Abstract
Background: Acetaminophen (APAP) overdose, which can lead to hepatotoxicity, is the most commonly reported poisoning in the United States and has the highest rate of mortality, with more than 100,000 exposures and 300 deaths reported annuallyCitation. The treatment of choice, N-acetylcysteine (NAC), is effective in both oral (PO) and intravenous (IV) formulations. The main difference in therapies, other than administration route, is time to complete delivery – 72 hours for PO NAC versus 21 hours for IV NAC, according to full prescribing information. This distinction is the primary basis for variation in management costs for hospitalized patients receiving these products.
Objectives: To quantify and compare full treatment costs from the provider perspective to manage acute APAP poisoning with either PO or IV NAC in a standard treatment regimen.
Methods: A cost model was developed and populated with published data comprising probabilities of potential clinical outcomes and the costs of resources consumed during patient care.
Results: For patients who present <10 hours post-ingestion, the estimated total cost of care with PO NAC in the treatment regimen is $5,817 (ICU patients) or $3,850, (ward patients) compared with $3,765 and $2,768 for similar care with IV NAC. Potential cost savings equal – $2,052 (–35%) or –$1,083 (–28%), respectively, in favor of IV NAC. Similar potential savings were estimated for patients presenting 10–24 hours post-ingestion.
Conclusion: IV NAC is the less costly therapeutic option for APAP poisonings, based on simulation modeling and retrospective data. The current economic evaluation is restricted by the absence of comparative data from head-to-head, matched-cohort studies and the limitations common to retrospective APAP toxicology datasets. Additional research could refine these results.
Key words::
Introduction
Acetaminophen (APAP) overdose is the most commonly reported poisoning in the United States and is associated with the highest rate of mortality, with more than 100,000 exposures and 300 deaths reported annuallyCitation1. It has replaced viral hepatitis as the most common cause of acute hepatic decompensation and is the second most common cause of hepatic failure requiring transplantationCitation2. These catastrophic outcomes and liver-related deaths are uncommon, however, in overdosed patients who present to the hospital within 16 hours of ingestionCitation3. Rapid identification of at-risk patients and early initiation of treatment are the keys to avoiding hepatic events.
The therapeutic standard for APAP toxicity, N-acetylcysteine (NAC), is effective in both oral (PO) and intravenous (IV) formulations, which appear to produce similar clinical outcomes based on decades of researchCitation3–9. If treatment begins within 10 hours of toxic ingestion, liver damage occurs in ≤6% of patients (range, 0–30% in notable studies)Citation3. On the other hand, PO or IV NAC administered 10–24 hours after toxic ingestion is less effective; the reported incidence of hepatotoxicity averages ≤30% (range, 8–53%) for PO- and IV-treated groupsCitation3. Worse yet, treatment initiated after 24 hours is associated with even higher rates of liver damage, with frequencies rising to more than 50% of cases. Higher rates and greater toxic severity produce occasional episodes of acute liver failure, which requires intense management with a broad range of costly resources and interventions including, in extreme cases, liver transplantation.
Although safety concerns are different for each formulation, the risks associated with both PO and IV NAC are manageable. Oral NAC is difficult to administer due to its foul odor and taste, often resulting in intolerance and occasional refusal. Specific complications include nausea, vomiting, and diarrhea, each with the potential to compromise dosing. Intravenous NAC is associated with flushing, erythema, and anaphylactoid reactions. The adverse events typically associated with both drug formulations must be competently addressed for PO or IV therapies to continue uninterrupted.
In approximately 95% of emetic cases, vomiting is controlled with antiemetic drugs but occasionally persists and necessitates a switch from the PO to the IV antidoteCitation10. The average time-to-switch and start IV NAC is about 4.5 hoursCitation10, potentially delaying effective treatment and increasing the risk of liver damage and sequelae, particularly in cases that would otherwise have been treated within 10 hours of ingestion. Sporadic anaphylactoid reactions are reported with use of the IV therapy and are controlled with antihistamine and/or reduction of the infusion rate during the loading dose.
A principal difference between the two therapies, other than administrative route, is the time required for complete delivery. The approved therapeutic duration for the PO product is 72 hoursCitation11 versus 21 hours (also known as the 20-hour protocol) for the IV productCitation12. This major distinction is the basis for potential cost disparity between PO and IV regimens and has been the focus of substantial discourse over the years.
Head-to-head prospective studies of PO versus IV therapies are currently unavailable, so a direct comparison has yet to be made. Retrospective evaluations to compare the two products have been limited by diverse clinical presentations coupled with incomplete datasets and absent cost accounting in individual studies. Even administrative databases that closely track resource consumption and related charges are hampered by dissimilar patient characteristics, indistinguishable acute versus chronic poisonings, varied treatment regimens, inadequately reported laboratory data, and missing time stamps that document the interval between APAP overdose and commencement of treatment. Consequently, after years of study, no clear picture has emerged to establish definitively which formulation is superior. As a result, they have been viewed to be similar: ‘The differences claimed between oral and intravenous N-acetylcysteine regimens are probably artifactual and relate to inappropriate subgroup analysis,’ concluded a previously published meta-analysis of pooled data from seven respected studiesCitation3.
The most recent evaluation and perhaps the most definitive to date – but unpublished during our analysis – compared multi-site data sets for both PO- and IV-treated patients with acute poisoningCitation14. The authors illustrated the dynamic nature of therapeutic effectiveness based on a continuum of data points from time of ingestion to treatment for the two study groups (PO- and IV-treated patients) and illustrated that: (1) IV NAC was more effective in the 0-to-12 hour post-ingestion period, (2) the two formulations were equivalent in the 12-to-18 hour post-ingestion period, and (3) PO NAC was found to be more effective from 18 to 24 hours post-ingestion. The study is retrospective and compares patients from different countries (Canada for IV-treated patients versus the United States for PO-treated patients). Study data were collected during different timeframes (1976–1985 for PO-treated patients and 1980–2005 for IV-treated patients), and no economic outcomes were captured or computed.
Lacking definitive, prospective, head-to-head comparative data, the present analysis was conducted under the assumption that PO and IV NAC produce similar outcomes for patients treated within 24 hours of APAP ingestion as reported in the meta-analysisCitation3 and numerous other publications. Thereafter, our analyses were segregated into <10 hours and 10-to-24 hour periods, based on the timeframes most commonly reported in contemporary literature, being thus constrained by their data sets. Clinical data within these timeframes do vary slightly, and those variations were included in the present analyses.
Objective
The objective of this cost analysis is to quantify and compare the full treatment costs to manage acute APAP poisonings with approved regimens of PO versus IV NAC in order to prevent or ameliorate ensuing hepatotoxicity that may result from toxic overdose. The time horizon of this provider-perspective study is from patient presentation in the emergency department (ED) to provider network discharge.
Methods
Health-economic modeling is a method that has broad applicability in the medical industry. It provides a template to help organize thoughts and logically structure chains of cause and effect. Additionally, models can help to depict influence between interacting elements in the economics of healthcare. Finally, models facilitate experimentation and scenario testing such as evaluating the effects of alternative treatment options or weighing the integrity of different data inputs and/or opinions and arguments regarding management practices.
The model developed for the current study () was used to estimate the total expected cost to manage a patient with documented acute APAP poisoning as described in . Conceptually, it simulates the ED presentation of a toxic patient, urgent evaluation and management of the toxicity, and subsequent in-patient care. Specifically, it accepts probability data for various clinical outcomes related to APAP toxicity and cost data for resources consumed in association with either PO or IV NAC treatment regimens. Alternately, it explores several clinical circumstances; i.e., patient presentations <10 hours and 10-to-24 hours post-ingestion and patient management in either the intensive care unit (ICU) or, less commonly, on the general medical ward. Finally, it facilitates comparisons of base-case, best-case, and worst-case scenarios for combinations of previously cited clinical circumstances; for example: (1) patients treated within 10 hours of ingestion and maintained in the ICU, (2) patients treated within 10 hours of ingestion and maintained on the ward, (3) patients treated 10–24 hours post-ingestion and maintained in the ICU, and (4) patients treated 10–24 hours post-ingestion and maintained on the ward. For analyses of extremes, the worst-case scenarios incorporated data points for each variable from the range of published data for that variable to estimate upper ends of expected costs. Conversely, the best-case scenarios used data points that estimated lower ends of expected costs. Between these two extremes of data sets and outcomes are the base-case scenarios, the outputs of which are the principal results of this studyCitation3–9.
Figure 1. PO or IV N-acetylcysteine regimen. Patients with documented acetaminophen poisoning enter the model and are treated with PO or IV N-acetylcysteine according to their approved uses. Patients are managed for the acute poisoning as well as subsequent potential clinical consequences including possible but unlikely liver failure.
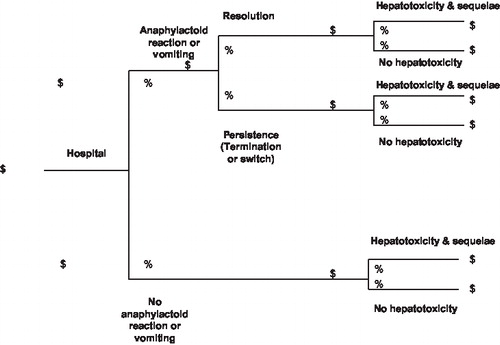
Table 1. Model assumptions.
Of the assumptions listed in the first table, one merits explanation. For IV-treated patients, half a day of care was added to total resource consumption because some patients on the 21-hour treatment regimen will be admitted and treated overnight following time spent in the ED for primary evaluation and initiation of therapy. Therefore, some percentage of IV-treated patients will incur a second in-patient day (common to typical hospital accounting). In consideration of this possibility, an additional half hospital day and related resource costs were incorporated into the IV assessment.
Data input and sources
Clinical data used to populate the model were abstracted from retrieved literature identified through Ovid, Medline, PubMed, MedScape, eMedicine, and open internet searches as well as reference citations in collected articles, which numbered 47 at final count. Key search terms included: ‘poisoning,’ ‘toxicity,’ ‘acetaminophen,’ ‘paracetamol,’ ‘acetylcysteine,’ ‘N-acetylcysteine,’ ‘economics,’ and ‘cost.’ Other related and combined terms included: ‘hepatotoxicity,’ ‘acute liver failure,’ ‘vomiting,’ ‘anaphylactoid reaction,’ and ‘liver transplantation.’ Retrieved publications reported clinical outcomes from prospective, randomized controlled trials; meta-analysis; nonrandomized observational studies; case series; case reports; and financial records. Principal data sources included those publications that are referenced frequently in other papers, that provide detailed patient and poisoning characteristics, and that evaluate a study population of meaningful size.
Financial data used in the model also were obtained from published and non-published sources including consultation with respected clinicians as well as direction from the Contracting and Analysis Department of University Hospital at University of Medicine and Dentistry of New Jersey (UMDNJ). The most complete listing of consumed ED and ICU resources and related charges was found in a recently published financial analysis of an individual patient who suffered varied consequences of APAP poisoningCitation13. Economic data identified as charges, regardless of source publication, were converted to costs by a factor of 0.33, which is more reflective of real-world circumstances than the commonly applied charge-to-cost ratio of 0.50, cited in contemporary medical literatureCitation15. Additionally, low and high values for both costs and probabilities were obtained from the retrieved literature and from UMDNJ's Contracting and Analysis Department to provide bounded ranges of model inputs to facilitate testing of the previously cited scenarios in analyses of extremes.
Although no randomized, comparative, head-to-head clinical studies were found in the literature, one often-cited article revealed the results of a meta-analysis of clinical outcomes from seven different studies (three PO NAC; four IV NAC) and also provided a comparison of pooled IV-NAC treatment data (n=341 patients) versus pooled PO-NAC treatment data, including data from the largest single series study reported to that point in time (n=1,462 patients)Citation3. Insight gained from the meta-analysis, review of individual studies, and consultation with highly respected clinical experts aided data selection. For the actual clinical and economic inputs used in the model, see and for base-case scenarios (<10 hours post-ingestion and 10-to-24 hours post-ingestion, respectively). and show calculated values for base cases.
Table 2. Model inputs. Base-case scenario, patient treated <10 hours post-ingestion.
Table 3. Model calculated costs. Base-case scenario, patient treated <10 hours post-ingestion.
Table 4. Model inputs. Base-case scenario, patient treated 10–24 hours post-ingestion.
Table 5. Model calculated costs. Base-case scenario, patient treated 10–24 hours post-ingestion.
Results
The estimated total expected cost associated with PO NAC is $5,817, if patients present for treatment within 10 hours of ingestion and are managed in the ICU and $3,850, if managed on the medical ward. This compares with $3,765 and $2,768 for similar management with IV NAC, for a cost differential of $2,052 (35%) and $1,083 (28%), respectively, in favor of IV NAC. Likewise, the total estimated cost associated with PO NAC is $6,200, if patients present for treatment 10–24 hours after ingestion and are managed in the ICU and $4,233, if managed on the medical ward. This compares with $4,293 and $3,296 for similar management with IV NAC, for a cost differential of $1,906 (31%) and $937 (22%), respectively, in favor of IV NAC. lists results for all scenarios.
Table 6. Analytic results all scenarios.
Discussion
The current economic evaluation computes and compares total expected costs from the provider perspective for approved treatment protocols of PO (72-hour administration) and IV NAC (21-hour administration) in the management of patients with acute APAP poisoning. It assumes that both formulations deliver similar outcomes in terms of preventing hepatotoxicity and related sequelae when patients with analogous levels of toxicity present in EDs within 24 hours of APAP ingestion, although some differences in outcomes have been reported for more tightly segmented, ingestion-to-treatment timeframes. To account for various clinical circumstances and a significant range of values for data inputs, several scenarios were tested.
A cost model followed patients through various levels of care and strongly reflected the economics of reduced hospital length of stay. It indicates that IV NAC can deliver systemic cost savings when used in hospital practice. When evaluating this result, several questions arise. First, is the assumption of clinical equivalence justified? To answer this question, a review of previously published as well as newly emerging data is required. Collectively, these data suggest that treatment with IV NAC may produce better clinical outcomes in the first 8–12 hours post-ingestion. Based on recently published outcomes data, this advantage seems to diminish between 12 and 18 hours post-ingestion, and swings in favor of PO NAC after 18 hoursCitation14. Over the entire post-ingestion period of 0–24 hours, however, the two formulations appear to deliver similar outcomes.
Next, how accurate is patient-derived information about the quantity of APAP consumed and the time from ingestion to ED presentation? This question addresses the correctness of recorded timeframes in previous research and the assignment of specific rates of hepatotoxicity based on the reported interval from ingestion to treatment. According to prominent experts in the field, the accuracy of patient-provided information is questionable, especially information from patients with potential psychiatric disorders who are under substantial duress.
Additionally, one must consider the treatment protocol that is used in actual clinical practice; is it truly 72 hours for PO NAC and 21 hours for IV NAC, or something different? Although full prescribing information recommends 72-hour treatment for PO NAC and 21-hour treatment for IV NACCitation11,12, some healthcare professionals may withdraw therapy sooner in response to declining APAP blood levels, which may shorten hospital stay and reduce costs. The current model was not designed specifically to evaluate such circumstances, nor do the authors advocate these practices without sufficient study and supportive data, but fractionation of per-patient estimated costs generated through the model could provide some insight into the potential economic consequences of these management decisions. In other instances, treatment duration may be extended, based on evolving clinical conditions. Again, the current model was not designed specifically to evaluate such circumstances but does account for rising resource consumption and costs based on a small proportion of patients who experience severe hepatotoxicity leading to acute liver failure and ultimately transplantation in rare cases. Customarily, however, treatment protocols and standards of care are closely followed, if for no reason other than to avoid potential litigation in the event of adverse clinical outcomes, but primarily to achieve the results that are expected based on efficacy claims approved by the FDA and documented effectiveness seen in routine practice.
Finally, are the results of the present analysis concordant with those of other pharmacoeconomic assessments? The most comparable and currently available assessment, published in 2005, concluded that the IV preparation was more cost-effective when given for 20 hours, based on drug costs and decreased hospital length of stayCitation10. In that study, the total cost of the 72-hour PO NAC protocol was reported as $10,801, compared to $4,068 for the 20-hour IV NAC protocol, for treatment delivered in the ICU. Equivalent clinical outcomes were assumed and ED costs were excluded. With the addition of ED costs and updated charge-to-cost data, these results are similar to those reported in our study for 3 days of ICU care for PO-treated patients and 1.5 days for IV-treated patients.
Limitations
The current economic evaluation is restricted by the absence of comparative data from head-to-head, matched-cohort studies and the previously mentioned limitations common to retrospective APAP toxicology datasets. Moreover, data were culled from reports of different studies, which could have dissimilar patients and poisoning characteristics. Point estimates that are representative of different categories of patients were used as data inputs, whereas, in reality, an infinite number of distinct scenarios exists but were impossible to model in totality because complete datasets were unavailable.
Conclusions
The results of this analysis strongly suggest that cost saving in a health system can be achieved if APAP poisonings are managed with IV NAC. When analyses are conducted to test best- and worse-case scenarios, IV NAC prevails in all circumstances, as does treatment on the ward. In large part, these results reflect the difference in the route of administration of each competitive product and the time required for complete treatment according to approved usage. Prospective comparative health-economic study of PO and IV NAC could refine current cost information and help to resolve questions about therapeutic selection. Until such data are available, model estimates can provide insight into relative estimated costs, thereby aiding product and protocol selections. A cost-effectiveness analysis of newly emerging clinical data may further clarify the value of any differences in treatment outcomes between PO and IV NAC regimens based on tightly segments timeframes from APAP ingestion to treatment.
Acknowledgment
Declaration of interest: This study was funded by Cumberland Pharmaceuticals.
A.M. and R.R. have disclosed that they are paid consultants for Med-ERA, Inc, and have received research and educational grants for their work with Cumberland Pharmaceuticals, Bristol-Myers Squibb, Intendis, Sanofi-Aventis, and Abbott Laboratories.
References
- Bronstein AC, Spyker D, Cantilena LR, 2007 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 25th annual report. Clin Toxicol 2008;46:927-1057.
- US Food and Drug Administration. Public health problem of liver injury related to the use of acetaminophen in both over-the-counter (OTC) and prescription (RX) products. Available at: http://www.fda.gov/AdvisoryCommittees/Calendar/ ucm143083.htm . Accessed August 25, 2009.
- Buckley NA, Whyte IM, O'connell DL, Oral or intravenous N-acetylcysteine: which is the treatment of choice for acetaminophen (paracetamol) poisoning? J Toxicol Clin Toxicol 1999;37:759-67.
- Prescott LF, Illingworth RN, Critchley JAJH, Intravenous N-acetylcysteine: the treatment of choice in acetaminophen poisoning. BMJ 1979;2:1097-100.
- Parker D, White JP, Paton D, Routledge PA. Safety of late acetylcysteine treatment in paracetamol poisoning. Hum Exp Toxicol 1990;9:25-7.
- Smilkstein MJ, Bronstein AC, Lindein C, Acetaminophen overdose: a 48-hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med 1991;20:1058-63.
- Rumack BH, Peterson RC. Acetaminophen overdose: incidence, diagnosis, and management if 416 patients. Pediatrics 1978;62:898-903.
- Rumack BH, Peterson RC, Koch GG, Amara IA. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med 1981;141(3 Spec No):380-5.
- Smilkstein MJ, Knapp GL, Kulig KW, Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose: analysis of the national multicenter study (1975 to 1985). N Engl J Med 1988;319:1557-62.
- Culley CM, Krenzelok EP. A clinical and pharmacoeconomic justification for intravenous acetylcysteine: a US perspective. Toxicol Rev 2005;24:131-43.
- Prescribing Information. Mucomyst? (acetylcysteine) Solution. Roxane Laboratories, Inc. Revised March 2007.
- Prescribing Information. Acetadote? (acetylcysteine) Injection. Cumberland Pharmaceuticals Inc., 2006.
- Ferris M, Hasket M, Pilkington S, Williams M. Financial analysis of acetaminophen suicide in a teen girl. Ped Nurs 2007;33: 422-541.
- Yarema MC, Johnson DW, Berlin RJ, Comparison of the 20-hour intravenous and 72-hour oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann Emerg Med. Published Online, 2009.
- Friedman B, De la mare J, Andrews R, McKenzie DH. Practical options for estimating cost of hospital inpatient stays. J Health Care Finance 2002;29:1-13.
- Kanter MZ. Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am J Health-Syst Pharm 2006;63:1821-7.
- Bond GR, Novak JE. The human and economic cost of acetaminophen (acetaminophen) overdose. Pharmacoeconomics 1995;8:177-81.
- Flanagan RJ, Meredith TJ. Use of N-acetylcysteine in clinical toxicology. Am J Med 1991;91:131-9S.
- Sunman W, Hughes AD, Sever PS. Anaphylactoid response to intravenous acetylcysteine. Lancet 1992;339:1231-2.
- Yip L, Dart RC, Hurlbut KM. Intravenous administration of oral N-acetylcysteine. Crit Care Med 1998;26:40-3.
- Bailey B, Mcguigan MA. Management of anaphylactoid reactions to intravenous N-acetylcysteine. Ann Emerg Med 1998;31:710-15.
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis 2007;11:525-48.
- Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin N Am 2008;92:761-94.
- Dasta JF. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med 2005;33:1266-71.
- Evans RW, Manninen DL, Dong FB. An economic analysis of liver transplantation, costs, insurance coverage, and reimbursement. Gastroenterol Clin North Am 1993;22:451