Abstract
Objectives: To assess the cost effectiveness of palivizumab, a humanised monoclonal antibody, used as prevention against severe respiratory syncytial virus (RSV) infection requiring hospitalisation, in infants with haemodynamically significant congenital heart disease (CHD) in the German healthcare setting.
Study design: A decision-tree model was used to estimate the cost effectiveness of palivizumab for a hypothetical cohort of patients. The analysis was based on a lifetime follow-up period in order to capture the impact of palivizumab on long-term morbidity and mortality resulting from an RSV infection. Data sources included published literature, the palivizumab pivotal trials, official price/tariff lists and national population statistics. The study was conducted from the perspective of society (primary analysis) and the healthcare purchaser (secondary analysis).
Results: From the societal perspective, use of palivizumab results in an incremental cost-effectiveness ratio (ICER) of €2,615 per quality-adjusted life-year (QALY) without discounting, which increases to €9,529/QALY after discounting. From the perspective of the German healthcare purchaser, use of palivizumab results in an ICER of €4,576/QALY without discounting, which increases to €16,673/QALY after discounting. Probabilistic sensitivity analyses confirmed the robustness of the model. The study is limited by a number of conservative assumptions. It was assumed that palivizumab only affects the occurrence of RSV hospitalisation and does not influence the severity of the RSV infection. Another assumption was that international clinical trial data and data on utilities could be applied to the German healthcare setting.
Conclusion: This analysis showed that palivizumab represents a cost-effective means of prophylaxis against severe RSV infection requiring hospitalisation in infants with haemodynamically significant CHD.
Introduction
Respiratory syncytial virus (RSV) is a common pathogen which is not restricted by geographical, cultural or economic factors. RSV is a leading cause of lower respiratory tract illness in children, the elderly and immunocompromised individuals. Children with congenital heart disease (CHD) are particularly at risk of severe or even fatal RSV infectionCitation1.
Incidence of RSV infection in children is high: by the age of 1 year, between 25 and 50% will have been infected at least once and by the age of 5 years, 95% will have been infectedCitation2. There are no epidemiological data on the incidence of RSV in infants with CHD in Germany, although there are limited data concerning the impact on RSV infection in other high-risk groups, such as infants born prematurely and those with bronchopulmonary dysplasia (BPD). In a retrospective study based on the charts of a university paediatric hospital, 8.9% of preterm infants of <37 weeks gestational age experienced hospitalisation due to RSVCitation3. Similarly, the Munich RSV study aimed to identify high-risk patient groups and to determine the incidence and risk factors for RSV hospitalisationCitation4. During the 1999-2000 RSV season, the risk for RSV related hospitalisation among infants with a gestational age of less than 35 weeks was 5.2%. Premature infants with BPD had a 15% risk of RSV-related hospitalisationCitation4. Annual hospitalisation rates in at-risk patients may be as high as 20%Citation5.
Data from Canada suggest that 25–36% of hospitalised infants with RSV and underlying prematurity, heart disease, or lung disease will require admission to an intensive care unit (ICU), and 18–25% of these will require mechanical ventilationCitation6. Other studies suggest that among infants with CHD who are hospitalised with RSV disease, 33% will require treatment in a paediatric intensive care unit (ICU), and 2.5–3.4% will die due to complications of severe RSV infectionCitation7, 8. Nosocomial RSV infection has a particularly adverse impact on infants and children who become infected in the immediate preoperative or postoperative period for heart surgeryCitation9, 10.
Investigations of the long-term prognosis of patients with RSV disease in infancy have shown measurable respiratory abnormalities that may persist for several years following infectionCitation11. Among young school children, previous RSV infection may increase the risk of asthma tenfoldCitation12.
Palivizumab, a humanised monoclonal antibody, is the only product approved in Germany for the prevention of severe RSV infection in children with CHDCitation13. Palivizumab is approved for the prevention of serious lower respiratory tract infection requiring hospitalisation caused by RSV in children at a high risk of RSV infection: children born at 35 weeks gestational age or less and who are less than 6 months old at the onset of the RSV season; children less than 2 years old who have received treatment for BPD within the proceeding 6 months; and children less than 2 years of age with haemodynamically significant CHD. The license for palivizumab use in children with CHD is supported by a large, international, phase III trial, in which use of palivizumab was associated with a 45% relative reduction in hospitalisations due to RSV infection (p=0.003) compared with the placebo armCitation14. The recommended dose is 15 mg/kg of body weight, given as an intramuscular injection once a month during anticipated periods of RSV risk in the communityCitation15.
To date, no economic analyses of the use of palivizumab in children with CHD in Germany have been published. Therefore, a model was developed to establish whether prevention of RSV infection with palivizumab is a cost-effective strategy in this high-risk group of children in the context of the 2005 German healthcare setting.
Materials and methods
The cost effectiveness of palivizumab was estimated using decision analytical techniques, a well-established methodology that has been employed in a number of published studies calculating the costs of medical strategiesCitation16.
The setting of the study was that of the German healthcare system in 2005. Data were drawn from a wide variety of sources in order to maximise the external validity of the analysis for the German setting, including the palivizumab clinical trials, published literature, official price/tariff lists and national population statistics. Clinical events and utilities are not country-specific and can therefore be derived from international studies. Country-specific data sources were used for economic measures and information on therapeutic choicesCitation17.
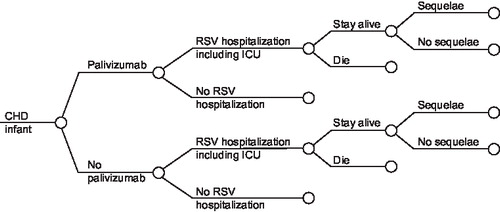
shows the structure of the model. Infants may develop an RSV infection leading to hospitalisation. The majority of these children will be managed in a paediatric ward, but some will require transfer to a neonatal or paediatric ICU. A small proportion of the children will die. Among the surviving children, a significant proportion may develop long-term sequelae (e.g., wheezing, asthma). The clinical pathway and overall structure of the model is identical for both the cyanotic and acyanotic sub-populations, with cyanotic patients defined as those with pulmonary atresia with ventricular septal defect, pulmonary atresia with intact septum, tetralogy of Fallot, single ventricle including hypoplastic left or right heart, tricuspid atresia, double-outlet right ventricle with transposed great arteries, Ebstein anomaly, or D-transposition of the great arteries with or without ventricular septal defect, with or without pulmonary stenosis.
Figure 1. Treatment pathway. CHD, congenital heart disease; RSV, respiratory syncytial virus; ICU, intensive care unit.
The analysis compares the clinical and financial consequences of palivizumab with no prophylaxis. The reduction in RSV hospitalisations due to palivizumab prophylaxis was limited to a single RSV season, corresponding to data available from the palivizumab clinical trials, although the base-case analysis considered a life-time follow-up period to capture the impact of palivizumab on long-term morbidity and mortality resulting from severe RSV infection.
In line with German recommendations for health economic assessmentCitation18, the primary perspective of the analysis was that of society, including costs associated with the purchase and administration of palivizumab, costs of RSV hospitalisation, costs relating to asthma treatment and indirect costs incurred due to lost productivity. The health insurance (third party payer) perspective was also considered, taking into account direct medical costs aloneCitation18.
The patients in the international, phase III trial by Feltes were followed over a period of 150 daysCitation14. The model extrapolated the efficacy data from the palivizumab clinical trial (reduction in the rate of RSV hospitalisations) to calculate the likely number of life-years gained (LYG) and Quality Adjusted Life Years (QALYs) from use of palivizumab prophylaxis. RSV hospitalisations are associated with clinical and economic consequences beyond the clinical trial period. A proportion of the children may develop long-term sequelae (e.g., wheezing, asthma) leading to a reduction of QALYs and additional medical costs. A small proportion of the children will die, which will lead to a life-time loss of productivity costs. The analyses were based on life-time horizon in order to capture long-term costs and morbidity beyond the RSV hospitalisation period, which include the medical costs for management of wheezing and future lost productivity of a child resulting from mortality.
In order to calculate the present value of costs and benefits accrued in the future, both were discounted at equal rates when the time horizon of the model extends beyond 1 year. The base-case analysis was based on a 5% discount rate, which was varied in sensitivity analyses from 3 to 10%Citation18, 19.
Hospitalisation, mortality and life expectancy
Rates of hospitalisation were derived from the pivotal phase III clinical trial in which palivizumab was compared with placebo in children with CHD ()Citation14. As stratification of the data did not yield statistically significant differences between palivizumab and placebo in cyanotic patients, the probability for hospitalisation in the overall CHD group was used in the base-case analysis. A scenario analysis was performed using the hospitalisation rate from the RSV immune globulin (RSV-IGIV) Cardiac study, which yielded a higher hospitalisation rate (15.0%) for children in the control group than the palivizumab trial (9.7%)Citation20. The rate of hospitalisation in the placebo arm of the Cardiac study was adjusted using the risk ratio from the palivizumab trial (5.3%/9.7%) to provide the likely rate of hospitalisation in palivizumab-treated patients.
Table 1. Hospitalisation and mortality inputs from the palivizumab trial.
Mortality rates ranging from 3 to 5% have been reported in the literatureCitation6, 21. To minimise uncertainty, the base-case analysis was performed using mortality rates estimated for the CHD sub-population of a previous meta-analysis, as outlined in a recently published cost-effectiveness analysis for palivizumab in the UKCitation22. Calculated mortality rates were 2.3% for children receiving palivizumab and 4.0% for the no prophylaxis group.
According to national statistics, the life expectancy of a healthy child in Germany is 78.5 yearsCitation23. For children with CHD, a correction for a small reduction in life expectancy was made according to the results of a UK study by Wren and O'SullivanCitation24. This study showed that 82% of children born with CHD survived to 1 year and 78% were predicted to survive to age 16. Thus, the value used in the model, the proportion surviving from age 1 to 16, was 95.3% with an annual mortality rate of 0.32%. It was assumed that beyond 16 years the children would have a normal life expectancy.
Utilities
A study by Greenough et al reported utilities in children with a history of RSV infection using the Health Utility Index (HUI), a multi-attribute score that includes measures for sensation, mobility, emotion, cognition, self-care, pain, vision, hearing, speech, ambulation and dexterityCitation25. The HUI 2 questionnaire has been designed to capture utility scores in the range –0.03 to 1.00 while the HUI 3 questionnaire captures scores from –0.36 to 1.00. For the base-case analysis it was assumed that high-risk children who did not experience a hospitalisation due to RSV infection would not have perfect health but would have a utility corresponding with a median HUI 2 for non-RSV infected children (u=0.95); high-risk children who experienced a hospitalisation due to a RSV infection would have a utility corresponding with a median HUI 2 for RSV-infected children (u=0.88); all patients above 16 years of age would have perfect health (u=1). As the median HUI 3 multi-attribute score did not differ significantly with RSV infection, a scenario analysis was conducted based on a utility of 0.93 (no RSV hospitalisation) for all children up to the age of 16 years.
Resource utilisation and costs
Resource utilisation and associated costs were determined from a literature review focused on Germany and an analysis of the palivizumab clinical trials. Palivizumab prophylaxis consists of 15 mg/kg body weight administered by intramuscular injection once a month for 4 or 5 months. In the base-case analysis it was assumed that each infant would receive 4.93 doses of palivizumab over the RSV season, corresponding with average resource use in the palivizumab trialCitation14. Use of palivizumab was based on the proportion of 50-mg and 100-mg vials used in the palivizumab trial, in which the average patient weight was 6.05 kg, resulting in an average cost of €1,538 per dose. In Germany, the first dose of palivizumab is administered during a hospital stay unrelated to RSVCitation26. As such, the administration cost is included within the DRG or daily rate relating to the indication for which the child has been hospitalised. The remaining 3.93 doses are given in hospital on an ambulatory basis at a cost of €80.75 each, resulting in a total administration cost of €317 for the entire RSV season.
Inpatient costs were based on the total number of RSV hospitalisations, according to the DRG funding system in Germany, which was introduced in 2004. The economic value of each DRG comprises points per indication-specific procedure multiplied by a basic hospital valuation. Using a weighted average of all relevant DRGs, the cost associated with inpatient treatment of RSV infection for all sub-populations was calculated to be €17,687 per hospitalisationCitation27.
Asthma
The base-case analysis takes into account long-term consequences beyond the period of RSV hospitalisation, including costs associated with subsequent development of asthma. The direct cost associated with grade 1 asthma was €1,060 per year for an adult patientCitation28.
Indirect costs (productivity losses)
German data were used to derive short-term indirect costs borne by parentsCitation26. It was assumed that administration of palivizumab would take 2 hours (1 hour at the hospital and 1 hour driving time) and that the infant would be accompanied by one parent for each visit. Based on an average wage per working hour of €18, total work-loss costs were calculated as €144 for the assumed four injections performed in the hospital per RSV seasonCitation26. The first administration of palivizumab corresponds with a planned hospital visit, unrelated to RSV. Therefore indirect costs associated with this first visit were not included in the base-case analysis.
If the infant was hospitalised due to RSV infection, parental work-loss costs were calculated by multiplying the average length of hospital stay by the average wage. Costs for a parent with a hospitalised child were calculated according to the number of risk factors, giving indirect costs per hospitalisation of €605, €1,093, €1,349 and €1,183 for children with one, two, three and four risk factors, respectivelyCitation26. However, as the model was not stratified according to risk, the base-case analysis assumed a total indirect cost of €1,093 per hospitalisation. This cost input was varied in sensitivity analyses from €605 to €1,349.
Long-term indirect costs (lost productivity following childhood mortality due to RSV infection) were based on estimates of the lifetime productivity of an average German inhabitant. The OECD estimates that an employed person in Germany works on average 1,446 hours per yearCitation29. Taking into account the average hourly wage, average annual productivity per employed person was calculated to be €26,028. Application of the 64.6% employment rate leads to potential annual productivity of €16,814 for an average German inhabitantCitation29. Discounting was applied to annual indirect costs to take into account the fact that individuals prefer to delay costs rather than incur them in the present. The costs were based on 2006 data. Costing data from previous years were updated to 2006 using an inflation correction.
Results
The use of palivizumab in the CHD population leads to an additional medical cost of €6,972 versus no prophylaxis, taking into account the costs of palivizumab and RSV hospitalisation. When the direct costs associated with asthma are included, the total incremental cost of prophylaxis falls to €6,364. From the societal perspective, which considers direct medical costs, costs associated with asthma and indirect costs resulting from lost productivity, use of palivizumab leads to an additional cost of €3,637 versus no prophylaxis.
In terms of clinical outcomes, use of palivizumab provides an additional 1.36 LYG (undiscounted) and 0.36 LYG (discounted) versus no prophylaxis. The inclusion of utilities leads to a gain of 1.39 QALYs (undiscounted) and 0.38 QALYs (discounted).
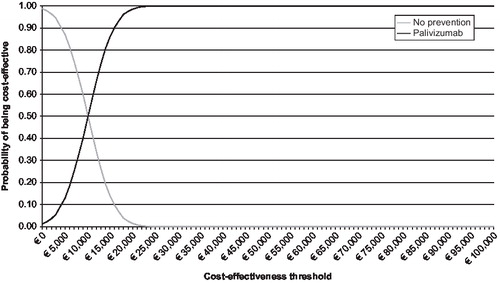
The base-case analysis from the societal perspective shows that the use of palivizumab results in an incremental cost-effectiveness ratio (ICER) of €2,675/LYG without discounting (). After discounting, the ICER is €10,116/LYG. When quality of life is taken into account, the ICER becomes slightly more favourable: €2,615/QALY (undiscounted) and €9,529/QALY (discounted). also shows the results from the health insurance perspective, based on direct medical costs alone. From this perspective, the ICERs are considerably less favourable for palivizumab. shows the cost-effectiveness acceptability curve from the societal perspective, based on a probabilistic sensitivity analysis with effectiveness discounted. This illustrates that there is a 99% chance that the ICER associated with use of palivizumab in the CHD population is less than €20,000/QALY.
Table 2. Cost-effectiveness results (total CHD population).
Outcomes of a scenario analysis based on hospitalisation rates from the Cardiac study are also strongly in favour of palivizumab (), resulting in ICERs considerably lower than the base case. Another scenario analysis was performed, excluding mortality from the model., which increases the ICER to €123,439/QALY. These results show that the outcomes of the analysis are sensitive to differences in hospitalisation rates. Sensitivity analyses () also demonstrate that the cost-effectiveness outcome is sensitive to discounting, and is moderately sensitive to utility and the value of the DRG.
Figure 3. Sensitivity analyses. DRG, diagnosis related group; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
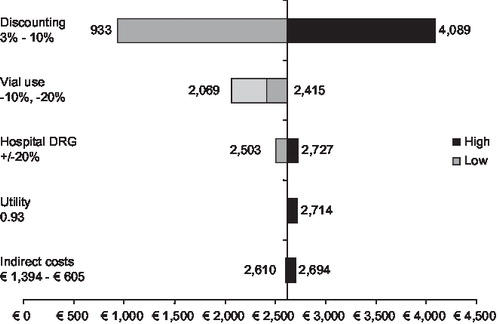
Table 3. Cost-effectiveness results (scenario analysis based on the Cardiac study).
Discussion
This analysis was performed using decision analytical techniques to establish the cost effectiveness of palivizumab in children with CHD in the German healthcare setting in 2005. From both the societal and healthcare purchaser perspectives, the use of palivizumab is cost effective. In most analyses, the ICER falls well below €30,000/QALY, a widely accepted cost-effectiveness thresholdCitation30.
This model provides a more favourable cost-effectiveness outcome for palivizumab than previously published studies in this area. The analysis presented here is the first to consider cost per QALY in Germany for children with CHD based on the results of a relatively large international randomised controlled trial. While the US study by Yount et al uses a similar analytical approach, it does not include the indirect costs resulting from lost lifetime productivity following the death of a childCitation31. The approach described above reflects a broader range of opportunity costs by including long-term downstream consequences of RSV infection such as asthma and productivity losses, and reconsiders mortality from a new analytical perspective. Inclusion of direct medical costs associated with asthma only leads to a moderately more favourable ICER for palivizumab. However, inclusion of the long-term indirect costs due to lost lifetime productivity following childhood mortality leads to a substantial improvement in the cost effectiveness of palivizumab.
Clinical studies evaluating the overall benefit of prophylaxis against RSV infections have tended to focus on the prevention of RSV-related hospitalisation as the single outcome measureCitation26,32,33. However, it was thought important to include mortality outcomes in this health economic evaluation, as a small reduction in the rate of mortality in children can lead to large increases in life expectancy, providing an important health benefit from both a clinical and societal perspective. Although the palivizumab trials were not powered to yield statistically significant differences in mortality, absolute mortality among children with CHD hospitalised due to RSV infection was lower in the palivizumab-treated group than in the placebo group (3.3 vs. 4.2%). It is important to note that the published studies that have reported mortality rates in RSV-infected children include preterm infants and children with BPD in addition to children with CHDCitation6, 21. As these populations have significantly lower mortality risks than children with CHD, these studies may underestimate the mortality risk of children with CHD.
Limitations
The study is limited by a number of conservative assumptions. It was assumed that palivizumab only affects the occurrence of RSV hospitalisation and does not influence the severity of the RSV infection. However, clinical trials suggest that palivizumab reduces the severity of the RSV infection. The Feltes et al trial showed an absolute reduction in probability of ICU admission for infants who received palivizumab (vs. placebo), which suggests a positive impact on the severity of the RSV infection, although the difference in the Feltes et al trial was not statistically significantCitation14. An analysis of the underlying Feltes et al study data showed palivizumab patients had significantly shorter LOS than patients without preventionCitation14. LOS may also be considered a measure of the severity of RSV infection, which confirms the previous hypothesis that palivizumab may reduce the severity of an RSV infection. As with other models, this study had to rely on clinical trial data from an international trial and the utilities of a UK studyCitation14, 25. The assumption in this model was that the probability of RSV hospitalisation and utilities are not country-specific, which corresponds with published recommendations for handling data in health economic modelsCitation17.
The results presented here for the German CHD population are in line with those reported recently from the perspective of the UK National Health Service and the health insurance fund perspective in Austria using a similar model structureCitation22, 34. In the UK, in babies with CHD, the use of palivizumab resulted in an ICER of £2,427/QALY without discounting outcomes and £6,664/QALY after discounting. From the health insurance fund perspective in Austria, the incremental cost-effectiveness ratio without discounting was estimated to be €2,668/QALY for the CHD population. When discounted, this figures rises to €9,754/QALY. This confirms that the cost-effectiveness outcomes for palivizumab are not dependent on country-specific input data, and suggests that the results for Germany, Austria and the UK may guide decision makers in other European countries.
Although modelling techniques cannot replace clinical experience, they do allow us to demonstrate the possible long-term health economic outcomes associated with palivizumab without the need for a naturalistic prospective study that may require a 5–10-year follow-up period. Use of a decision-tree model allows us to extrapolate clinical outcomes beyond the duration of the existing clinical trials. Scenario and sensitivity analyses were performed to ensure robustness of the model. These analyses show that, although there is some sensitivity to variations in the underlying assumptions and data, the overall outcome is robust: palivizumab remains cost effective in all analyses.
Finally, all analyses were performed with and without discounting. However, as the majority of costs (costs of prophylaxis and hospitalisation) occur in the short-term, discounting mainly affects effectiveness (LYG and QALY). This explains the substantially higher ICER outcomes after discounting, although palivizumab remains cost effective.
Conclusion
The use of palivizumab represents a cost-effective means of prophylaxis against RSV infection in children with haemodynamically significant CHD in Germany. Even when administered for just one RSV season, the positive clinical and economic benefits may persist in a lifetime analysis.
Transparency
Declaration of funding: This research was funded by Abbott, the manufacturer of palivizumab.
Declaration of financial/other relationships: W.W. has disclosed that he is an employee of Abbott. M.L. has disclosed that he is an employee of University of Sheffield, Sheffield, United Kingdom. M.N. has disclosed that he has served as consultant to Abbott and was paid by Abbott to conduct the analyses presented in this article.
Acknowledgment:
The authors have disclosed that they had no outside editorial assistance in preparing this manuscript.
References
- Simoes E. Respiratory syncytial virus infection. Lancet 1999;354: 847–852.
- Hall C. Respiratory syncytial virus . In: Feigin R, Cherry J, eds. Infectious Diseases. Philadelphia: W.B. Saunders, 1998.
- Berner R, Schwoerer F, Schumacher RF, Community and nosocomially acquired respiratory syncytial virus infection in a German paediatric hospital from 1988 to 1999. Eur J Pediatr 2001;160:541–547.
- Liese JG, Grill E, Fischer B, Munich RSV Study Group. Incidence and risk factors of respiratory syncytial virus-related hospitalizations in premature infants in Germany. Eur J Pediatr 2003;162:230–236.
- Canfield S, Simoes E. Prevention of respiratory syncytial virus (RSV) infection: RSV immune globulin intravenous and palivizumab. Pediatric Ann 1999;28:507–514.
- Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr 1995;126:212–219.
- Navas L, Wang E, de Carvalho V, Robinson J. Pediatric Investigators Collaborative Network on Infections in Canada: improved outcome of respiratory syncytial virus infection in a high-risk hospitalized population of Canadian children. J Pediatr 1992;121:348–354.
- Moler FW, Khan AS, Meliones JN, Respiratory syncytial virus morbidity and mortality estimates in congenital heart disease patients: a recent experience. Crit Care Med 1992;20:1406–1413.
- Khongphatthanayothin A, Wong PC, Samara Y, Impact of respiratory syncytial virus infection on surgery for congenital heart disease; postoperative course and outcome. Crit Care Med 1999;27:1974–1981.
- Altman CA, Englund JA, Demmler G, Respiratory syncytial virus in patients with congenital heart disease: a contemporary look at epidemiology and success of preoperative screening. Pediatr Cardiol 2000;21:433–438.
- Malhotra A, Krilov L. Influenza and respiratory syncytial virus. Update on infection, management and prevention. Pediatr Clin North Am 2000;47:353–372.
- Bont L, Aalderen WM, Kimpen JL. Long-term consequences of respiratory syncytial virus (RSV) bronchiolitis. Paediatr Respir Rev 2000;1:221–227.
- European Medicines Agency. http://www.emea.eu.int/humandocs/Humans/EPAR/synagis/Synagis.htm.
- Feltes TF, Cabalka AK, Meissner HC, Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003;143:532–540.
- Palivizumab prescribing information.
- Nuijten, MJC. Cost-effectiveness of fluvoxamine in the treatment of recurrent depression in France. Pharmacoeconomics 1998;14: 433–445.
- Nuijten MJ. The selection of data sources for use in modelling studies. Pharmacoeconomics 1998;13:305–316.
- Brecht JG, Jenke A, Kohler ME, Recommendations of the German Society of Clinical Pharmacology and Therapy for implementing and evaluating pharmaco-economic studies. Med Klin (Munich) 1995;90:541–556.
- International Society for Pharmacoeconomics and Outcomes Research (ISPOR). German recommendations on health economic evaluation studies. Revised version of the Hannover Consensus, 1995.
- Simoes EA, Sondheimer HM, Top FHJr, ; Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus disease in infants and children with congenital heart disease. The Cardiac Study Group. J Pediatr 1998;133: 492–499.
- Rodriguez WJ, Parrott RH. Ribavirin aerosol treatment of serious respiratory syncytial virus infection in infants. Infect Dis Clin North Am 1987;1:425–439.
- Nuijten MJ, Wittenberg W, Lebmeier M. Cost effectiveness of palivizumab for respiratory syncytial virus prophylaxis in high-risk children: A UK analysis. Pharmacoeconomics 2007;25:55–71.
- Statistisches bundesamt Deutschland. www.destatis.de.
- Wren C, O'Sullivan JJ. Survival with congenital heart disease and need for follow up in adult life. Heart 2001;85:438–443.
- Greenough A, Alexander J, Burgess S, Health care utilisation of prematurely born, preschool children related to hospitalization for RSV infection. Arch Dis Child 2004;89:673–678.
- Roeckl-Wiedmann I, Liese JG, Grill E, Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. Eur J Pediatr 2003:162;237–244.
- 3M Medica - Health Information Systems. DRG online grouper http://www.grdrg.de/GrouperCGI/OnlineGrouper.exe.
- Graf von der Schulenburg JM, Greiner W, Molitor S, Cost of asthma therapy in relation to severity. An empirical study. Med Klin (Munich) 1996;91:670–676.
- Organisation for Economic Co-operation and Development (OECD). OECD Employment Outlook 2004. www.oecd.org.
- Laufer F. Thresholds in cost-effectiveness analysis – more of the story. Value Health 2005;8:886–887.
- Yount LE, Mahle WT. Economic analysis of palivizumab in infants with congenital heart disease. Pediatrics 2004;114:1606–1611.
- Wegner S, Vann JJ, Liu G, Direct cost analyses of palivizumab treatment in a cohort of at-risk children: evidence from the North Carolina Medicaid Program. Pediatrics 2004;114:1612–1619.
- Lofland JH, O'Connor JP, Chatterton ML, Palivizumab for respiratory syncytial virus prophylaxis in high-risk infants: a cost-effectiveness analysis. Clin Ther 2000;22:1357–1369.
- Resch B, Gusenleitner W, Nuijten MJC, Cost-effectiveness of palivizumab against respiratory syncytial viral infection in high-risk children in Austria. Clin Ther 2008;30:749–760