report from the third national health and nutrition examination survey. Archives of Internal Medicine 2001;; 161((13):)1621–1628.
- Whitney EJ, Krasuski RA, Personius BE, et al. A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: effects on progression of coronary heart disease and clinical events. Archives of Internal Medicine 2005;; 142((2):)95–104.
- Blankenhorn DH, Nessim SA, Johnson RL, Sanmarco ME, Azen SP, Cashin-Hemphill L. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. Journal of the American Medical Association 1987;; 257((23):)3233–3240.
- Baigent C, Keech A, Kearney P, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;; 366((9493):)1267–1278.
- Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. New England Journal of Medicine 1987;; 317((20):)1237–1245.
- Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs High-Density Lipoprotein Intervention Trial (VA-HIT). Archives of Internal Medicine 2002;; 162((22):)2597–2604.
- Pedersen TR, Kjekshus J, Berg K, et al. Cholesterol lowering and the use of healthcare resources: results of the Scandinavian Simvastatin Survival Study. Circulation Journal of the American Heart Association 1996;; 93((10):)1796–1802.
- Prosser LA, Stinnett AA, Goldman PA, et al. Cost-effectiveness of cholesterol-lowering therapies according to selected patient characteristics. Annals of Internal Medicine 2000;; 132((10):)769–779.
- Scuffham P, Chaplin S. An economic evaluation of fluvastatin used for the prevention of cardiac events following successful first percutaneous coronary intervention in the UK. Pharmacoeconomics 2004;; 8: 525–535.
- Scuffham PA, Chaplin S. A cost-effectiveness analysis of fluvastatin in patients with diabetes after successful percutaneous coronary intervention. Clinical Therapy 2005;; 27((9):)1467–1477.
- Nyman JA, Martinson MS, Nelson D, et al. Cost-effectiveness of gemfibrozil for coronary heart disease patients with low levels of high-density lipoprotein cholesterol: the Department of Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial. Archives of Internal Medicine 2002;; 162((2):)177–182.
- Hay J, Sterling K. Cost effectiveness of treating low HDL-Cholesterol in the primary prevention of coronary heart disease. Pharmacoeconomics 2005;; 23((2):)133–141.
- Feher M, Langley-Hawthorne C, Byrne C. Cost-outcome benefits of fibrate therapy in type 2 diabetes. British Journal of Diabetes & Vascular Disease 2003;; 3: 124–130.
- Roze S, Ferrieres J, Bruckert E, et al. Cost-effectiveness of raising HDL cholesterol by adding prolonged-release nicotinic acid to statin therapy in the secondary prevention setting: a French perspective. International Journal of Clinical Practice 2007;; 61((11):)1805–1811.