Abstract
Objectives: A database analysis evaluating the comparative costs and outcomes of asthma treatment with breath-actuated inhalers (BAIs) and metered-dose inhalers (MDIs) has previously been undertaken in 2001. This analysis found that, despite the higher acquisition cost associated with BAIs, economies elsewhere meant that the overall cost associated with BAIs was lower than MDIs. Between 2001 and 2007, the comparative price of MDIs was significantly reduced thus widening the gap between the comparative acquisition cost of MDIs and BAIs. Furthermore, the introduction of specific targets for asthma review included a requirement for regular checks on inhaler technique. Such initiatives may be expected to enhance the overall effectiveness of MDIs. Given the potential impact of such changes, it appeared to be timely to update the original database analysis to assess the extent to which the original findings have been altered by changes in the clinical and economic environment over the past 5 years.
Methods: As in all chronic diseases, it is important that economic analyses evaluate cost effectiveness over as long a period as possible, and so the 2006 analysis was conducted over a 12- and a 24-month time period.
Results: The results emphasised that the clinical benefits associated with BAIs for certain patients can still be translated into greater cost effectiveness, but that altered cost structures required a longer time period for the greater cost effectiveness of BAIs to become evident.
Conclusion: Since completing this study, the reimbursement costs for beclometasone MDIs have increased significantly (since October 2007) due to the discontinuation of Becotide and Becloforte. As a consequence of this higher acquisition cost for MDI inhalers, the current cost-effectiveness advantages of BAI compared to MDI can be expected to be even greater than that identified in the study.
Aim and objectives
In 2001, a retrospective analysis was undertaken to quantify and compare the healthcare resources consumed by asthma patients who were prescribed either a metered-dose inhaler (MDI) or breath-actuated inhaler (BAI)Citation1. All patients were initially using MDI and an index event was identified as a switch from their existing MDI to either a BAI or a different MDI. The 2001 analysis used the Doctors Independent Network (DIN-LINK) primary care database to assess the extent to which patients switched to BAI (Easi-Breathe®) differed from patients switched to a different MDI. A detailed analysis of the asthma-related healthcare resource costs of both cohorts was undertaken in the 12-month period following this switch in inhaler provision.
The aim of the current study is two-fold. The first aim is to assess whether the clinical and economic advantages identified in 2001 for BAI (Easi-Breathe®) over a traditional MDI still persist. In order to achieve this, the authors replicated the data analysis and methodology employed in the 2001 analysis to quantify and compare current healthcare resource use. The entry criteria, patient cohorts, data sources and structure of analysis were specifically designed to ensure comparability between the current study and the 2001 analysis. The second aim is to extend the period covered by the analysis to evaluate comparative cost effectiveness from a longer term perspective. Asthma is a chronic disease and any interventions at each stage of the disease process should ideally be assessed from a lifetime perspective. Although data constraints would not allow this, the analysis was conducted over both 12 months (the period covered by the earlier analysis) and 24 months in an attempt to assess longer term trends in cost effectiveness.
The aim of this analysis is largely to raise hypotheses concerning the impact of changes in relative prices and the clinical environment on the comparative cost effectiveness and utilisation of BAIs and MDIs through the analysis of patients in the process of switching from their current therapy. Within this specified cohort, the reasons for switching to a specified new form of therapy are likely to be complex and multi-factoral. Identifying the factors relating to both the patient and clinician that underpin the choice of BAI or MDI for any particular patient would require a detailed qualitative analysis and is therefore outside the scope of this study. Also outside the scope of this study is the impact of recent increases in the cost of beclometasone MDIs. Since October 2007, the acquisition cost of MDI inhalers has more than doubled. Additional analysis (not reported here) is ongoing with regard to the impact of this price change.
Study design
The 2001 study included 1,856 existing asthma patients, 1,481 of whom used an MDI for the study duration and 375 used a BAI. The 2006 study included 635 existing asthma patients, 573 of whom used an MDI for the study duration and 62 used a BAI. Although this comparison may appear to be unbalanced numerically, it reflects the dominance of MDIs in the asthma marketplace. On both occasions, the proportionate breakdown between MDI and BAI patients reflected real-life clinical practice.
To ensure comparability, both analyses utilised the same electronic UK primary care database that was used in the original analysis (DIN-LINK) to obtain a representative sample of general practioner (GP) practices in Great BritainCitation2. In comparing the 2001 and 2006 analyses, the BAI and MDI cohorts were equivalent with regard to gender, socio-economic status (ACORN scores)Citation3 and all other measured variables. In addition, as far as the limited data can be interpreted it would appear to indicate that all potential baseline confounders (age, sex, severity) are similar in both the BAI and MDI cohorts. A separate analysis was undertaken for children (0–12 years) and adults (13+ years). In the 2001 analysis, 392 patients (21%) were children (defined as 0–12 years) compared to 204 patients (19%) in the comparable 1-year analysis undertaken in 2006.
All patients identified in the database with a diagnosis of asthma were eligible for inclusion in both analyses if they met the following criteria:
Their inhaled corticosteroid (ICS) therapy had been changed from an existing MDI to a different MDI or to a BAI (the ‘index event’).
They solely used the same MDI or BAI for all ICS medication for a full 12-month period following this index event.
The BAI cohort received only the Easi-Breathe® for ICS therapy.
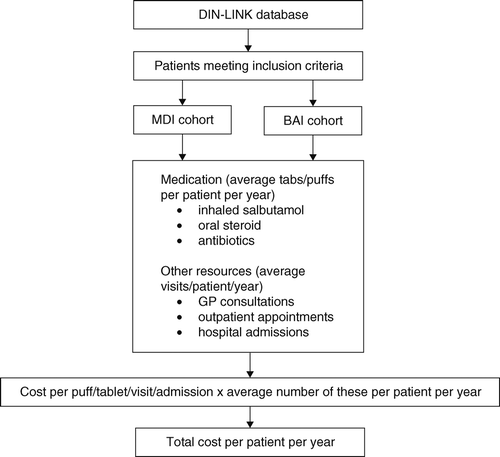
Medical records of patients meeting the inclusion criteria were analysed to identify their asthma-related resource use over the 12 months following their therapeutic switch. Both analyses, therefore, concentrated entirely on evaluating resource use among existing asthma patients who were subject to a switch in inhaler therapy during the period analysed. A description of the patient cohorts used in both analyses is provided in .
Table 1. Cohort definitions.
The study design employed in both analyses is outlined in . The consumption of National Health Service (NHS) resources was analysed for both patient cohorts with appropriate unit costs being applied and summed over the period of analysis. The study design therefore allows us to estimate and compare the average cost imposed on the NHS by supporting asthma patients on either MDI or BAI inhalers in real-world clinical practice in both 2001 and 2006.
Resource consumption
Medication resource use
Pharmaceutical resource use was analysed for each patient over a 12-month period for direct comparison with the analysis undertaken in 2001. In addition, the resource use analysis for both BAI and MDI was extended to 24 months to assess cost effectiveness over a longer time period. The 12-month analysis relating to prescribing data contained 1,086 patients (204 children and 882 adults). The 24-month analysis incorporated a total of 1,306 patients for whom prescribing data was available within this wider timeframe. Four classes of drugs were analysed. First, the amount of inhaled salbutamol consumed in both the MDI and BAI cohorts was analysed as the basis for the pharmaceutical cost analysis. This is in line with guidelines that recommend using the amount of short-acting β2-agonist usage as a measure of asthma controlCitation4. In addition, two other classes of prescribing costs were analysed. Oral steroid courses were included as real-life markers of significant asthma exacerbations as their usage is recommended in acute exacerbationsCitation4. Patients with uncontrolled asthma are more prone to suffer from respiratory infections and therefore antibiotic usage was also analysed to obtain potentially useful additional information about asthma control. In addition, the cost of spacers utilised to improve inhaler technique and reduce oral deposition was also included in the cost analysis. Spacers reflect a potentially important means of improving drug delivery and any improvements in clinical outcome relating to their use would implicitly be incorporated into the analysis in the form of a reduction in resource use in the MDI cohort.
Non-medication resource use
The non-medication resource use analysis hypothesises that primary care consultations and hospital interventions are indicative of poorly controlled asthma. The DIN-LINK database has a more limited coverage of such non-medication costs. Data relating to 635 patients (114 children and 521 adults) was analysed in the 12-month analysis with 937 patients being analysed over the 24-month time frame. The frequency of GP consultations for asthma-related symptoms or respiratory infections is likely to be closely related to the quality of asthma controlCitation5. The frequency of outpatient appointments or hospital admissions for asthma is likely to indicate more severe problems with the degree of control that individual patients are experiencingCitation5. A decrease in asthma stability has been shown to be associated with poor inhaler technique9. Hospital referral or outpatient attendance for significant asthma exacerbations represents a relatively rare occurrence in this relatively mild cohort of patients. In general, in both the 2001 and 2006 analyses, the number of inpatient episodes was too small to undertake any meaningful analysis. The exceptional circumstances leading to secondary care intervention are likely to be multi-factorial rather than simply indicative of the quality of day-to-day asthma control. However, to ensure the completeness of the analysis, secondary care resource use was incorporated into the analysis.
Resource costs
Information on the proportionate use of the various strengths of MDI and BAI inhalers were obtained from the DIN-LINK database to enable a weighted cost to be calculated. Medication costs for branded drugs were obtained from the Monthly Index of Medical SpecialitiesCitation6. Medication costs for generic drugs were obtained from the Prescription Pricing AuthorityCitation7. An example of the process undertaken to ensure comparability in unit cost data between the 2001 and 2006 analyses is provided in .
Table 2. Other medication costs7.
Non-medication resource costs were obtained from the Personal Social Services Research UnitCitation8 or from national reference costs. provides details of the unit cost data applied to non-medication resource use data in both the 2001 and 2006 analyses.
Table 3. Resource costs.
In general, the unit costs of the major resources consumed in support of asthma patients remained fairly constant. However, there was one major area with regard to the relative prices of BAIs and MDIs. Although the price of BAI (Easi-Breathe®) remained relatively constant over the period (with a small price increase occurring in October 2006) there was a significant reduction in the price of MDIs. This reduction greatly increased the price gap between the two forms of inhalers. Thus, although the actual price of BAIs was not increased, the collapse in the price of MDIs, particularly from April 2005 () meant that the comparative acquisition cost of the two forms of inhalers had altered significantly in favour of MDIs.
Table 4. Example of metered-dose inhaler price reductions during the study period.
The impact of the significant change in unit costs on comparative cost effectiveness between 2002 and 2007 is analysed in and .
Since completing this study, the reimbursement costs for beclometasone MDIs have increased significantly subsequent to the discontinuation of Becotide®Footnote† and Becloforte®Footnote‡. The price changes since October 2007 are provided in . As can be seen from and , the post-study acquisition cost of MDIs returned largely to the levels exhibited in the 2002 study.
Table 5. Example of MDI price increases – post-study period.
Comparative medication costs
presents the comparative medication costs estimated in 2001 for children and adults in both the BAI and MDI cohorts. The total medication costs per patient estimated in 2001 were higher in the BAI group for both children (an additional cost of £16.83 per patient) and adults (an additional cost of £3.02 per patient). provides comparative medication costs for 2006. Although the general pattern of costs is similar, the price reductions in MDIs have been reflected in a far greater gap in the cost of inhaled steroid prescriptions. This occurred despite the fact that the cost per patient associated with BAI inhaled steroid prescriptions has fallen over the period for both adults (a reduction of 3%) and children (a reduction of 21%). The reduction in MDI inhaled steroid costs over the period were far greater, with the costs being approximately halved for children (a reduction from £53.66 to £28.64) and adults (from £68.80 to £33.83).
Table 6. Medication costs per patient per year for BAI users compared to MDI users (children and adults) in 2001.
Table 7. Medication costs per patient per year for BAI users compared to MDI users (children and adults) in 2006.
Comparative non-medication costs
presents the non-medication resource costs for MDI and BAI patients for the 2001 analyses. The non-medication resource costs for both children and adults using BAI were significantly lower than in the MDI groups. A child using BAI in 2001 exhibited non-drug resource costs, which were £46.57 less than a child using an MDI. Similarly, an adult using the BAI in 2001 had non-drug resource costs which were £69.09 lower than adults using an MDI.
Table 8. Non-medication costs per patient per year for BAI users compared to MDI users (children and adults) in 2001.
Comparative non-medication resource use in 2006 is provided in . emphasises that the comparative use of such resources has become far more even between the two groups. In 2006, far less use was made of hospital admissions with no adult patients in either group being admitted to hospital in the 2006 analysis. In general, the extent of non-pharmacological support provided to MDI and BAI patients appeared to have evened out considerably in the more recent analysis, perhaps reflecting the development of a standard ‘package’ of care provided to all asthma patients irrespective of the actual needs for such support. The reduced non-medication costs for MDIs may also partly reflect the success of enhanced training packages aimed at improving inhaler techniques for MDI patients.
Table 9. Non-medication costs per patient per year for BAI users compared to MDI users (children and adults) in 2006.
shows how healthcare costs (medication costs plus non-medication resource usage) have changed between 2001 and 2006.
Table 10. Total NHS costs per patient, per year for BAI users compared to MDI users (12-month analysis).
Overall, total NHS costs imposed by a child using BAI in 2001 were on average £29.74 less per year than for a child using an MDI. NHS costs for an adult using BAI in 2001 were on average £66.06 less per year than for an adult using an MDI. In contrast, the 2006 analysis indicates a cost penalty associated with the use of BAI of £8.85 for adults and £31.99 for children.
The comparative cost analysis between 2001 and 2006 clearly emphasises the seismic change in the asthma marketplace that has occurred over the past 5 years. Both adults and children switched to BAI in 2001 exhibited annual asthma medication costs £16.83 higher than a child switched to an MDI. In comparison, an adult switched to BAI was found to exhibit medication costs that were £3.02 higher than adults switched to MDI. These additional medication costs largely arose from the higher acquisition cost of the BAI as antibiotic and oral steroid costs were found to be lower in the BAI cohort. The higher medication costs identified in the BAI cohorts, however, were more than offset by lower non-medication costs (£46.57 less for children and £69.09 less for adults) arising for patients on BAI. The study therefore identified a lower overall cost to the NHS for BAI users compared to MDI users, indicating that the additional clinical benefits associated with BAIs in certain patients could be obtained while simultaneously optimising the use of NHS resources. The significant reduction in the acquisition price of MDIs has transformed the cost-effectiveness calculations undertaken in 2001. Although no price increase has occurred for BAIs, the fact that they have not mirrored the cost reductions undertaken by MDIs has inevitably increased the price gap between these two forms of inhalers leading to a significant cost penalty associated with the use of BAI over a 12-month period of analysis. The following section extends the timescale of the analysis to 24 months to assess whether the greater clinical efficacy of BAIs leads to a greater degree of comparative cost effectiveness over a longer period of analysis.
Comparative cost effectiveness – the long-term perspective
Asthma is a chronic episodic condition, and patients may experience a ‘career’ of ill health that may persist over time. In such circumstances it becomes necessary to assess the comparative value of interventions in optimising quality of life and resource use over the lifetime of the asthma patient. A more expensive intervention may be justified if it slows the rate at which the patient progresses through the stages of severity and maintains the patient for a longer period in the less severe (and cheaper) early stages of the disease.
Unfortunately, retrospective analyses of this type do not necessarily lend themselves to lifetime cohort analyses of this kind. However, in order to move towards a longer term conceptualisation of cost effectiveness, the original 12-month database analysis was extended to 24 months to assess comparative cost effectiveness between BAIs and MDIs over this extended time period. presents the comparative medication costs for both children and adults estimated in 2006 over the extended 2-year period of analysis.
Table 11. Medication costs per patient over 2 years for BAI users compared to MDI users (children and adults), 2006.
Although the total cost of asthma-related medication is still higher for BAI in children (+£24.84), it has actually become lower in the adult group (-£15.01). provides the non-medication resource usage over the 24-month period of analysis. Over this extended time period, the much greater utilisation of non-pharmacological support by MDI patients becomes more evident.
Table 12. Resource costs per patient over 2 years for BAI users compared to MDI users (children and adults), 2006.
In particular, a far greater number of GP consultations were required by both child and adult MDI patients. combines both pharmacological and non-pharmacological sources of support to obtain an overall estimate of the cost burden imposed on the NHS by both groups of patients over the 24-month period of analysis.
Table 13. Total NHS costs per patient per year for BAI users compared to MDI users (24 months analysis, 2006).
The new comparative cost structures between MDIs and BAIs mean that, although the superior clinical efficacy of BAIs ultimately asserts itself, it takes longer for this to manifest itself in terms of superior cost effectiveness. Comparison of the results in (12-month analysis) and (24-month analysis) emphasises that a significantly different picture emerges with regard to comparative cost effectiveness over the two time periods analysed. The 12-month period of analysis indicates an annual cost penalty of £31.99 (child) and £8.85 (adult) for each asthma patient supported on BAI. In contrast, the 24-month period of analysis indicates an annual cost saving of £34.49 (child) and £40.96 (adult) for each asthma patient supported on BAI over this longer time frame of analysis.
Discussion
Choosing the device that is best suited to each individual patient represents a critical decision in the management of each patient's asthma. The choice of inhaler device should be made jointly by the clinician and the patient. Clinicians must consider the pulmonary function of the patient, and patients must emphasise their preference and acceptance of the device. Furthermore, both clinician and patient should be aware of the limitations of each type of device and the optimum methods of use. Once an appropriate device has been selected, it is essential that patients receive adequate instruction and guidance to ensure good inhalation technique. The increased focus on asthma that has occurred over the past 5 years should effectively contribute to such guidance. The comparative acquisition costs of different forms of inhalers therefore merely represent a single (if important) element that the physician should take into account in their decision making. As has been emphasised, this study covered a period of unprecedented volatility with regard to the price of MDIs. Between 2002 and 2006, the price of MDIs fell substantially before returning close to its original level in 2007. The reasons for such a significant change in price are poorly understood, but perhaps reflect an aggressive pricing response to the increased utilisation of BAIs in the NHS. The introduction of a ‘new’ technology frequently leads to a price reduction in an ‘old’ technology. For example, there was a significant reduction in the price of bare-metal stents in response to the introduction of drug-eluting stents in an attempt to maintain market share. Such reductions implicitly acknowledge the clinical superiority of treatment alternatives and reduce their price to enhance the cost effectiveness of the ‘old’ technology.
This analysis has demonstrated the extent to which comparative healthcare resource use of patients treated with BAI or MDI has altered over the past 5 years. It has achieved this by comparing evidence on the comparative cost effectiveness of the Easi-Breathe® BAI and MDIs in asthma treatment. Because of the size of the database analysis undertaken and the variety of GP practices covered, the results are likely to be representative of how the cost effectiveness of different inhalers has altered throughout the NHS. An increasing body of evidence over this period has shown that many patients find MDIs difficult to operate. While patient education and training has improved over the past 5 years, benefits derived from BAI for each individual patient will be directly proportional to the level of difficulties that they experience with MDIs. The ability of the NHS to afford such benefits, however, is intrinsically linked to the comparative costs associated with both forms of inhalers. As this analysis has shown, this cost-benefit ratio has been significantly altered by the recent price reductions in MDIs.
Having emphasised the strengths of observational database analyses, it is equally important to highlight weaknesses resulting from the non-randomised and retrospective nature of such analyses. The quality of any database analysis is intrinsically linked to the extent to which the cohorts being analysed can be meaningfully compared. While a range of potentially confounding factors (age, gender, socioeconomic status) is similar, there is always the possibility that unknown or unknowable confounders retain a degree of influence on the results. However, given the efforts that have been made to ensure comparability between the two analyses, the authors are hopeful that the impact of such confounders on the results of the analyses have been minimised.
Conclusion
Health economic evaluations based on observational databases provide clinicians with useful information from real-life patients treated by the NHS. Strict controls, necessary to maximise the internal validity of a randomised controlled trial (RCT), limit the applicability of their results to mainstream clinical practice. This poor external validity also eliminates the influence of patient choice and preference in drug delivery systems. Observational studies are able to address key questions concerning long-term effectiveness and the effectiveness of treatment in patients normally excluded from RCTs.
In the UK, increasing asthma morbidity places a significant burden on national primary and secondary healthcare resources. This high-level resource use is partly caused by sub-optimal control of asthma resulting from poor inhaler technique. However, the standard MDI is still perceived as being the most cost-effective inhaler device available due to its lower acquisition cost. A comprehensive economic evaluation must however consider the impact of poor inhaler technique on drug wastage, clinical effectiveness, and the need for additional drug therapy and increased clinical workload in both primary and secondary care.
The 2001 analysis indicated that primary care physicians were able to attain, for appropriate patients, the clinical benefits of using a BAI while simultaneously achieving efficiencies in resource utilisation that could translate into significant cost savings to the NHS. The situation in 2006 appeared to be less straightforward. The price reduction in MDIs over the intervening period significantly widened the gap between the acquisition costs of BAIs and MDIs. This reduction in acquisition cost significantly enhanced the cost effectiveness of MDIs. However, despite this change, the analysis undertaken in 2006 still found that patients using BAIs consume fewer healthcare resources than those using MDIs although it takes longer for the differences to emerge. The recent price increases in MDIs (implemented since this study was undertaken) would be expected to have significantly reinforced this greater cost effectiveness of BAIs.
Acknowledgements
Declaration of interest: The study was supported by an unrestricted educational grant from TEVA. The authors would like to thank Ruben Mujica-Mota for data presentation and analysis.
Notes
*Easi-Breathe® is a registered trademark of Teva UK Ltd.
†Becotide is a registered trademark of GlaxoSmithKline.
‡Becloforte is a registered trademark of GlaxoSmithKline.
References
- Haycox A, Mitchell G, Niziol C, Featherstone R. Cost effectiveness of asthma treatment with a breath-actuated pressurised metered dose inhaler (BAI) - a prescribing claims study of 1856 patients using a traditional pressurised metered dose inhaler (MDI) or a breath-actuated device. Journal of Medical Economics 2002;; 5: 65–77.
- DIN-Link Database, Compufile Ltd. Available from: http://www. cegedim-uk.com/
- ACORN scoring system, CACI Ltd. Available from: http://www.caci.co.uk
- British Thoracic Society/Scottish Intercollegiate Guidelines Network.. British guideline on the management of asthma in adults. Thorax 2003;; 58((1):)i1–i94.
- Morice A. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Respiratory Medicine 2001;; 95: 851–856.
- Monthly Index of Medical Specialities. Haymarket Publishing, London: 2006
- Prescription Cost Analysis 2006. Available from: http://www.dh. gov.uk/en/Publicationsandstatistics/index.htm
- Netten A, Curtis L, Harrison G. Unit cost of health and social care. Personal Social Services Research Unit (PSSRU), University of Kent:, Canterbury 2001
- Giraud V, Roche N. Misuse of corticosteroid metered dose inhaler is associated with decreased asthma stability. European Respiratory Journal 2002;; 19((2):)246–251.