Abstract
Introduction: Canadian, Inuit, full term infants have the highest rate of respiratory syncytial virus (RSV) infection globally, which results in substantial costs associated hospitalisation.
Methods: Decision-analytical techniques were used to estimate the incremental cost-effectiveness ratio (ICER) for palivizumab compared to no prophylaxis for Inuit infants of all gestational age. The time horizon was that of life-time follow-up, and costs and effectiveness were discounted at 5% per year. Costs (2007 CAD$) for palivizumab, hospitalisation (including medical evacuation, intensive care unit [ICU]), physician visits, and transportation were calculated based on the Canadian payer's perspective. Benefits on decreasing RSV hospitalisation were expressed as quality-adjusted life-years (QALYs). One-way and probabilistic sensitivity analysis (PSA) were conducted, varying: mortality rates, utilities, length of stay in hospital and ICU.
Results: For all of Baffin Island infants (<1 year), the ICER was $39,435/QALY. However, when infants were grouped by age and area of residence, those residing in Iqaluit (<1 year) had an ICER of $152,145/QALY, while those residing in rural areas (outside of Iqaluit) had an ICER of $24,750/QALY. Prophylaxis was a dominant strategy (cost saving) for rural infants under 6 months of age, with the PSA demonstrating that it was dominant 98% of the time.
Conclusions: The ICERs suggested that palivizumab is a cost-effective option for the prevention of RSV for Inuit infants on Baffin Island compared to no prophylaxis. Palivizumab is highly cost effective in Arctic infants <1 year of age specifically residing outside of Iqaluit and is a dominant strategy for those under 6 months of age in rural areas. However, palivizumab is not cost effective compared to no treatment for infants of all ages residing in Iqaluit.
Introduction
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections (LRTIs) in infants and young childrenCitation1–3, with particularly high rates in Canadian Inuit infantsCitation4–7. Approximately 40% of infants infected with RSV have lower respiratory tract (LRT) involvement, namely bronchiolitis or bronchopneumoniaCitation8. RSV is estimated to cause 50–90% of paediatric hospitalisations of bronchiolitis and 25–50% of paediatric hospitalisations for pneumoniaCitation9. RSV is a major cause of morbidity and mortality after the neonatal period, particularly for premature infants and infants with underlying medical risk factors such as chronic lung disease (CLD), congenital heart disease (CHD), or immune deficiencyCitation3. RSV is an important viral respiratory pathogen in children in terms of individual morbidity and societal costs.
Palivizumab is a humanised monoclonal antibody administered by intramuscular injection at monthly doses of 15 mg/kg. It was shown to reduce RSV related hospitalisations by 78% in preterm infants without CLDCitation10. However, due to the high acquisition cost of palivizumab, restrictive guidelines govern the use of palivizumab and limit it to premature infants or those with bronchopulmonary dysplasia (BPD)/CLD who meet specific criteria. In addition, the Canadian Paediatric Society indicated that ‘children born between 33 and 35 weeks’ gestation in isolated communities where hospital care is not readily accessible’ may be given special consideration for RSV prophylaxisCitation11. These isolated communities would include those living in the Eastern Canadian Arctic. In a study by Banerji et alCitation12, 86% of infants that were hospitalised for RSV in the Eastern Canadian Arctic were term, Inuit infants and would not be eligible for prophylaxis under the current guidelines.
In fact, different risk factors may be present in the Eastern Arctic. In a recent case control study analyzing risk factors for LRTI in the Eastern Canadian Arctic by Banerji et al Citation13, prematurity was not found to be a significant risk factor (p=0.10) for hospitalisation due to LRTIs, while residing in communities outside of Iqaluit (OR=2.7, 95% CI: 1.0–7.2), full Inuit race (OR=3.8, 95% CI: 1.1–12.8), and overcrowding (OR=2.5, 95% CI: 1.1–6.1) were significant when cases were matched for age. All of the infants who required ICU admission resided in the rural communities. Other studies have shown rates of RSV hospitalisation for Inuit infants to range from 6.3 to 51.2% over a 1-year period, having a much higher risk than preterm infants in the IMpact trialCitation4–7,14.
In Canada, infection due to RSV is a large public health burden, accounting for an estimated 5,800 hospitalisations annuallyCitation15. Langley et al (1997) estimated that the annual cost of treating RSV-associated illness in Canada was approximately US$18 million (1993)Citation15. The largest component of direct expenditures (62%) was for inpatient hospital care. Expenditures for ambulatory patients accounted for 38% (or US$6.8 million) of the total cost of RSV illness. The authors concluded that the greatest reductions in the economic cost of RSV infection would be found in interventions that reduce duration of or prevent hospital stay. In a Canadian cost-effectiveness analysis of palivizumab by Lanctôt et alCitation16, the authors found that palivizumab was cost effective for infants born at 32–35 weeks’ gestational age, especially those with more than two risk factors or those categorised at least at a moderate level of risk. However, the costs in that study were based on RSV hospitalisations in large urban centres and are not reflective of the Canadian Arctic. In 2005, Creery et al determined the average cost of admission for infants living in Nunavut with bronchiolitis to be $14,273, $12,029 for those admitted to Baffin Regional Hospital (BRH) and $45,688 for those admitted to the Children's Hospital of Eastern Ontario (CHEO)Citation17. In a cost impact analysis by Banerji et al Citation12, authors demonstrated that palivizumab prophylaxis in rural communities on Baffin Island results in cost savings from the payer's perspective due to additional transportation costs incurred from remote areas. Although Banerji et al addressed the feasibility of implementing a palivizumab prophylaxis programme for term Inuit infants, the cost effectiveness of palivizumab still needs to be determined for this special population.
The primary objective was to conduct a cost-effectiveness analysis to determine the value of universal prophylaxis for all infants less than 1 year of age on Baffin Island. As a secondary objective, the impact of age and location of residence within the Baffin region on cost effectiveness was also evaluated.
Methods
Model design
Decision-analytical techniques were used to estimate the cost effectiveness of palivizumab compared to no prophylaxis on Baffin Island, Nunavut in the Eastern Canadian Arctic. The Microsoft Excel model was adapted from a Canadian palivizumab cost-effectiveness model published by Lanctôt et al Citation16, and originally adapted from a model by Nuijten et alCitation18 and was applied to a real cohort of infants on Baffin Island in 2002 who were less than 1 year of age. A decision-tree model was designed to compare the costs and benefits of palivizumab in children less than 1 year of age and 6 months of age on Baffin Island as outlined in . The clinical pathways simulated included a consideration of the following events: with or without prophylaxis, these infants may develop an RSV infection leading to hospitalisation and admission to BRH (now the Qikitani General Hospital). Those who require ICU are medically evacuated to the CHEO in Ottawa. The course of the illness may result in survival or mortality. The analysis was performed from the payer's perspective (base-case) and societal. The payer's perspective is the publicly funded health care system (provincial Ministries of Health) and includes all direct medical costs while the societal perspective includes all direct and indirect costs. Indirect costs encompass future lost productivity of the child. The time horizon of the analysis was that of life-time follow-up. The impact of the reduction of RSV infections was limited to prophylaxis during a single RSV season.
Figure 1. Decision-analytical model outlining the clinical pathway for term Inuit infants on Baffin Island with respiratory syncytial virus (RSV). The no prophylaxis branch is the same as the prophylaxis branch (not repeated). Model adapted from Lanctôt et al. BRH, Baffin Regional Hospital; CHEO, Children's Hospital of Eastern Ontario; ICU, intensive care unit.
Lifetime costs and outcomes were determined for each arm – palivizumab treatment and no prophylaxis treatment. The incremental cost-effectiveness ratio (ICER) of palivizumab treatment compared to no prophylaxis was calculated by dividing the differences in costs by the differences in outcomes. Outcomes were expressed as quality adjusted life-years (QALY) gained. QALYs were obtained by multiplying time spent in the specific health state by the utility value associated with that health state.
Palivizumab effectiveness
The point estimates of the input parameters have been included in . The efficacy of palivizumab was demonstrated in randomised placebo-controlled trials by a decrease in RSV positive hospitalisationsCitation19–21. There were no statistically significant differences in severity markers such as incidence of intubation and total days of mechanical ventilation. In this model, palivizumab effectiveness was estimated based on the IMpact trial and thus, a 78% decrease in RSV positive hospitalisation was assumed. Palivizumab effectiveness estimates were applied to actual hospitalisation rates reported in the case control study of Inuit children in the Eastern Canadian arcticCitation12. Banerji et al. examined 99 of the 108 infants less than one year of age admitted to BRH with LRTI during a one-year period. These infants were tested for RSV in order to determine RSV hospitalization rate for this regionCitation12. These hospitalisation rates were extracted and disaggregated by age and location of the infant. They ranged from 6.3% (for those under the age of 1 year residing in Iqaluit) to 51.2% (for those under the age of 6 months residing in a high risk rural area)Citation12.
Table 1. Point estimates and probabilistic sensitivity analysis (PSA) distributions for key input parameters.
To estimate mortality in those hospitalised for RSV, the 1% mortality reported in the IMpact study was used and was applied to both arms of the decision-tree model. RSV mortality rate has been a controversial issue due to the lack of data regarding the RSV mortality rate in this population. To maintain a conservative model, the value of 1% from the IMpact study, one of the lowest reported RSV mortality rates in the literature, was usedCitation19–21. Sensitivity analyses were performed to examine the impact of applying other RSV mortality rates in the model. The probability of dying from RSV was calculated by multiplying the RSV hospitalisation rate by the RSV mortality rate. As shown in the model decision tree (), RSV related deaths were dependent on prior hospitalisation of the infant. Of all Inuit infants, a certain proportion were hospitalised with RSV and a fraction of those infants eventually died due to the disease. The following calculations relate to the base-case analysis with the Impact-RSV hospitalisation rate and mortality data. For Inuit infants who did not receive prophylaxis, the probability of dying from RSV was estimated to be 0.166% (16.6% [hospitalisation rate] × 1% [mortality rate])Citation20,21. For a similar population receiving palivizumab, the probability of dying from RSV was estimated to be 0.037% (3.7 × 1%).
Differences in health outcomes between prophylaxis and no prophylaxis were determined by comparing the life-years lost and QALYs in each arm. For life-years lost, the mean life expectancy from birth for Inuit infants in Canada is 69.55 years for males and females. Since mean age for RSV infection is during the first year, it is estimated that the mean life expectancy after RSV infection is 68.55 years. The difference in life-years lost between the two groups gave the difference in outcomes, which was then used in the calculation of the ICER (difference in costs/difference in outcomes).
In accordance with the current Canadian guidelines regarding economic evaluationCitation22, the primary economic analysis was expressed in terms of incremental cost per QALY gained. The concept of QALY incorporates both the quantity and quality of life in the assessment of a therapeutic intervention, which may be more appropriate than looking only at life-expectancy. QALYs were calculated by using the health utilities (u) for children with or without a previous RSV infection. These values were obtained from the UK Greenough et al study which used the Health Utility Index Mark 2 (HUI 2)Citation23. According to Greenough et al, a significant difference (p=0.0088) between the groups of infants with (u=0.88) and without (u=0.95) previous RSV infection was evident using the HUI 2. This difference in health-utility values attests to the effect of RSV infection on the quality of life of these children. The utility values from Greenough et al were applied until 16 years of age after which it was assumed that the utilities for both groups were equal in the lifetime follow-up model. This was based on previous studies investigating the association between childhood RSV and long-term respiratory sequelae that found a gradient effect until adolescenceCitation24,25. Death and full health were respectively assigned health utility scores of 0 and 1. The RSV-related values from the HUI 2 scale of the Greenough et al study were applied in the base-case analysis. QALYs were computed by adjusting the life expectancy of each time period (less than 16 years of age, above 16 years of age until death) by the corresponding utility value assigned.
Direct costs
The resources used in association with hospitalisation for RSV infection were based on a cost analysis by Banerji et alCitation12. All direct costs used in the model have been reported in 2007 Canadian dollars using the Bank of Canada inflation calculator if applicable and have been summarised in . Drug acquisition costs were obtained from Abbott Laboratories Limited for both size vials of palivizumab marketed in Canada: 50 mg and 100 mg. Acquisition costs (2007) were assumed by the payers and, in keeping with the current palivizumab administration procedures in Canada they did not include any mark-up. The recommended dose of palivizumab depends on the infant's weight and is given as monthly intramuscular injections of 15 mg/kg. The total dose used was calculated according to the Health and Nutrition Examination Survey growth curves for infants less than 6 months of age and less than 1 year of age by averaging the 50th percentile weight for each month of age up to 6 months and 12 months (1 year), respectivelyCitation26. The cost of drug acquisition including nursing administration cost is $220/kg of infant weight for each injection. It was assumed that each infant would receive five injections throughout the season. The mean length of stay (LOS) for children hospitalised with RSV infection at BRH and CHEO were calculated from patient level data. Cost for medical evacuation, return trip to community and hostel stays were calculated from patient level data provided by the Government of Nunavut. There were no costs related to adverse drug events included in the model.
Table 2. Direct costs (2007 $CAD) associated with a RSV hospitalisation in the Eastern Canadian Arctic.
Indirect costs
The societal perspective model incorporated indirect costs associated with future lost productivity. The average Canadian weekly earnings in 2007 were reported as $770.82 by Statistics CanadaCitation27. This was multiplied by 52 weeks to account for the annual wage per person and was adjusted by the employment rate which was 63.8% in November 2007Citation28. Together these data were used to estimate the average yearly earnings per person. This value was used to represent the annual wage lost for each infant who died prematurely. Years of lost productivity were considered to be from 16 to 65 years of age. To maintain a conservative model, northern cost of living allowances (typically 30%) were not added to the wage rate.
Discounting
Health outcomes and costs accrued beyond 1 year were discounted at 5% for the base-case analysis as recommended by the Canadian Agency for Drugs and Technologies in Health (CADTH)Citation22. In addition, non-discounted results were also included in keeping with CADTH guidelines.
Scenario analyses
To determine the groups with optimal cost effectiveness, scenarios included prophylaxing all infants <1 year of age or just infants <6 months of age during the RSV season. In addition to calculating the cost effectiveness of palivizumab for Baffin Island, estimates were also made for Iqaluit (urban, capital of Nunavut), rural areas (defined as outside of Iqaluit) and high risk areas (defined as having hospitalisation rates over 500/1000 live births).
Sensitivity analyses
To examine the robustness of the model, sensitivity analyses were conducted on three key point estimates. For mortality, rates of 3.3% and 4.2%, derived from Orr et al Citation29 and Feltes et alCitation19, respectively were used. Orr et al demonstrated a 3.3% mortality rate for Inuit infants with LRTI over a 1-year period while Feltes et al showed a 4.2% mortality rate in patients with congenital heart disease, a particularly vulnerable population. The Nunavut Report on Comparable Health Indicators found that Inuit infants had a threefold higher mortality rate than the general population based Canadian infantCitation30. Therefore, a mortality rate of 3% over a year is a reasonable mortality rate for RSV if the 1% from the IMpact studyCitation20 is assumed and multiplied by 3. The second sensitivity analysis used discount rates of 0% and 3% as recommended by the CADTHCitation22. These were applied to both costs and outcomes.
Probabilistic sensitivity analysis (PSA)
A PSACitation31 was also performed to demonstrate the robustness of the model against input assumptions. Using Monte Carlo techniques, all model parameters were varied simultaneously according to pre-specified distributions based on trial data. Distributions were assigned according to the inherent characteristics of each parameter according to the accepted conventionsCitation31, recommending the use of beta distributions for probabilities and utilities and gamma distributions for resource utilisation and costs. Hence, beta distributions were used to model the variability in the mortality rate, utilities and BRH and CHEO length of stay (LOS). presents the point estimates used in the model and the distributions for the PSA. The PSA used discounted values for direct costs (indirect costs were excluded) and effectiveness. Results were based on 10,000 Monte Carlo simulations.
Results
shows the health and economic outcomes for term Canadian Inuit infants. When compared with no prophylaxis, Palivizumab prophylaxis led to an additional medical cost of $5,057.The inclusion of indirect costs (future lost productivity) reduced the additional cost to $4,753. Use of palivizumab led to a gain in 0.23 QALYs without discounting and 0.1286 QALYs after discounting. also shows the cost-effectiveness results for this population. Palivizumab prophylaxis resulted in an ICER of $39,435/QALY after discounting. From the societal perspective, the inclusion of indirect costs lowered the ICER to $37,070/QALY after discounting.
Table 3. Costs and health outcomes for palivizumab versus no prophylaxis in term Inuit infants in the Eastern Canadian Arctic.
shows the results of the sensitivity analyses for the base-case analysis. The results were insensitive to varying the RSV mortality rate. Using a mortality rate of 3.3%, palivizumab prophylaxis resulted in an ICER of $28,289/QALY after discounting. The results were not sensitive to varying the discounting rate. At 0% discounting, prophylaxis with palivizumab resulted in an ICER of $21,737/QALY. At 3% discounting, the use of palivizumab lead to an ICER of $32,709/QALY.
Table 4. Results of sensitivity analysis for the base-case analysis using alternate mortality rates, discounting rates, health utilities.
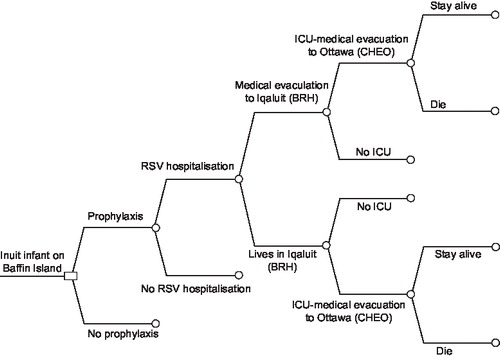
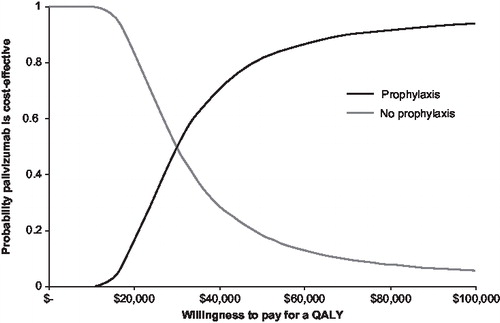
The results of the probabilistic sensitivity analyses for all of Baffin Island (<1 year of age) and high risk rural areas (<1 year of age) are shown in and . presents the 10,000 Monte Carlo simulations in terms of incremental costs (y-axis) and QALYs (x-axis) taking no prophylaxis as reference. presents the cost-effectiveness acceptability curves (CEACs) for the incremental cost per QALY gained. The way to interpret this CEAC is to consider a threshold that decision makers might be willing to pay for a unit of effect (i.e., willingness to pay per QALY gained) along with the horizontal axis and read along the vertical axis the probability that the treatment is cost effective after accounting for uncertainty. As shown in and , if decision makers were willing to pay $50,000 per QALY, the probability of palivizumab being cost effective was 0.82, and 0.92 if decision makers were willing to pay $75,000 per QALY.
Figure 2. Incremental cost-effectiveness plane for palivizumab prophylaxis in term Inuit infants in the Eastern Canadian Arctic (all of Baffin, under 1 year of age). Results are based on 10,000 Monte Carlo simulations shown in terms of cost (y-axis) and quality adjusted life-years (QALYs). The solid line represents an ICER threshold of $75,000/QALY while the dotted line represents an ICER threshold of $50,000/QALY. Simulations indicate a probability of 82.7% that the ICER falls below $50,000/QALY and 92.3% probability that it falls below an ICER of $75,000/QALY.
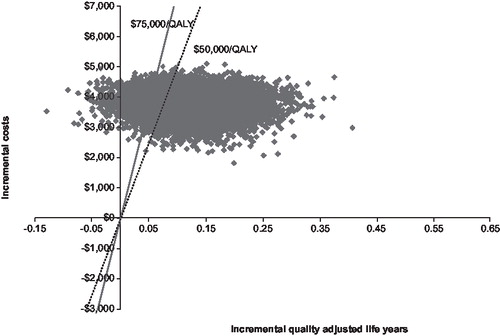
Figure 3. Cost-effectiveness acceptability curve (CEAC) for palivizumab prophylaxis in term Inuit infants. Results are based on probabilistic sensitivity analysis. CEACs can be interpreted by considering a threshold that decision makers might be willing to pay for a unit of effect (i.e., willingness to pay per QALY gained) along the horizontal axis and reading along the vertical axis the probability that the treatment is cost effective after accounting for uncertainty.
Discussion
Results from this study demonstrated an ICER of $39,435/QALY after discounting for all infants less than 1 year of age on Baffin Island. These results are similar to a previous cost-effectiveness analysis of premature infants born at 32–35 weeks’ gestational age in Canada by Lanctôt et al Citation16, which demonstrated an ICER of $20,924/QALY after discounting, including the benefits of asthma in the base-case analysis. There are several differences between that study and the current study. That study used a mortality rate of 8.1% based on SampalisCitation21, while this study used a much lower mortality rate of 1%. In sensitivity analyses, when the mortality rate was increased to the conservative estimate of 3.3% for the Inuit population, the ICER decreased to $27,138/QALY, comparable to the results of Lanctôt et al. The benefits of palivizumab prophylaxis on the subsequent reduction of asthma were not included in this study, as there is a paucity of research in this special population. This also makes this analysis more conservative.
Importantly, this study showed that when location of residence and age were considered, the ICER values obtained were very different compared to that for all of Baffin Island (base-case analysis). The ICER value for those living in Iqaluit (<1 year of age) was $152,145/QALY, much higher than that for all of Baffin Island. These high ICERs for those living in Iqaluit can be attributed to the low rate of hospitalisation for RSV and the fact that there are no transportation costs associated with a RSV hospitalisation. Banerji et al Citation12 suggested that there may also be a protective effect of having a hospital within the community with regard to the rates and severity of RSV. The ICERs of those infants less than 6 months of age living in rural areas or high risk rural areas were dominant (i.e., resulted in cost savings) and were much lower than that of the entire Baffin Island. These dominant ICERs can be attributed to the higher hospitalisation rates and high transportation costs associated with residing in the rural areas. The inclusion of indirect costs (lost productivity) further decreases the ICERs in this study, and makes palivizumab very cost effective for all infants less than 6 months of age, living in rural areas and high risk rural areas (<1 year of age).
There are no specific thresholds of cost effectiveness in Canada. However, in a study by Laupacis et alCitation32, ICERs between $0 and $20,000 represent strong evidence for adoption; between $20,000 and $100,000 represent moderate evidence for adoption while ICERs above $100,000 represent weak evidence for adoption. In Canada, interventions with ICERs between $50,000 and $75,000 are commonly adopted, with the highest ICER being $113,000/QALY recommended for adoption by the Common Drug Review (CDR) up to May 31st, 2008Citation33. Probabilistic sensitivity analyses demonstrated that 82% of the ICERs fell below $50,000/QALY and that 92% of the ICERs were below $75,000/QALY for the base-case analysis (all of Baffin Island). Sub-group PSA demonstrated that 98% of the ICERs were dominant for high-risk rural infants under 1 year of age. Thus depending on the location and age of the infant, there is strong to moderate evidence for the adoption of palivizumab prophylaxis in both the payers’ and societal perspective.
Lanctôt et alCitation16 included the benefits of preventing asthma after palivizumab prophylaxis. There have been many studies published regarding the sequelae of reactive airways disease such as asthma and wheezing after RSV infectionCitation23,34,35. Studies have demonstrated that RSV hospitalisation in Alaska Native children were associated with increased wheezing, LRTIs, and asthma diagnosed in the first 4 years of lifeCitation36 and other studies have shown that LRTI in the Inuit is associated with abnormal pulmonary functions testing and a high risk of bronchietasisCitation37–40. To maintain a conservative model, the benefits of treating or preventing asthma was not included in the study. However, if the estimated cost of asthma for this population is at least the same as the general Canadian population, further cost effectiveness would be observed in this model. The lack of information regarding asthma in this vulnerable population highlights the need for more research in this area.
The true efficacy of palivizumab in this population is not known. However, the 78% reduction in hospitalisation based on the IMpact study for premature children without chronic lung disease is a reasonable estimate for term Inuit infants. Motavizumab, a sister drug of palivizumab, demonstrated a 83% reduction in RSV hospitalisation in healthy, American-Native infantsCitation41. This model assumes that there is no wastage of palivizumab (e.g., vial sharing), a common practice in clinicsCitation42,43. However, it is unknown the degree to which vial sharing could be achieved in the more remote communities. This analysis included the cost of administration, and assumed that it would take nurses at most 30 minutes to administer the injection. The cost of readmissions, follow-up visits, health professionals accompanying the child during the return flights were not included in this cost-effectiveness analysis and may underestimate the true cost of a RSV hospitalisation in the Eastern Canadian Arctic. Moreover, while this study assumed five monthly injections as a full course for prophylaxis, the variability of the Arctic RSV season may significantly influence the duration of prophylaxis.
Conclusions
In conclusion, this study demonstrates that palivizumab is cost effective when given to all infants less than 1 year of age residing in the Eastern Canadian Arctic. In particular, palivizumab is the dominant strategy for those under 6 months of age living in rural areas from the payer's perspective and a dominant strategy for those under 6 months of age, living in rural areas and for those under 1 year of age, living in high risk rural areas. A cost impact analysis by Banerji et al Citation12 suggested that implementation of prophylaxis for all infants living in rural areas is affordable. Current guidelines need to be changed to reflect the need for prophylaxis of those living in remote, rural areas. Changes in these policies will not only improve the lives of aboriginal people but can also result in cost savings for the payers.
Acknowledgements
Declaration of interest: B.P. and K.L. have disclosed that they are principal investigators and C.H. is a site investigator on an investigator-initiated grant from Abbott Laboratories, Ltd. in support of a registry for palivizumab. A.B. (principal investigator) and B.P., K.L. and C.H. (co-investigators) have disclosed that they have received a grant from Abbott International for similar research. K.L. and A.B. have disclosed that they have received honoraria for presentations at unrestricted educational events sponsored by Abbott International. D.Y.T. and J.T. have disclosed they have no relevant financial relationships.
References
- Levy BT, Graber MA. Respiratory syncytial virus infection in infants and young children. J Fam Pract 1997;45:473-81.
- Ottolini MG, Hemming VG. Prevention and treatment recommendations for respiratory syncytial virus infection. Background and clinical experience 40 years after discovery. Drugs 1997;54: 867-84.
- Simoes EA. Respiratory syncytial virus infection. Lancet 1999;354:847-52.
- Banerji A. High rates of hospitalisation for bronchiolitis in Inuit children on Baffin Island. Int J Circumpolar Health 2001;60:375-9.
- Banerji A, Bell A, Mills EL, Lower respiratory tract infections in Inuit infants on Baffin Island. CMAJ 2001;164:1847-50.
- Carson JB, Postl BD, Spady D, Lower respiratory tract infections in Inuit infants on Baffin Island. Int J Circumpolar Health 2001;84:226-8.
- Young M, Kandola K, Mitchell R, Hospital admission rates for lower respiratory tract infections in infants in the Northwest Territories and the Kitikmeot region of Nunavut between 2000 and 2004. Paediatr Child Health 2007;12:563-6.
- Hall CB. Respiratory syncytial virus. In: Feigin R, Cherry J , eds. Infectious Diseases. Philadelphia: W.B. Saunders, 1998.
- Anderson LJ, Hierholzer JC, Tsou C, Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis 1985;151:626-33.
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The Impact-RSV Study Group [see comments]. Pediatrics 1998;102:531-7.
- Canadian Pediatric Society. Palivizumab and respiratory syncytial virus immune globulin intravenous for the prophylaxis of respiratory syncytial virus infection in high risk infants. Paediatr Child Health 1999;4(7):474-80.
- Banerji A, Lanctôt KL, Paes BA, Comparison of the cost of hospitalization for respiratory syncytial virus disease versus palivizumab prophylaxis in Canadian Inuit infants. Pediatr Infect Dis J 2009;28:702-6.
- Banerji A, Greenberg D, White LF, Risk factors and viruses associated with hospitalization due to lower respiratory tract infections in Canadian Inuit children: a case-control study. Pediatr Infect Dis J 2009;28:697-701.
- Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics 1997;99:93-9.
- Langley JM, Wang EE, Law BJ, Economic evaluation of respiratory syncytial virus infection in Canadian children: a Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. J Pediatr 1997;131:113-17.
- Lanctôt KL, Masoud ST, Paes BA, The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32-35 weeks: a Canadian-based analysis. Curr Med Res Opin 2008;24:3223-7.
- Creery D, Lyer P, Samson L, Costs associated with infant bronchiolitis in the Baffin region of Nunavut. Int J Circumpolar Health 2005;64:38-45.
- Nuijten MJ, Wittenberg W, Lebmeier M. Cost effectiveness of palivizumab for respiratory syncytial virus prophylaxis in high-risk children: a UK analysis. Pharmacoeconomics 2007;25(1): 55-71.
- Feltes TF, Cabalka AK, Meissner HC, Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003;143:532-40.
- IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 1998;102:531-7.
- Sampalis JS. Morbidity and mortality after RSV-associated hospitalizations among premature Canadian infants. J Pediatr 2003;143:S150-6.
- Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada [3rd edn]. Ottawa, 2006.
- Greenough A, Alexander J, Burgess S, Health care utilisation of prematurely born, preschool children related to hospitalisation for RSV infection. Arch Dis Child 2004;89:673-8.
- Sigurs N, Gustafsson PM, Bjarnason R, Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005;171:137-41.
- Stein RT, Sherrill D, Morgan WJ, Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541-5.
- Centers for Disease Control and Prevention. Clinical Growth Charts. [cited 2008; Available from: http://www.cdc.gov/growthcharts/clinical_charts.htm.
- Statistics Canada: Canada's National Statistical Agency. Earnings, average weekly, by province and territory (Table). In 2007.
- Statistics Canada: Canada's National Statistical Agency. The Daily: Labour Force Survey. 2007 [cited; Available from: http://www.statcan.ca/Daily/English/071207/d071207a.htm.
- Orr P, McDonald S, Milley D, Bronchiolitis in Inuit children from a Canadian central Arctic community, 1995-1996. Int J Circumpolar Health 2001;60:649-58.
- Healey S, Plaza D, Qayyum A, Nunavut report on comparable health indicators. In: Department of Health and Social Services, Government of Nunavut, editors, 2004.
- Briggs A, Sculpher MJ, Claxton K. Decision Modelling for Health Economic Evaluation. Handbooks in Health Economic Evaluation, volume 1. Oxford: Oxford University Press, 2006.
- Laupacis A, Feeny D, Detsky AS, How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473-81.
- Canadian Agency for Drugs and Technologies in Health (CADTH). Common Drug Review Database. [cited July 31, 2008]; Available from: http://www.cadth.ca/index.php/en/cdr/search? &status=complete&order_field=drug_name.
- Greenough A, Cox S, Alexander J, Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child 2001;85:463-8.
- Perez-Yarza EG, Moreno A, Lazaro P, The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatr Infect Dis J 2007;26:733-9.
- Singleton RJ, Redding GJ, Lewis TC, Sequelae of severe respiratory syncytial virus infection in infancy and early childhood among Alaska Native children. Pediatrics 2003;112:285-90.
- Singleton R, Morris A, Redding G, Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatr Pulmonol 2000;29:182-7.
- Fleshman JK, Wilson JF, Cohen JJ. Bronchiectasis in Alaska native children. Arch Environ Health 1968;17:517-23.
- Redding G, Singleton R, Lewis T, Early radiographic and clinical features associated with bronchiectasis in children. Pediatr Pulmonol 2004;37:297-304.
- Hemmelgarn B, Ernst P. Airway function among Inuit primary school children in far northern Quebec. Am J Respir Crit Care Med 1997;156:1870-5.
- Chandran A, Millar EV, Weatherholtz R, Safety and efficacy of motavizumab in the prevention of RSV disease in healthy infants. In: Pediatric Academic Societies, 4 May 2008; Hawaii. Abstract 4460.5.
- Gooding J, Millage A, Rye A-K, The cost and safety of multidose use of palivizumab vials. Clin Pediatr (Phila) 2007;47: 160-3.
- Wills S, Simpson JH, Coutts J. Cost minimisation of RSV prevention with palivizumab. Arch Dis Child 2006;91:717