Abstract
Objective: A cost analysis of once-daily insulin glargine versus three-times daily insulin lispro in combination with oral antidiabetic drugs (OADs) for insulin-naive type 2 diabetes patients in Germany based on the APOLLO trial (A Parallel design comparing an Oral antidiabetic drug combination therapy with either Lantus once daily or Lispro at mealtime in type 2 diabetes patients failing Oral treatment).
Methods: Annual direct treatment costs were estimated from the perspective of the German statutory health insurance (SHI). Costs accounted for included insulin medication, disposable pens and consumable items (needles, blood glucose test strips and lancets). Sensitivity analyses (on resource use and unit costs) were performed to reflect current German practice.
Results: Average treatment costs per patient per year in the base case were €1,073 for glargine and €1,794 for lispro. Insulin costs represented 65% vs. 37% of total costs respectively. Acquisition costs of glargine were offset by the lower costs of consumable items (€380 vs. €1,139). Sensitivity analyses confirmed the robustness of the results in favour of glargine. All scenarios yielded cost savings in total treatment costs ranging from €84 to €727.
Conclusions: Combination therapy of once-daily insulin glargine versus three-times daily insulin lispro both with OADs, in the management of insulin-dependent type 2 diabetes offers the potential for substantial cost savings from the German SHI perspective.
Introduction
A recent post-trial follow-up of the UK Prospective Diabetes Study (UKPDS) clearly demonstrated a legacy effect of earlier glucose control with a significant reduction of both, microvascular and cardiovascular eventsCitation1.
Newer therapies such as insulin glargine, used in combination with oral antidiabetic drugs (OADs) which carry a lower risk of hypoglycaemia, may help patients achieve improved glucose control, defined in terms of low HbA1c and reduce the risk of diabetic complicationsCitation2. Current American and European consensus statementsCitation3 and German-specific clinical guidelinesCitation4 recommend the initiation of basal or prandial insulin injections in type 2 diabetes after failure of lifestyle intervention and therapy with metformin (step 1).
The primary objective of this analysis was to compare the costs of the two treatment strategies investigated in the APOLLO trial (A Parallel design comparing an Oral antidiabetic drug combination therapy with either Lantus once daily or Lispro at mealtime in type 2 diabetes patients failing Oral treatment)Citation5. In this randomised, controlled (RCT) multicentre study, patients recruited from study sites across Europe and Australia with type 2 diabetes inadequately controlled by oral medication were assigned to treatment with either once-daily injection of insulin glargine or three-times daily injections of insulin lispro for 44 weeks. Analysis of the APOLLO trial was per protocol with 205 patients randomly assigned to insulin glargine and 210 to insulin lispro (plus continuation of previous oral hypoglycaemic agents). Both insulins were self-administered and self-monitored. Non-inferiority was demonstrated in terms of the decrease in mean HbA1c between regimes. More patients with lispro achieved HbA1c levels of 7% or less than with glargine (69 vs. 57%, p=0.025). In those taking insulin glargine, HbA1c dropped by an average of 1.7%; in those taking insulin lispro, HbA1c dropped by an average of 1.9%. Patients reported greater treatment satisfaction with insulin glargine, and it was also associated with less risk of hypoglycaemia, fewer injections, and less need for glucose self-monitoring. The APOLLO trial demonstrated that insulin glargine provides equivalent efficacy with superior safety and convenience compared with insulin lispro in patients with type 2 diabetes. A cost analysis in which only the costs of the alternative treatment strategies are compared is a justified economic evaluation method in the current study since evidence from the APOLLO study demonstrated that the alternative strategies are clinically equivalent. Such economic evaluation approaches are useful to support decision making on the financing of (initiating) insulin therapies in patients with type 2 diabetes. Moreover, since transferability from one country to another is usually restricted, country-specific evaluations are required that take into account country-specific features such as treatment policies, epidemiology of diabetes, service patterns, unit costs and reimbursement regulationsCitation6. The economic implications of both regimes have not yet been evaluated, and thus was the purpose of the present analysis.
Patients and methods
The APOLLO trial was designed as a non-inferiority trial with intermediate outcome measures such as HbA1c. and fasting blood glucose. The present study conducted a direct costs comparison between the two different insulin analogue treatment strategies in combination with OADs (glimepiride and metformin) in type 2 diabetes based on the findings of the APOLLO study for the German healthcare setting. A number of assumptions made in the modelling analysis have a certain degree of uncertainty, but in general the values chosen for calculation adopted a conservative approach, generally biased against the treatment arm with insulin glargine. The present evaluation was performed as a cost minimisation analysis.
Perspective and time horizon
The economic analysis involved an assessment of direct healthcare costs only and therefore takes the costs perspective of the German statutory health insurance (SHI), in an open care setting. The time period is the first year after initiating insulin treatment.
Cost determinants
The data concerning insulin utilisation were based on the actual quantities used in the APOLLO study. Assessment of all other included healthcare resources assumed constant use over the entire treatment period, based on the data collected in the clinical trial (i.e., per protocol) and used for the calculations.
Identification, measurement and valuation of the key cost determinants comprise the main activities in conducting a cost analysis. In the present study, the unit costs were taken from official price lists and sources for the current price year, 2008. The cost determinants that were included, resource utilisation in each case, and the unit costs applied, are presented in detail below. The assumptions characterise the base case of the cost analysis and were further investigated in sensitivity analyses.
Insulins
In the APOLLO RCT, the starting glargine dosage was 10 U per injection, once daily at the same time and was closely titrated to reach target blood glucose values of fasting blood glucose (FBG) ≤ 100 mg/dl (5.5 mmol/l). The insulin lispro titration regime was also closely titrated to achieve preprandial BG ≤ 100 mg/dl (5.5 mmol/l) and postprandial BG ≤ 135 mg/dl (7.5 mmol/l). The starting dose for insulin lispro was 4 U, given three times daily before breakfast, lunch and dinner. The actual quantities of insulin glargine consumed in APOLLO up to week 44 were used for the base case analysis. From week 44 onwards, constant insulin consumption was assumed (i.e., the quantities consumed for weeks 45–52 were held at week 44 consumption rates). In the base case utilisation of disposable injection devices (insulin pens) were assumed in accordance with the APOLLO clinical trial.
Prices were taken as the pharmacy sales price according to the official drug price list including VAT as reimbursed by SHICitation7. Insulin glargine is available on the market in Germany as Lantus (Sanofi-Aventis). The formulation for insulin lispro used in the trial was Humalog (Eli Lilly). Insulin injection was carried out using the disposable devices Optiset (Sanofi-Aventis) and HumalogPen (Eli Lilly) for glargine and lispro, respectively. Each cartridge contains 3 ml solution (1 ml contains 100 U). For insulin prices, see . It was assumed that insulin glargine treatment starts with a prescription for the smallest available pack and is prescribed according to the distribution of pack size consumption in APOLLO thereafter. Alternative assumptions were investigated in the sensitivity analysis. For treatment with insulin lispro it is assumed that insulin treatment starts with a prescription for the smallest available pack and the largest pack size is prescribed thereafter.
The costs for the two types of insulin in the 1-year analysis period were calculated as follows:
Where, CInsG = costs of insulin glargine, in € and CInsL = costs of insulin lispro, in €
Uy = insulin consumption in U during 1 year;
NsP = contents of the small pack, in U
NlP = contents of the large pack, in U
NmP = contents of the medium pack, in U
PsP = insulin price using the small pack size, in €/U
PmP = insulin price using the medium pack size, in U
PlP = insulin price using the large pack size, in €/U
RsP = proportion of insulin prescriptions using the smallest pack size
RmP = proportion of insulin prescriptions using the medium pack size
RlP = proportion of insulin prescriptions using the largest pack size
U = insulin units
As other short-acting insulin analogues (e.g., Aspart, Glulisin) are available at similar prices, a uniform price in the comparator group was assumed.
Oral antidiabetic drugs, OADs (glimepiride and metformin)
On the basis of the APOLLO results, the dose of OADs was kept stable during the 4-week screening period and the 44-week treatment phase (i.e., the dose of glimepiride or other oral hypoglycaemic agents remained unchanged throughout the study). After randomisation, most patients received metformin therapy throughout the study: 156 (76%) and 153 (74%) in the insulin glargine and insulin lispro treatment groups, respectively. Most patients in both treatment groups were given glimepiride, with only 11 (6%) patients assigned to insulin glargine and 14 (7%) to insulin lispro not receiving glimepiride. Subjects pre-treated with sulphonylurea changed to the equivalent dose of glimepiride or were kept on their glimepiride dose. On the basis of APOLLO, glimepiride (blister pack) is administered in the morning before breakfast in a stable dose, tablets containing 2, 3 or 4 mg. Since differences in the utilisation of OADs were negligible (1–2%), an assessment of costs related to OADs in the base-case cost calculations was not included. However, this practice may not be entirely realistic in the actual clinical practice setting in GermanyCitation8. This assumption was therefore tested in the sensitivity analyses.
Consumable items: needles, blood glucose test strips and lancets
As mentioned above, utilisation of disposable pens was assumed in the present analysis. With respect to needles, it was assumed, as earlier in the LAPTOP cost analysisCitation9, that a new needle would be used for each insulin injection. However, findings from a recent European diabetes patient survey reported that 93% of men with diabetes in Germany used the same needle several timesCitation10, on average 9.2 injections with the same needleCitation11. Therefore, the number of needles used relative to the number of injections was evaluated using modified assumptions in the sensitivity analysis for a scenario assuming one needle per ten injections. ClickFine (Ypsomed, Germany) needles were assumed for all scenarios as these can be fitted in all makes of pens. The manufacturer's recommended price was used as the basis for the cost calculationsCitation12. One lancet and one glucose test strip are required for each blood glucose measurement. Instructions in the APOLLO trial protocol were that during the first 8 weeks of the treatment phase, forced titration phase, subjects on combination therapy with insulin glargine plus OADs tested fasting blood glucose (FBG) once daily, and subjects on lispro insulin measured blood glucose (BG) values three times daily – pre-breakfast, pre-lunch and pre-dinner. After the forced titration phase, subjects on basal insulin glargine continued to measure FBG once daily while subjects on prandial insulin lispro continued to measure three times daily unless more BG values per day were required for the investigator's medical judgement. Thus, in the base case, a single daily measurement was assumed for glargine and three times daily for lispro. As in the LAPTOP cost analysisCitation9, the number of measurements corresponded to the number of injections. These assumptions were varied in the sensitivity analyses. It was assumed that Softclix (Roche) lancets were used. As a wide range of blood glucose test strips are available at almost identical prices, a uniform priceCitation13 could be established so that cost was independent of any particular measuring system (see ).
Further resource utilisation
The cost for glucose monitoring devices and injection aids were not taken into account as patients in both therapeutic groups needed a blood glucose reader/meter, and the lifespan of such devices depends more on its chronological age than on the number of measurements performedCitation10. In addition, according to APOLLO trial protocol, patients were trained to self-monitor blood glucose with the blood glucose meter provided by the sponsor.
In APOLLO, the incidence of hypoglycaemia was higher with insulin lispro compared to glargine, which may give rise to a potential difference in associated hypoglycaemic treatment resource use and costs between the two therapeutic groups. APOLLO defined this as an event with or without symptoms consistent with hypoglycaemia, not needing the assistance of another person, and associated with blood glucose concentrations less than 3.3 mmol/l. Three types of hypoglycaemia were measured in APOLLO: symptomatic, nocturnal and severe. Severe hypoglycaemia was defined as an event with symptoms consistent with hypoglycaemia, requiring the assistance of another person, associated with a blood glucose concentration less than 2.0 mmol/l, or recovery after oral carbohydrate, intravenous glucose, or glucagon administration. Instructions in the trial protocol stipulated that whenever subjects felt a symptomatic hypoglycaemia he/she should measure their blood glucose value so that an actual blood glucose value was available.
The cost of hypoglycaemia is a complex issue as hypoglycaemia affects the diabetic patient in several ways, and also because of the varying definitions of hypoglycaemic events. Detailed information on resource use relating to hypoglycaemia was not recorded in the APOLLO study and therefore not included in the base case. However, an assessment of the potential costs associated with hypoglycaemia was included in the sensitivity analysis, specifically with respect to confirmed symptomatic and severe hypoglycaemia. A number of studies on the costs of hypoglycaemia in type 2 diabetes have been published suggesting that there are significant cost implications relating to such events. A UK studyCitation14 has estimated the cost of severe hypoglycaemia to be about £1,764 per event in Scotland (ambulance, emergency attendance and ward admission) and a French study estimated the total hospital cost of a stay for hypoglycaemia to be FF14,000 (US$2,100)Citation15. In Germany, the direct medical costs of severe hypoglycaemia have been estimated to be US$44,338 per 100,000 inhabitantsCitation16. In the German study16 (90% patients with type 2), severe hypoglycaemia was defined as a symptomatic event requiring intravenous glucose or glucagon injection and was confirmed by a blood glucose measurement. Most patients experiencing severe hypoglycaemia present to the medical emergency department or are attended by the emergency team. Moreover, whereas 60% (55 of 92) of hypoglycaemic patients with type 1 diabetes were treated only at the scene of the emergency by an emergency physician or in the hospital emergency department, hospitalisation of patients with type 2 diabetes was usually unavoidable (95%, 141 of 148).
Data management and calculations
Data on resource use and unit costs were entered into Microsoft Excel 2003 spreadsheets. All calculations, as well as presentations of results, were carried out using prices (Euro) to two decimal places. Calculations, tables and graphs were generated using Excel 2003.
Allowance for parameter uncertainty: sensitivity analysis
A number of sensitivity analyses were performed to explore the robustness of the study results to changes in the value of key cost parameter estimates.
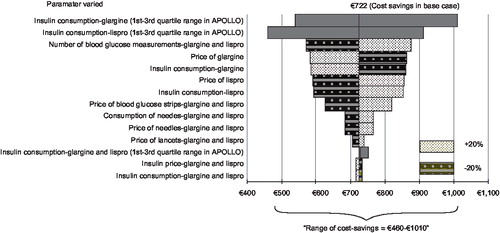
First, the assumptions made in the base case analysis were varied in one-way sensitivity analyses in order to test the robustness of the base case results to alternative assumptions in price, resource use, uncertainty in other assumptions and possible deviations from the APOLLO trial results for routine clinical care. In the first part of the sensitivity analyses, the most important cost determinants were altered independently of one another by ±20% (unless otherwise stated). Some parameters relate to only one of the treatment regimes while other parameters relate to both groups. The sensitivity analyses are summarised in a tornado plot showing the cost drivers in descending order of importance (). The diagram, however, does not take into account how realistic or likely these variations are. Therefore further sensitivity analyses with modified assumptions were carried out to test specifically variations more applicable to routine care in Germany. To address further shortcomings in performing only univariate sensitivity analysis, probabilistic sensitivity analysis (PSA) was also performed taking the form of a Monte Carlo simulation (MCS) with respect to insulin consumption based on APOLLO in which mean daily consumption values (second-order uncertainty) over the course of 1 year of treatment were simultaneously varied by replacing parameter estimates with an appropriate distribution. Output from this MCS (1,000 iterations, using RiskAmp Add-In for Microsoft Excel 2007, Structured Data, LLC, New York, US. www.riskamp.com) generates estimates of the likelihood of different levels of cost-differences in insulin costs between the two groups given the uncertainty in insulin consumption. The range of values for insulin consumption was defined probabilistically to assess more appropriately this parameter uncertainty. Values were varied according to a gamma distribution between the upper a lower limits recorded in APOLLOCitation5.
As a conservative estimate in the sensitivity analysis, it was assumed that episodes of severe hypoglycaemia were associated with additional resource use in the form of a single glucagon injection, GlucaGen HypoKit (GG, Novo Nordisk Pharma GmbH). Resource use was valued using current market prices for glucagonCitation7. Resource use relating to severe hypoglycaemia such as contact with medical personnel, attendance at a hospital emergency department or hospitalization was excluded.
The modified scenarios chosen to be tested also included: insulin utilisation of prescribed pack sizes different to the base case; the number of blood glucose measurements could be different in routine care (additional measurements in lispro were justified by the recommendations of the APOLLO study protocol); costs associated with/without the use of OADs.
Results
Base case analysis
The identification, measurement and valuation of the relevant cost items are presented in . Based on the assumptions made for the base case, once-daily insulin glargine in combination with OADs generated average cost savings amounting to €722 per patient per year compared to insulin lispro combination therapy. Calculated on a daily basis, this provided savings of approximately €2 per patient per day assuming all patients were using disposable pens. It is of interest to note that the overall benefit in terms of cost savings is due to the lower costs associated with insulin administration (in terms of needles) and blood glucose measurements (test strips and lancets) with once-daily insulin glargine combination therapy compared to thrice-daily insulin lispro combination therapy. Moreover, in the base case, insulin glargine is associated with an additional €37 per patient per year in terms of insulin consumption itself. Bearing in mind that OADs are excluded in the base case the role of different cost determinants are summarised in . Insulin comprises the largest proportional cost (64.6%), with insulin glargine and blood glucose test strips (40.2%) the largest cost component with insulin lispro combination therapy.
Sensitivity analysis
Univariate sensitivity analysis is summarised in as a tornado diagram showing the cost savings of insulin glargine resulting from changes in different cost determinants in descending order. The length of the horizontal bars corresponds to the difference in average costs between glargine and lispro groups over the specified variables of interest depicted on the y-axis. The vertical line transecting the bars represents the cost difference between the groups in the point-estimate (non-probabilistic) base case. The base case with a cost saving of €722 per patient per year is represented by the central axis. The tornado diagram ranks the cost parameters based on the magnitude of their impact on the cost differences between the two treatment groups. The results show clearly that the insulin consumptions and insulin price have the highest impact. For all these variations, positive cost advantages and thus real cost savings were seen with insulin glargine. The advantage of insulin glargine was robust to the range of variations of parameter values tested in respect of the base case (i.e., ±20%). However, as already mentioned in the methods section, the one-way sensitivity analysis does not take into account how realistic or likely these variations and corresponding results are. To investigate this issue further, parameter uncertainty was explored with respect to insulin consumption (the largest cost determinant in applying the interquartile range for insulin consumption based on APOLLO) in probabilistic sensitivity analysis using Monte Carlo simulation. The insulin consumption parameter in the model was assumed to follow a gamma distribution. Results from the PSA, in which base case insulin consumption estimates were replaced with an appropriate distribution generated a difference in annual insulin cost of €29 (5th/95th percentiles: cost saving €92 to additional cost of €151). The probability that annual insulin costs per se were lower in the glargine versus lispro group was estimated at around 32% (i.e., 320/1,000 model simulations) as shown in . Thus, total cost savings ranged from €608.05 to €850.75 (5th/95th percentiles) with insulin glargine combination therapy ().
In the second part of the sensitivity analyses, selected parameters were altered from the base case to reflect ‘routine care’ conditions considered more applicable in Germany. The results shown in are presented as costs savings of insulin glargine combination therapy. The tested scenarios were chosen because the APOLLO study does not permit any reliable conclusions about insulin utilisation outside the trial setting because prescribed pack sizes and their distribution could differ from the base case and the number of blood glucose measurements could be different in routine care. Additional measurements with insulin lispro combination therapy, which were justified by the recommendations of the APOLLO study protocol, increase the cost savings of once-daily insulin glargine combination therapy. However, even if only one glucose measurement was performed each day in both treatment groups, insulin glargine would still result in a cost advantage of €159. In addition, if only one needle was used per ten injections in both groups the base case cost advantage would be reduced by €177 (i.e. from €722 to €545). To account for the possibility of OADs being discontinued on initiation of treatment with insulin, exclusion of these costs from the lispro group was assumed but the case was biased for the glargine group by assuming continued use of them In a further scenario the use of lispro versus human insulin (Insuman Rapid) was explored, which is another possible alternative treatment choice relevant for Germany. For this sensitivity analysis only the unit price of glargine was replaced with that of human insulin. The results showed that the use of human insulin is cost-saving compared to lispro. However, compared to glargine the cost advantage is reduced by 23% (from €722 to €556 per patient/year). Finally, with disease progression, it has been reported that prandial boluses of insulin added to basal insulin (e.g., glargine) will be required in about one-third of patients to sustain daytime controlCitation17. To test the impact on the base case results a sensitivity analysis was therefore conducted in which it was assumed that the addition of prandial boluses involving lispro three times daily would be required in a third of patients in the glargine treatment group. This would also require a corresponding increase in consumption of needles, test strips, etc. in this proportion of patients. From a clinical practice viewpoint a basal insulin therapy may also be added to the lispro group; however, a conservative economic approach was followed, i.e. biasing against the glargine treatment group. From the analysis it was estimated that even with increases in consumption of short acting insulin and other disposable items the glargine group still generated cost-saving (≈€84) per patient/year.
In all the cases tested in the sensitivity analysis, shows clear cost advantages for insulin glargine with each of the variations carried out independently.
Discussion
To the authors' knowledge, the current study is the first to compare the economic implications of once-daily basal insulin glargine and three-times daily prandial insulin lispro from the perspective of the German SHI. The economic analytical framework for the present evaluation was performed as a cost minimisation analysis. The evaluation was based on the clinical results of the APOLLO RCT with patients recruited mainly in central Europe, while the economic perspective was based on routine care in Germany. Therefore some clinical aspects of the APOLLO study may not present a representative basis for this economic evaluation based on the German SHI. Sensitivity analyses with wide ranges were therefore performed to account for these uncertainties.
As shown in the base case, insulin costs were somewhat higher in the basal therapeutic group, because of the slightly higher insulin price associated with insulin glargine. The insulin medication formed the main cost block for the basal therapeutic regime. On the other hand, the costs for blood glucose monitoring (test strips and lancets) represented the main cost block for the prandial therapeutic regime. The cost of lancets represented the lowest cost component in both groups.
Costs associated with medical intervention and hospital admissions (i.e., associated with hypoglycaemia) were not taken into account in the base case, mainly due to lack of data on resource use on the specific categories of hypoglycaemia in APOLLO. However, on the basis of the APOLLO results, the incidence of hypoglycaemia was higher with insulin lispro compared to insulin glargine and, based on the published literatureCitation18, it is plausible that the costs of hypoglycaemia are underestimated in the present study. As shown in the second part of the sensitivity analysis, and under conservative resource-use assumptions concerning the potential costs of hypoglycaemia, the base case results did not change markedly. The utilisation and price of insulin have the greatest influence on the cost difference between the treatment regimes, provided these are altered independently of each other for the two types of insulin. The next most influential parameters were the number of blood glucose measurements and the price of blood glucose test strips. When both groups are similarly affected, variations in the number of blood glucose measurements have even greater impact on the result than variations in the price or utilisation of insulin glargine (). In the second part of the sensitivity analysis, the number of blood glucose measurements (both groups once daily) was shown to be the major cost determinant with important influence on the cost difference between the treatment groups.
In all scenarios investigated, insulin glargine therapy was found to be cost-saving. The lowest benefit with insulin glargine that could be expected was estimated when addition of short-acting insulin lispro three times daily to one-third of patients initiated on insulin glargine was assumed.
The current study has a number of limitations. Firstly, the costs of hypoglycaemic events may be underestimated in this analysis due to the conservative approach adopted. The calculated costs associated with hypoglycaemia did not include intervention in the form of direct medical attention (e.g., a physician visit or hospitalisation requiring inpatient care). The APOLLO trial showed that hypoglycaemic events in patients with type 2 diabetes were higher in the lispro group. Notably, only one episode of hypoglycaemic coma was documented in APOLLO and occurred in the lispro group (unpublished data, Sanofi-Aventis). However, data regarding the cost consequences of hypoglycaemia in this context were not collected. Resource use for (all categories of) hypoglycaemia for therapy involving insulin glargine in combination with OADs compared to prandial insulin lispro with OADs in the German healthcare setting are therefore important areas for further research.
Secondly, the practice of starting insulin therapy in type 2 diabetes with three-times daily insulin lispro without addition of basal insulin according to APOLLO study protocol has been argued to be an uncommon treatment approachCitation8 and not standard clinical practice in GermanyCitation18. However, current guidelines of the German Diabetes Association do indeed recommend insulin initiation either with basal or with prandial insulinCitation20. In the context of the current study a sensitivity analysis was performed in which it was assumed that one-third of patients treated initially with once-daily insulin glargine also received thrice-daily short-acting insulin lispro. It was found that the addition of short-acting insulin to the glargine-treated group still generated cost-savings in favour of glargine.
Data from APOLLOCitation21 and the 4-T trialCitation22 have shown that prandial insulin formulations carry a higher risk of hypoglycaemia. In APOLLO, overall incidence rates were four-fold increased in the lispro group. It has been suggested that the continued administration of sulphonylurea (glimepiride) in the prandial lispro groupCitation8,18,23, and the higher frequency of self-monitoring blood glucose valuesCitation8, may have contributed to the higher rate of overall hypoglycaemia. However, Linn et al Citation17 have argued that the relative frequencies of hypoglycaemic events by hour of day showed a similar distribution profile, with peaks at 2 p.m. for lispro and 3 p.m. for glargine. In addition, a sub-group analysis of patients not given glimepiride showed a three-fold higher incidence of hypoglycaemic events per patient in those on lispro: 7.2 versus 2.3Citation17. Linn et al Citation17 also pointed to the fact that the frequency of unconfirmed symptomatic hypoglycaemia as well as perceived hypoglycaemia was higher in the lispro group – important results which could not be explained simply by the higher frequency of self-monitored blood glucose.
A further limitation relates to the time horizon of the current economic analysis. Economic model-based evaluations provide a useful tool that extrapolates what is relatively short-term data (e.g., 52 weeks in the current study) to longer term clinical and economic outcomes. However, more studies are needed on the longer-term costs and benefits of the alternative insulin regimes using glargine and lispro in type 2 diabetes. Incidence of hypoglycaemia and frequency of daily administrations may also have important implications for other patient-orientated outcomes such as quality of life. In this context, and over longer time horizons, conducting a cost-utility analysis, with effectiveness measured as quality-adjusted life-years (QALYs) may be considered to be an appropriate economic evaluation method.
It should be mentioned that the study design of the original clinical RCT (APOLLO) was not fully ‘blinded’ due to different approaches to the two insulin therapies studied. Therefore there is some uncertainty as to how this might impact on resource utilisation of the alternative therapies. This issue was beyond the scope of the current economic analysis but is an important area for further clinical study.
As a final point, the situation in Germany in relation to prescribing of insulin analogues at the present time should be highlighted. It is notable that in July 2006, the German Joint Committee (G-BA) decided to discontinue the reimbursement for short-acting insulins for people with type 2 diabetes unless they were no more expensive than human insulin. The German authorities took this action in light of a report published by the German Institute for Quality and Efficiency in Healthcare (IQWiG) which suggested that there was a lack of demonstrated benefits of short-acting insulin analogues in type 2 diabetes. The 2006 G-BA decision further stipulated that health insurance companies and the pharmaceutical industry can negotiate discount contracts for short-acting insulin analogues, thereby maintaining treatment options accessible to people with type 2 diabetes. In the context of the comparator short-acting analogue treatment arm in APOLLO, the manufacturer gives rebates to statutory sick fundsCitation19,24. Taking this situation into account in the context of the current analysis would lower the cost of insulin in the lispro group. However, since cost savings are largely driven by other resource use components including blood sugar monitoring and needles, the results are not altered markedly. Combination therapy with once-daily basal insulin glargine is still much cheaper than thrice-daily insulin lispro.
Conclusion
The APOLLO RCT demonstrated therapeutic equivalence of insulin glargine to that of insulin lispro in terms of clinical efficacy. The trial results showed no difference in the primary endpoint (decrease in mean HbA1c) between the two therapeutic regimes for demonstrating non-inferiority. In addition, the incidence of hypoglycaemic events was statistically significantly less with insulin glargine than with prandial lispro (5.21 vs. 24.00 events per patient per year, p<0.0001); also fewer injections and less glucose self-monitoring led to greater improvement of treatment satisfaction. The addition of insulin glargine to OADs (basal supported oral insulin therapy (BOT)) can be regarded as first-line insulin initiation approach and is in line with the consensus guidelines the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)Citation25.
From the perspective of the SHI in Germany, combination therapy with once-daily basal insulin glargine for initiating insulin therapy in patients with type 2 diabetes after failure of monotherapy with OADs is estimated to yield average annual cost savings of €722 per patient compared to thrice-daily insulin lispro. Analyses undertaken on uncertainty in base case cost parameter estimates, including those relating to insulin utilisation, price and administration, use of OADs, blood glucose measurements and hypoglycaemia resource use, indicated that the cost advantage savings were robust to the most plausible values for model assumptions. The present study provides information on the costs of insulin glargine versus alternative formulas and has shown that, in terms of its economic impact, insulin glargine is cost saving, i.e. offsetting initial insulin acquisition costs by a reduction in total therapy costs. Therefore, once daily administration of insulin glargine combined with OADs such as glimepiride and metformin is a cost-saving treatment alternative in initiation of insulin therapy in type 2 diabetes patients with suboptimal control on oral therapy alone.
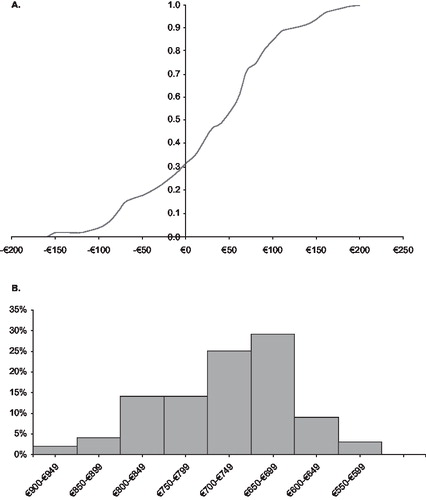
Table 1. Resource use, unit costs and cost estimates in €/patient/year: base case cost analysis.
Table 2. Sensitivity analysis: results with modified assumptions.
Erratum [Early Online citation]
The Early Online version of this article published online ahead of print on 28 May 2009 contained some labelling errors in (p. 92) [reversal of +/- 20% pattern filling for four of the scenarios, parameters 8-11 from top] and an error in (p 95) [correction to the second from last entry where “insulin glargine” should read “insulin lispro”]- this has been corrected in the version of the article shown here (and as reposted on 10 July 2009).
Acknowledgements
Declaration of interest: The study was funded by Sanofi-Aventis Deutschland GmbH.
R.G.B., T.L. and A.R.N. have been consultants for Sanofi-Aventis Deutschland GmBH. F.W.D. is an employee of Sanofi-Aventis Deutschland GmbH. R.G.B. and A.R.N. contributed equally to the work as lead authors.
Notes
References
- Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589
- Amiel SA, Dixon T, Mann R, et al. Hypoglycaemia in type 2 diabetes: Review Article. Diabet Med 2008;25:245–254
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32(1):193–203
- Matthaei S, Häring HU. Behandlung des Diabetes mellitus Typ 2. Diabetologie 2008;3(Suppl 2):S157–S161
- Bretzel RG, Nuber U, Landgraf W, et al. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084
- Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions. Value Health. 2009; forthcoming
- Rote Liste® Service GmbH, Arzneimittelverzeichnis für Deutschland (einschließlich-EU-Zulassungen und bestimmter Medizinprodukte), Frankfurt/Main, 2008;. Available at: www.rote-liste.de
- Oliveira JH, Kazda C, Simpson A, et al. Basal insulin glargine vs. insulin lispro in type 2 diabetes. Correspondence. Lancet 2008;372:371–372
- Janka HU, Högy B. Economic evaluation of the treatment of type 2 diabetes with insulin glargine based on the LAPTOP trial. Eur J Health Econ 2008;9:165–170
- Roper Western Europe Diabetes Patient Survey 2004; from GfK Healthcare. Available at: www.diabetes-world.net/Portal-f%C3%BCr-Patienten-und-Interessierte/Diabetes-behandeln/Insulininjektion/Pennadeln – Viele-sparen-am-falschen-Ende.htm?ID=3367
- Pinget, M: Pressekonferenz “Vom richtigen Umgang mit dem Insulinpen - Weil es unter die Haut geht ”, 28.03.2006;. Available at: http://diabetesprofi.diabetes-kids.de/index2.php?option= com_content&do_pdf=1&id=46
- Ypsomed GmbH, Otto-Volger Strasse 7c, 65843 Sulzbach/Taunus. Available at: www.ypsomed.de
- Florian Müller GmBh – the friendly mail-order for diabetics, Wichmannstrasse 4, 22607 Hamburg. Available at: www.florian-mueller.de
- Leese GP, Wang J, Broomhall J, et al. Frequency of severe hypoglycaemia requiring emergency treatment in type 1 and type 2 diabetes. Diabet Med 2003;26:1176–1180
- Allicar MP, Megas F, Houzard S, et al. Frequency and costs of hospital stays for hypoglycaemia in France in 1995. Presse Med 2000;29:657–661
- Holstein A, Plaschke A, Egberts EH. Incidence and costs of severe hypoglycaemia. Diabetes Care 2002;25:2109–2110
- Linn T, Landgraf W, Bretzel RG. Basal insulin glargine vs. prandial lispro in type 2 diabetes – Authors' reply. Correspondence. Lancet 2008;372:372
- Heinemann L. Control group does not reflect treatment reality in Germany. Lancet 2008;371(9618):1047–1048
- Schwabe U, Paffrath D. Arzneiverordnungs - Report 2007, Aktuelle Daten, Kosten Trends und Kommentare. Heidelberg: Springer Medizin Verlag, 2008
- Matthaei S, Bierwirth R, Fritsche A, et al. Medikamentöse antihyperglykämische Therapie des Diabetes mellitus Typ 2. Update der Evidenzbasierten Leitlinie der DDG, 13.10.2008;. Available at: http://www.deutsche-diabetes-gesellschaft.de/
- Bretzel RG, Eckhard M, Landgraf W, Linn T. Initiating insulin therapy in type 2 diabetic patients failing improvement with oral hypoglycemic agents: Basal or prandial insulin? The APOLLO trial. Diabetes Care 2009 (in press)
- Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357(17):1716–1730
- Arnolds S, Rave K. Basal insulin glargine vs. prandial insulin in type 2 diabetes. Correspondence. Lancet 2008;372:370–71
- Frick M, Knollmeyer J, Riederer H, et al. Modern insulins – comments on facts and assumptions in a recent Editorial. Letter. Diabetologia 2008;51:689–691
- Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy. Diabetologia 2008;51(1):8–11