Abstract
Objectives: The aim of this analysis was to evaluate the long-term clinical and economic outcomes associated with insulin detemir and neutral protamine Hagedorn (NPH) insulin in combination with mealtime insulin aspart in patients with type 1 diabetes in Belgian, French, German, Italian and Spanish settings.
Methods: The published and validated IMS CORE Diabetes Model was used to make long-term projections of life expectancy, quality-adjusted life expectancy and direct medical costs. The analysis was based on patient characteristics and treatment effects from a 2-year randomised controlled trial. Events were projected for a time horizon of 50 years. Potential uncertainty using a modelling approach was addressed.
Results: Basal-bolus therapy with insulin detemir was projected to improve quality-adjusted life expectancy by 0.45 years versus NPH in the German setting, with similar improvements in the other countries. Insulin detemir was associated with cost savings in Belgium, Germany and Spain. In France and Italy, lifetime costs were slightly higher in the detemir arm, leading to incremental cost-effectiveness ratios of €519 per QALY gained and €3,256 per QALY gained, respectively.
Conclusions: Compared to NPH, insulin detemir is likely to be a dominant treatment strategy in Belgium, Germany and Spain and highly cost-effective in France and Italy in patients with type 1 diabetes.
Introduction
Type 1 diabetes mellitus (T1DM) is associated with a wide range of complications such as cardiovascular disease, nephropathy, retinopathy, neuropathy and foot ulceration. These micro- and macrovascular complications are a key driver of costs. In a review, Liebl reported that diabetes is responsible for over 14% of the total direct medical cost burden in Germany, costing in excess of €60 million annuallyCitation1. A cost-of illness study in Spain by Oliva et al found that 6.3–7.4% of the total National Health System expenditures went towards to diabetes care, accounting for €2.4–2.7 billionCitation2. Even if patients with T1DM only make an estimated 10% of the patients diagnosed with diabetes, the financial burden of diabetes is still substantial.
Various studies like the Diabetes Control and Complications Trial (DCCT)Citation3 have shown that intensive glycaemic control can delay or reduce the risk of long-term complications in T1DM. However, intensive therapy with conventional insulins is associated with a greater risk of major hypoglycaemia and increased weight gain. Insulin detemir is a long-acting basal soluble insulin with a protracted action profile designed to avoid the variation in absorption and release typically seen with standard long-acting insulins like neutral protamine Hagedorn (NPH). In clinical trials, insulin detemir has demonstrated comparable HbA1c reduction, lower within-subject fasting blood glucose variability, less hypoglycaemia and less weight gain compared to basal-bolus regimens using NPH insulinCitation4–10. Many of these trials were, however, of too limited duration to evaluate long-term effects. In a recently published 2-year, multi-national, open-label, randomised, controlled trial (RCT), Bartley et al studied the long-term efficacy and safety of insulin detemir versus NPH in a total of 497 patients with T1DM. Basal insulin, either detemir or NPH, was individually titrated aiming for pre-breakfast and pre-dinner targets of ≤ 6.0 mmol/l. Insulin aspart was injected immediately before each meal, and was titrated to achieve a postprandial plasma glucose level of ≤ 9.0 mmol/l. After 24 months, insulin detemir was associated with significant improvements in glycaemic control (HbA1c 7.36 vs. 7.58%, mean difference -0.22%, p = 0.022) and major hypoglycaemic events (69% risk reduction, p = 0.001) versus NPH. Patients treated with detemir gained less weight (1.7 vs. 2.7 kg, p = 0.024)Citation11.
We designed and performed a computer simulation modelling analysis to estimate the long-term clinical and economic outcomes associated with insulin detemir and NPH in combination with mealtime insulin aspart in patients with T1DM, based on these short-time findings. The cost-effectiveness of insulin detemir versus insulin NPH was evaluated in Belgium, France, Germany, Italy and Spain – five large European economies with high expenditures on health care.
Methods
Model
The IMS CORE Diabetes Model (CDM) is an internet-based computer model developed to determine the long-term health outcomes and economic consequences of interventions in diabetes patientsCitation12. Disease progression is based on a series of inter-dependent semi-Markov sub-models that simulate progression of disease-related complications. Each sub-model uses time, state and diabetes type-dependent probabilities derived from published sources. Each sub-model utilises tracker variables to overcome the memory-less properties of standard Markov models and allows interconnectivity and interaction between individual complication sub-models. Clinical and economic outcomes (means and standard deviations) are calculated within the model using a non-parametric bootstrapping approach. This process simulates the lifetime progression of diabetes in cohorts of 1,000 hypothetical patients and repeats the process 1,000 times. This produces 1,000 mean values of clinical effectiveness and lifetime costs which are then used to generate a scatter plot diagram and acceptability curve to express the likelihood of a treatment being cost-effective versus a comparator. The reliability of simulated outcomes has been tested, with results validated against those reported by clinical trials and epidemiological studiesCitation13.
The model was used to project life expectancy, quality-adjusted life expectancy, complication rates, time to onset of complications and direct medical costs. For the estimation of quality-adjusted life expectancy, utility scores were derived wherever possible from diabetes populations and have been published previouslyCitation14–17.
Simulation cohorts
Country-specific simulation cohorts were generated based on patients' characteristics from the Bartley trialCitation11. The mean patient age at baseline was 35 years, with 54.7% male and an average duration of diabetes of 13 years. Mean HbA1c at baseline was 8.3% and body mass index (BMI) was 24.7 kg/m2. Full cohort characteristics including standard deviations used for all country simulations are given in . The prevalence of pre-existing complications in each country cohort was taken from recently published country-specific dataCitation5,18–31. Patient management practices in terms of the proportion of patients regularly screened for retinopathy and nephropathy were derived from the Bartley trial. Data about the use of concomitant cardiovascular medications such as aspirin, statins and ACE-inhibitors for primary and secondary prevention were taken form the EUROASPIRE II Euro Heart Survey ProgrammeCitation32.
Costs
Costs were accounted from a third party payer perspective. Direct medical costs of complications were derived from published country-specific sourcesCitation33–65. Acquisition costs (public prices) of insulin detemir (Levemir), NPH insulin (e.g., Insulatard) and insulin aspart (Novorapid), as well as needles and devices for self-monitoring of blood glucose were obtained from public pharmacies (Belgium, Germany, Spain) or national health authorities and health-care payers (National Health Service, Italy; RIZIV, Belgium). The annual costs of insulin were calculated based on the reported end-of-trial doses of insulin detemir, NPH and aspart. Costs were inflated as required, using country-specific indices, to 2006 values.
Discounting and time horizon
Future costs and clinical benefits were discounted at country-specific rates in line with published guidancesCitation66–70 (Belgium 3% costs, 1.5% benefits; France 3% both; Germany 5% both; Italy 3% both; Spain 6% both). Sensitivity analysis was performed using a range of discount rates between 0% and 10% for costs and clinical outcomes, based on the recommendations by the same authors. A time horizon of 50 years was used in the base-case analysis to capture all relevant long-term complications, their associated costs and impact on life expectancy and quality-adjusted life expectancy. Sensitivity analysis was performed using time horizons of 5, 10, 15, 20, 30 and 40 years.
Sensitivity analyses
One-way sensitivity analyses were performed around key inputs in the base-case analysis. Parameters were varied over a range of possible scenarios to assess their impact on health economic outcomes. Time horizon was varied between 0 and 50 years (here we report values at 5, 10, 15, 20, 30 and 40 years). Discount rates for costs and health outcomes were applied according to country-specific recommendationsCitation66–70 (Belgium 0% and 6%; France 0% and 5%; Germany 0%, 3% and 10%; Italy 0% and 8% and Spain 0% and 10%). The influence of changes in HbA1c levels on long-term clinical and economic benefits was assessed by abolishing and doubling the HbA1c benefit of insulin detemir over NPH. The influence of hypoglycaemic events was also evaluated: in one sensitivity analysis the same major hypoglycaemic rates were applied to both treatment arms, in another the same minor hypo-glycaemic rates. To investigate the impact of variation in BMI, simulations were run using the same effect on BMI for both the insulin detemir and the NPH treatment. To assess the view of the societal perspective, sensitivity analyses capturing indirect costs using a human capital approach (lost productivity) were performed.
In addition to the one-way sensitivity analyses, second order simulations were performed by entering associated standard deviations and selecting to run the simulation with sampling. In this case the parameter was then selected at random from the associated distribution thus accounting for uncertainty surrounding the input variable of interest.
Statistical methodology
For each analysis in the base case and sensitivity analysis, a simulated cohort of 1,000 patients was run through the model 1,000 times using a non-parametric bootstrapping approach. From which, mean values and standard deviations were generatedCitation71. One thousand mean values (each of 1,000 patients) of incremental costs and incremental effectiveness in terms of quality-adjusted life expectancy were plotted as scatter plots on a cost-effectiveness plane. For interventions that were not dominant (cost saving with benefits in terms of life expectancy or quality-adjusted life expectancy), it was planned to generate an acceptability curve by calculating the proportion of points below a range of willingness-to-pay thresholds.
Results
Main findings
Clinical outcomes
Basal-bolus therapy with insulin detemir was projected to improve both mean life expectancy (LE) and quality-adjusted life expectancy in all five countries analysed (). The magnitude of improvement in discounted life expectancy varied in the range of 0.07 to 0.15 years across the countries. In Germany, insulin detemir was associated with a benefit in LE of 0.09 years (12.80 vs. 12.71 years). In France and Italy the benefits were slightly higher, with 0.13 years (15.63 vs. 15.50 years) and 0.15 years (16.21 vs. 16.06 years), respectively. The smallest benefit was observed in Spain with 0.07 years (12.04 vs. 11.97 years). In Belgium, projected LE was extended by 0.14 years (14.36 vs. 14.22 years) with detemir treatment.
Improvements in discounted quality-adjusted life expectancy in the detemir group ranged from 0.40 to 0.58 years. In Germany, insulin detemir treatment was projected to improve quality-adjusted life expectancy with 0.45 years (7.04 vs. 6.59 QALYs). The values were higher in France and Italy with 0.55 (8.47 vs. 7.92 QALYs) and 0.58 (8.98 vs. 8.39 QALYs) years, respectively. In Spain and Belgium the projected improvements with detemir were 0.40 (6.59 vs. 6.19 QALYs) and 0.52 years (7.85 vs. 7.33 QALYs), respectively.
The time to onset of any diabetes-related complication in the German setting was delayed by 0.08 years in the detemir arm (1.18 vs. 1.10 years). Similar effects were observed in the other four countries. gives an overview on time free of the most prominent complications for both treatments in all countries studied.
Cumulative incidence of diabetic eye and renal disease, neuropathy and amputations were generally decreased for detemir-based therapy, with greatest benefits observed in renal disease. The cumulative incidences of heart failure, angina and stroke were slightly raised in the detemir-based treatment arm, which is likely attributable to longer survival of patients and therefore higher exposure to macrovascular diseases. shows the cumulative incidences of the most prominent complications in the German setting.
Lifetime costs and cost-effectiveness
Insulin detemir was associated with savings in direct costs in Germany (€74,880 vs. 75,734), Spain (€44,085 vs. 44,661) and Belgium (€122,737 vs. 134,679). In France and Italy, lifetime direct costs were higher in the detemir arm (€63,605 vs. 63,321 and €92,036 vs. 90,139, respectively). shows the total direct costs over patient lifetimes. The breakdown of direct medical costs demonstrated that the key drivers were higher treatment costs in the detemir arm, and higher major hypoglycaemic event rates in the NPH insulin arm. Overall costs of complications in all countries studied were higher in the NPH treatment group, except for cardiovascular disease (CVD), were they were marginally lower. The higher direct medical costs in France and Italy led to incremental cost-effectiveness ratios of €519 per QALY gained and 3,256 per QALY gained, respectively. Capturing lost productivity costs using a human capital approach suggested that detemir treatment was likely to be cost saving in France and Italy as well. Due to the lower rate of acute events like hypoglycaemia or ketoacidosis and fewer diabetes-related complications, lifetime indirect costs with detemir treatment were €7,935 lower than with NPH in France, and €10,318 lower in Italy, respectively. Combining both direct and indirect costs, insulin detemir resulted in being a dominant treatment versus NPH in both France and Italy.
shows an incremental cost-effectiveness scatterplot for quality-adjusted life expectancy generated from the mean values of 1,000 × 1,000 nonparametric bootstrap simulations in the German setting. Most of the points lying in the bottom right quadrant of the plane show the dominant nature (increased effectiveness at lower overall costs) of detemir.
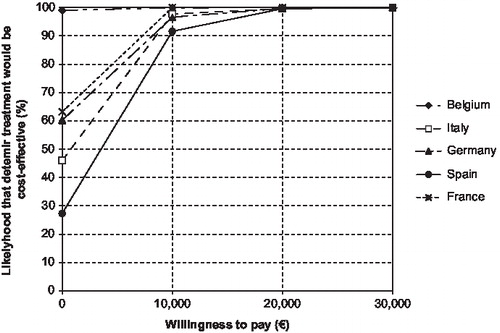
At a willingness-to-pay threshold of €50,000, the likelihood of detemir treatment being cost-effective has shown to be 100% in all five countries ().
Sensitivity analyses
One-way sensitivity analyses in the German setting revealed that results were most sensitive to differences in major hypoglycaemic event rates (). Abolishing the difference between the treatments while setting the effect of detemir to the same level as with NPH resulted in a decrease in quality-adjusted life expectancy with detemir treatment of 0.09 QALYs. Costs in the detemir group simultaneously increased by €3,472, resulting in detemir still being a cost-effective but not dominant treatment versus NPH. Assuming no difference in minor hypoglycaemic events between the two treatments resulted in a greater decrease in quality-adjusted life expectancy in the detemir group, however the overall outcome remained unchanged.
Applying the same change in HbA1c for detemir as applied for NPH resulted in a slight reduction of quality-adjusted life expectancy in the detemir arm (a decrease of 0.09 QALYs from the base case). With an increase in lifetime direct costs of €922, the ICER of detemir treatment versus NPH became €210 per QALY. When the HbA1c benefit associated with insulin detemir treatment was doubled, quality-adjusted life expectancy increased with costs slightly decreasing while detemir treatment remained dominant.
Variations in time horizon had a noticeable impact on the mean quality-adjusted life expectancy and total direct medical costs. Increases in the simulated time horizon resulted in elevated quality-adjusted life expectancy and direct costs for both treatment arms. Shorter time horizon failed to capture the long-term clinical outcomes and end-stage complications, resulting in small benefits at lower costs.
Discounting had little impact on the relative outcomes and detemir treatment remained dominant through the whole range of discount rates applied.
Sensitivity analyses also revealed that varying the treatment effects in terms of BMI had no impact on quality-adjusted life expectancy and only a marginal raising effect on costs of detemir, so that overall outcomes did not change.
The same patterns were observed in extensive one-way sensitivity analyses performed in the Belgian, French, Italian and Spanish settings (data not shown).
In France, clinical benefits in terms of quality-adjusted life expectancy improved with time horizon, with greater effects in the detemir group. With costs of both treatments being almost equal, the ICER of detemir versus NPH decreased with time horizon with a trend towards cost-neutrality. Doubling the efficacy of detemir with regard to change in HbA1c resulted in detemir being dominant versus NPH.
In the Italian setting, in all scenarios of the sensitivity analysis, quality-adjusted life expectancy in the detemir treatment group remained higher than in the NPH group. The same applied to lifetime direct costs, resulting in ICERs in the range of €1,929–8,129 per QALY gained for detemir versus NPH treatment.
In Belgium and Spain, detemir treatment was dominant in all sensitivity analyses performed, except when major hypoglycaemic event rates were the same number in the detemir as in the NPH arm. This led to ICERs of €14,797 and €5,942, respectively, per QALY gained for detemir versus NPH treatment.
The results of the second-order simulations did not reveal major differences to the results observed in the base-case setting ().
Discussion
Long-term projections using the CORE Diabetes Model, based on intervention effects from the Bartley trial, demonstrated that treatment with insulin detemir is associated with improvements in life expectancy and quality-adjusted life expectancy in comparison to NPH insulin in patients with type 1 diabetes in all five countries studied. The higher rate of major hypoglycaemic events in the NPH insulin arm (approximately three-fold higher versus insulin detemir) had a deleterious impact on the quality of life of patients. Variations in life expectancy and quality-adjusted life expectancy between the five countries were mainly due to differences in non-specific mortality (life tables) and different proportions of pre-existing diseases in the baseline cohorts. For instance, the Belgian population with most underlying diseases had the shortest undiscounted life expectancy. Additionally, clinical benefits were discounted at different rates varying between 1.5% and 6% in the five countries.
Interestingly, treatment with insulin detemir reduced the cumulative incidence of most diabetes-related complications and was associated with a delay in time to onset of all complications over patient lifetimes compared to NPH insulin. The cumulative incidences of heart failure, angina and stroke were slightly raised in the detemir-based treatment arm as overall survival was increased, exposing these patients to a longer ongoing risk of these events.
In the base case, insulin detemir was associated with savings in direct medical costs in Germany, Spain and Belgium. In France and Italy, lifetime costs were slightly higher than with NPH, but detemir treatment still remained a very cost-effective treatment. The high direct costs in Belgium were mostly due to a relatively sick baseline population, with high proportions of pre-existing cardiovascular and renal diseases. This population had an early onset of complications (on average already after 0.58 years) and a high cumulative incidence in costly conditions such as end-stage renal disease. Treatment costs of frequently occurring nephropathy were also relatively high compared to other countries. Spanish costs were the lowest of all five countries, due to low costs for treating complications and a relatively high discount rate of 6% in the base case.
Sensitivity analysis showed that results were highly sensitive to hypoglycaemic event rate. Assuming the same number of events with detemir treatment than with NPH, quality-adjusted life expectancy in the German setting decreased by 0.09 QALYs compared to base case in the detemir group. Due to the higher hypoglycaemic event rate lifetime direct costs with detemir increased by €3,472 compared to base case, resulting in an ICER of €7,393 per QALY gained. This analysis shows that by avoiding a higher number of major hypoglycaemic events, the higher acquisition costs of detemir treatment can be compensated. Abolishing the benefit of detemir on HbA1c decreased QALYs, inflated costs due to an increased number of complications and led to higher ICERs in all countries. Changing the treatment effect on BMI had a small effect on costs only. BMI in the model is not accounted as an independent risk factor and only affects congestive heart failure in women. All other cardiovascular risk formulae rely on systolic blood pressure and serum lipid markers insteadCitation72,73. Several analyses with varying time horizons also showed that insulin detemir is cost-saving compared to NPH already after 5 years. As long-term clinical outcomes and complications are only captured later on, its benefits clearly increase with time horizon.
Using a modelling approach to make long-term projections based on short-term trial data is a potential weakness of this analysis. In the model, we assumed that HbA1c benefits are maintained and hypoglycaemic event rates remain constant over a long time period, which is both supported by data in the DCCTCitation74. Like in all modelling analyses, many assumptions around the progression of the disease and associated costs had to be made, resulting in mean outcome values with large distributions. Additionally, the differences in clinical and economic outcomes were quite small, as we were comparing two potent and highly efficient treatments. Consequently, no claim about statistical significance between the observed results can be made. However, the CDM is one of the only two currently available models with published validations demonstrating the reliability of the outcomes when recreating the observations made in long-term clinical trials.
Conclusions
The findings of this analysis suggest that, compared to NPH, insulin detemir is likely to improve life expectancy, delay the onset of and reduce the cumulative incidence of most diabetes-related complications. Detemir treatment is considered to be dominant in Belgium, Germany and Spain and highly cost-effective in France and Italy in patients with type 1 diabetes.
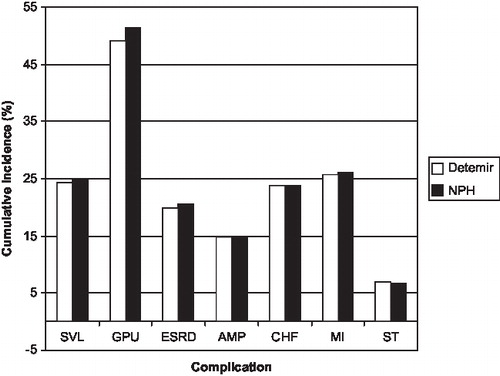
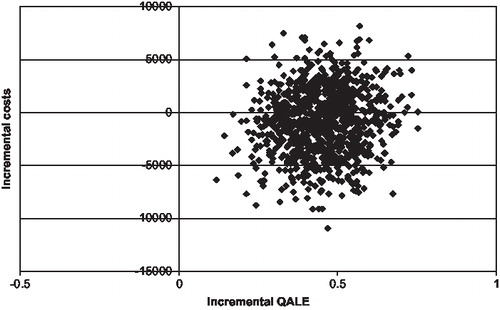
Table 1. Baseline demographic characteristics of all patients.
Table 2. Summary of clinical outcomes in five European countries.
Table 3. Time alive and free of complications (years).
Table 4. Total direct costs over patient lifetimes and ICERs.
Table 5. Sensitivity analysis comparing insulin detemir versus NPH: Germany.
Table 6. Probabilistic sensitivity analysis for all countries (2nd order with sampling).
Acknowledgements
Declaration of interest: This study was supported by an unrestricted grant from Novo Nordisk, Denmark. Manuela Gschwend and William Valentine are current employees of IMS Health, which has received consulting fees from Novo Nordisk for the use of the IMS CORE Diabetes Model, the performance of the analyses and the writing of this manuscript. Mark Aagren is a current employee of Novo Nordisk.
Manuela Gschwend was responsible for the overall design, model inputs, performance of the analysis, collection and interpretation of data and writing of this manuscript. William Valentine gave scientific advice and input into the design of the analysis and critically reviewed the manuscript. Mark Aagren was involved in choosing model input parameters and has given final approval of the version to be published. All authors read and approved the final manuscript.
Notes
* Levemir, Insulatard and Novorapid are all registered trademarks of Novo Nordisk A/S
References
- Liebl A. [Costs involved in the early and late phases of diabetes mellitus.]. Internist (Berl) 2007 Jun 19.
- Oliva J, Lobo F, Molina B, Monereo S. Direct health care costs of diabetic patients in Spain. Diabetes Care 2004;27:2616–21.
- The Diabetes, Control and Complications, Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86.
- Vague P, Selam JL, Skeie S, et al. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart. Diabetes Care 2003;26:590–6.
- Hermansen K, Fontaine P, Kukolja KK, et al. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia 2004;47:622–9.
- Russell-Jones D, Simpson R, Hylleberg B, et al. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal-bolus regimen. Clin Ther 2004;26:724–36.
- Home P, Bartley P, Russell-Jones D, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial. Diabetes Care 2004;27:1081–7.
- Raslova K, Bogoev M, Raz I, et al. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract 2004;66:193–201.
- Robertson KJ, Schoenle EM, Gucev Z, et al. Benefits of insulin detemir over NPH insulin in children and adolescents with type 1 diabetes: lower and more predictable fasting plasma glucose and lower risk of nocturnal hypoglycemia. Diabet Med 2005;22(Suppl. 2):45.
- De LI, Vague P, Selam JL, et al. Insulin detemir used in basal-bolus therapy in people with type 1 diabetes is associated with a lower risk of nocturnal hypoglycaemia and less weight gain over 12 months in comparison to NPH insulin. Diabetes Obes Metab 2005;7:73–82.
- Bartley PC, Bogoev M, Larsen J, Philotheou A. Long-term efficacy and safety of insulin detemir compared to Neutral Protamine Hagedorn insulin in patients with Type 1 diabetes using a treat-to-target basal-bolus regimen with insulin aspart at meals: a 2-year, randomized, controlled trial. Diabet Med 2008;25:442–9.
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(Suppl 1):5–26.
- Palmer AJ, Roze S, Valentine W, et al. Validation of the CORE diabetes model against epidemiological and clinical studies. Curr Med Res Opin 2004;20(Suppl 1):S27–40.
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ 5D (UKPDS 62). Med Decis Making 2002;22:340–9.
- AIHW. The Burden of Disease and Injury in Australia. Australian Institute of Health and Welfare, 2003;. Available from: http://www.aihw.gov.au/publications/health/bdia/bdia-x05.pdf.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583–637.
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523–34.
- Microvascular and acute complications in IDDM patients: the EURODIAB IDDM Complications Study. Diabetologia 1994;37:278–85.
- Sjolie AK, Stephenson J, Aldington S, et al. Retinopathy and vision loss in insulin-dependent diabetes in Europe. The EURODIAB IDDM Complications Study. Ophthalmology 1997;104:252–60.
- Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–50.
- Debacker N, Nobels F, Vandenberghe H, et al. Organization of a quality-assurance project in all Belgian multidisciplinary diabetes centres treating insulin-treated diabetes patients: 5 years' experience. Diabet Med 2008;25:179–85.
- Currie CJ, Poole CD, Tetlow T, et al. The outcome of care in people with type 1 and type 2 diabetes following switching to treatment with either insulin glargine or insulin detemir in routine general practice in the UK: a retrospective database analysis. Curr Med Res Opin 2007;23(Suppl 1):33–9.
- Currie CJ, Poole CD, Woehl A, et al. The financial costs of healthcare treatment for people with Type 1 or Type 2 diabetes in the UK with particular reference to differing severity of peripheral neuropathy. Diabet Med 2007;24:187–94.
- Romon I, Fosse S, Eschwege E, et al. Prevalence of macrovascular complications and cardiovascular risk factors in people treated for diabetes and living in France: The ENTRED study 2001. Diabetes Metab 2008;34:140–7.
- Lievre M, Marre M, Robert JJ, et al. Cross-sectional study of care, socio-economic status and complications in young French patients with type 1 diabetes mellitus. Diabetes Metab 2005;31:41–6.
- Tapp RJ, Balkau B, Shaw JE, et al. Association of glucose metabolism, smoking and cardiovascular risk factors with incident peripheral arterial disease: the DESIR study. Atherosclerosis 2007;190:84–9.
- Boutoille D, Leautez S, Maulaz D, et al. [Skin and osteoarticular bacterial infections of the diabetic foot. Ulcers of the diabetic foot: epidemiology and physiopathology]. Presse Med 2000;29:389–92.
- Renal involvement in type 1 (IDDM) diabetes in Spain. ESTUDIO DIAMANTE. Diabetes Res Clin Pract 1997;38:129–37.
- Harvey JN, Rizvi K, Craney L, et al. Population-based survey and analysis of trends in the prevalence of diabetic nephropathy in Type 1 diabetes. Diabet Med 2001;18:998–1002.
- Younis N, Broadbent DM, Harding SP, Vora JR. Prevalence of diabetic eye disease in patients entering a systematic primary care-based eye screening programme. Diabet Med 2002;19:1014–21.
- Chaturvedi N, Sjolie AK, Stephenson JM, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus [see comments]. Lancet 1998;351:28–31.
- Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J 2001;22:554–72.
- Palmer AJ, Annemans L, Roze S, et al. An economic evaluation of irbesartan in the treatment of patients with type 2 diabetes, hypertension and nephropathy; cost-effectiveness of Irbesartan in Diabetic Nephropathy Trial (IDNT) in the Belgian, and French settings. Nephrol Dial Transplant 2003;18:2059–66.
- Ray JA, Valentine WJ, Secnik K, et al. Review of the cost of diabetes complications in Australia, Canada, France, Germany, Italy and Spain. Curr Med Res Opin 2005;21:1617–29.
- Neeser K, Lubben G, Siebert U, Schramm W. Cost effectiveness of combination therapy with pioglitazone for type 2 diabetes mellitus from a German statutory healthcare perspective. Pharmacoeconomics 2004;22:321–41.
- Szucs TD, Smala A, Fischer T. Costs of intensive insulin therapy in type 1 diabetes mellitus: experiences from the DCCT study (in German). Fortschr Med 1998;116:34–8.
- Levy E, Gabriel S, Dinet J. The comparative medical costs of atherothrombotic disease in European countries. Pharmacoeconomics 2003;21:651–9.
- MulsE, Van Ganse E, Closon MC. Cost-effectiveness of pravastatin in secondary prevention of coronary heart disease: comparison between Belgium and the United States of a projected risk model. Atherosclerosis 1998;137(Suppl):S111–16.
- Statistisches Bundesamt Deutschland. Preisindizes für die Lebenshaltung. Gesundheitsbericht fur Deutschland. Statistisches Bundesamt Deutschland 2005; 1998; (7.8)(Stuttgart: Metzler Poeschel, 1998:410-13). Available from: www.statistisches-bundesamt.de
- Statistisches Bundesamt Deutschland. Diagnosedaten der Krankenhauspatienten von 1997. Wiesbaden. Krankenhausdiagnosestatistik 1999.
- Aros F. Guias de actuacion clinica de la Sociedad Espanola de Cardiologia en el infarto agudo de miocardio. Rev Esp Cardiol 1999;52:919–56.
- Velasco J. Guias de practica clinica de la Sociedad Espanola de Cardiologia en prevencion cardiovascular y rehabilitacion cardiaca. Rev Esp Cardiol 2004;53:1095–120.
- Palmer AJ, Annemans L, Roze S, et al. [Health economic consequences of the use of irbesartan in patients in Germany with type 2 diabetes, nephropathy and hypertension]. Dtsch Med Wochenschr 2004;129:13–18.
- Mullins CD, Sikirica M, Seneviratne V, et al. Comparisons of hypertension-related costs from multinational clinical studies. Pharmacoeconomics 2004;22:1001–14.
- Rychlik R, Pfiel T, Daniel D, et al. Socioeconomic relevance of treatment of chronic heart failure stage NYHA 2 with crataegus extract WS 1442- a prospective 3-year pharmacoeconomic study. Value Health 2004;7:695.
- Laaser U, Breckenkamp J, Niermann U. Cost-effectiveness analysis of medical interventions in patients with stroke in Germany under special consideration of “stroke units”. Gesundh okon Qual Manag 1999;4:176–83.
- Bastida J, Aguilar S, Alvarez M, Gonzalez D. The economic burden of stroke in Spain. Value Health 2003;6:614.
- Annemans L, Lamotte M, Levy E. Cost-effectiveness analysis of clopidogrel versus aspirin in patients with atherothombosis based on CAPRIE trial. J Med Econ 2003;6:55–68.
- Real Decreto 1247/2002 de 3 de diciembre por el que se regula la gestion del fondo de cohesion sanitaria, BOE numero 290, Real Decreto 1247/2002 de 3 de diciembre por el que se regula la gestion del fondo de cohesion sanitaria, 2004.
- Lamas J, Alonso M, Saavadra J, et al. Costes de la dialisis cronica en un hospital publicoL mitos y realidades. Nefrologia 2002;22:269–76.
- Lecomte P, Chaib Eddour D, Squifflet J. The cost of renal transplantation in Belgium. Value Health 2004;7:1088.
- Schadlich PK, Brecht JG, Brunetti M, et al. Cost effectiveness of ramipril in patients with non-diabetic nephropathy and hypertension: economic evaluation of Ramipril Efficacy in Nephropathy (REIN) Study for Germany from the perspective of statutory health insurance. Pharmacoeconomics 2001;19:497–512.
- Palmer AJ, Annemans L, Roze S, et al. Cost-effectiveness of irbesartan in patients with type 2 diabetes, hypertension and nephropathy: a Spanish perspective. Nefrologia 2004;24:231–8.
- O'brien JA, Shomphe LA, Kavanagh PL, et al. Direct medical costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care 1998;21:1122–8.
- Köhler A, Hess R. Kölner Kommentar zum EBM 2002. Deutscher Ärzte-Verlag, 2006.
- Kelm-Kahl I, Ohrloff C. Minimal invasive Chirurgie beim Grauen Star: Was zahlt die Kassee-Medizin and Gesdunheit. Available at: http://www3 lifeline de/yavivo/Verfahren/Augenheilkunde/Katarakt/35kosten html 2001
- Oliva J, Lobo F, Molina B, Monereo S. Direct healthcare costs of diabetes mellitus patients in Spain. Diabetes Care 2004;27:2616–21.
- Institute de Salud Carlos III. Analisis coste-efectividad de diferentes estrategias para el cribado y tratamiento de la retinopatia diabetica en pacientes con diabetes mellitus. Min de Sanidad Consumo 2004;00/10116.
- Van Acker K, Oleen-Burkey M, De Decker L, et al. Cost and resource utilization for prevention and treatment of foot lesions in a diabetic foot clinic in Belgium. Diabetes Res Clin Pract 2000;50:87–95.
- Ghatnekar O. Cost-Effectiveness of treating deep diabetic foot ulcers with Promogran in four different European countries. J Wound Care 2002;11:70.
- Ghatnekar O, Persson U, Willis M, Odegaard K. Cost effectiveness of Becaplermin in the treatment of diabetic foot ulcers in four European countries. Pharmacoeconomics 2001;19:767–78.
- Dahmen HG. Das diabetische Fußsyndrom und seine Risiken: Amputation, Behinderung, hohe Folgekosten. [Diabetic foot syndrome and its risks: amputation, handicap, high-cost sequelae]. Gesundheitswesen 1997;59:566–8.
- Reparaz L, Martinez I, Ligero J. Epidemiologia y analisis cost/efectividad de la angiopatia diabetica en cirugia vascular. Angiologia 1992;6:225–33.
- Calle-Pascual AL, Redondo MJ, Ballesteros M, et al. Nontraumatic lower extremity amputations in diabetic and non-diabetic subjects in Madrid, Spain. Diabetes Metab 1997;23:519–23.
- Rubio-Terres C, Rodriguez J, Bolinder B, de Pablos P. Cost-utility analysis of insulin glargine compared with NPH insulin in patients with type 1 diabetes in Spain. Diabetes 2004;53(Suppl 2): A-291-2.
- The Draft, Pharmacoeconomic Belgian Guidelines. KCE reports vol 28, 2008.
- Collège des économistes de la SANTé. Guide méthodologique pour l'évaluation économique des stratégies de santé. Available at: http://www ispor org/PEguidelines/source/PE%20Guide_2003 pdf 2008
- Graf von der Schulenburg JM, Greiner W, Jost F, et al. German recommendations on health economic evaluation: third and updated version of the Hanover consensus. Value Health 2008;11:539–44.
- Garattini L, Grilli R, Scopelliti D, Mantovani L. A proposal for Italian guidelines in pharmacoeconomics The Mario Negri Institute Centre for Health Economics. Pharmacoeconomics 1995;7:1–6.
- Rovira J, Antonanzas F. Economic analysis of health technologies and programmes. A Spanish proposal for methodological standardisation. Pharmacoeconomics 1995;8:245–52.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327–40.
- Kannel WB, D'agostino RB, Silbershatz H, et al. Profile for estimating risk of heart failure. Arch Intern Med 1999;159: 1197–204.
- D'agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J 2000;139:272–81.
- The DCCT, Research GROUP. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86